Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micropropagation and Genetic Transformation of Potato Plants
2.2. Acclimatization of Potato
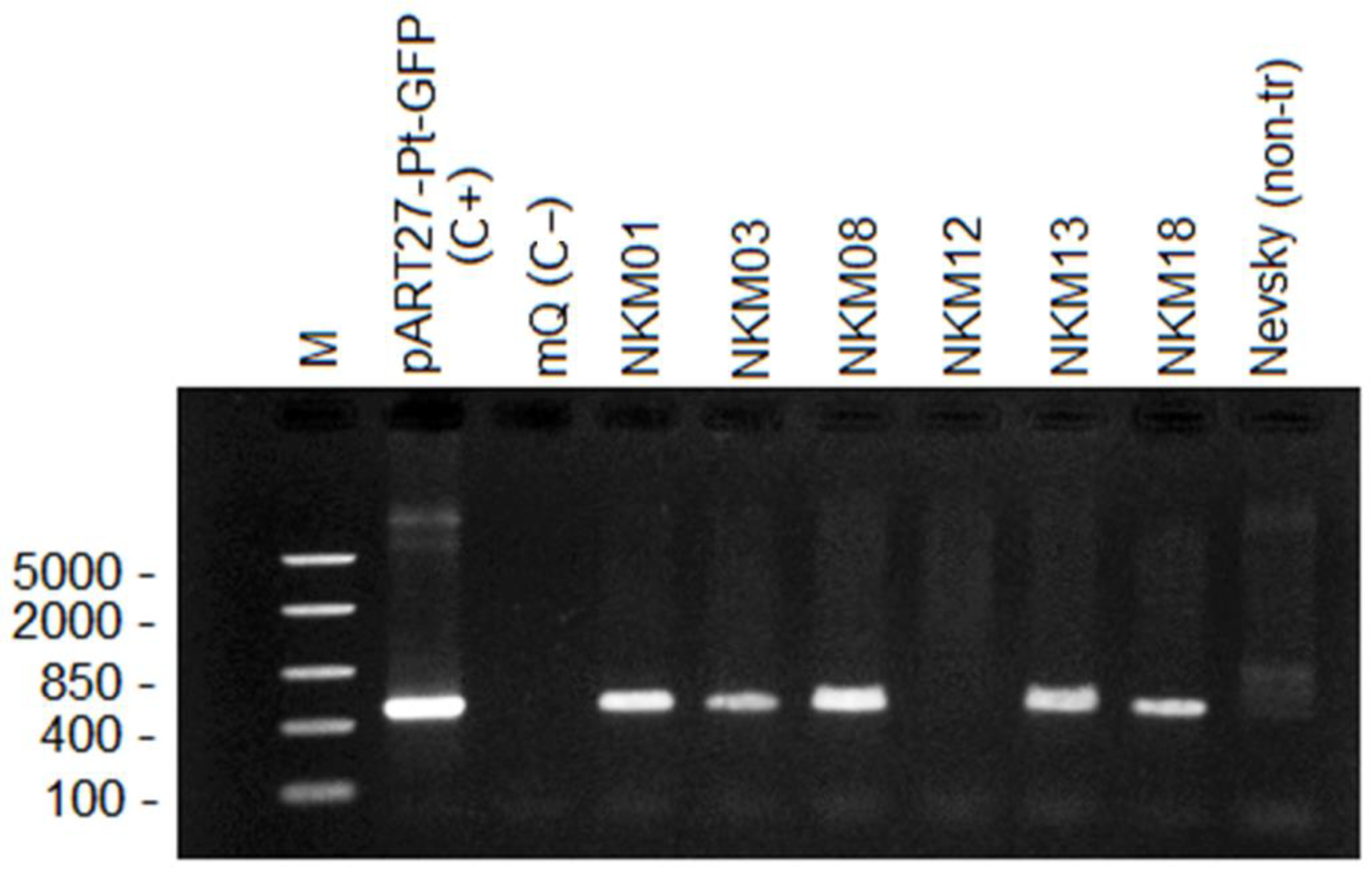
2.3. PCR Analysis of Transgenic Plants
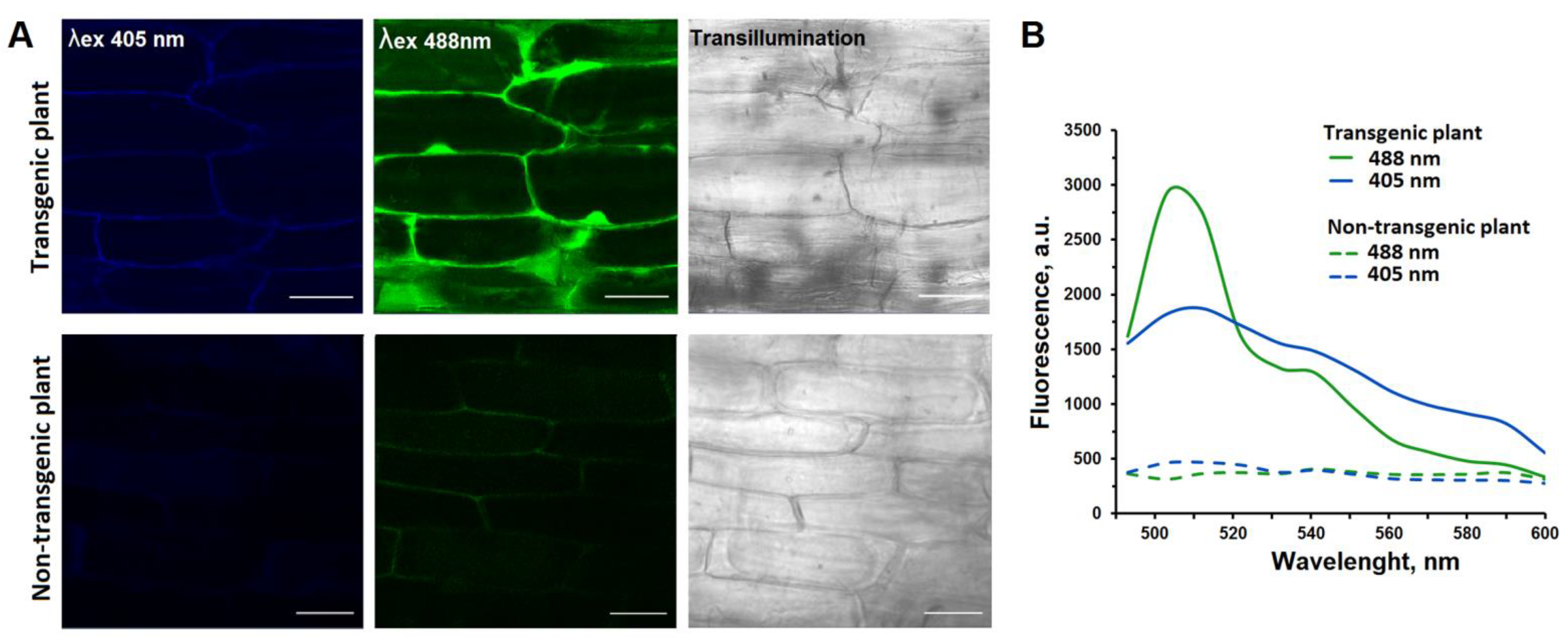
2.4. Confocal Laser Scanning Microscopy (CLSM)
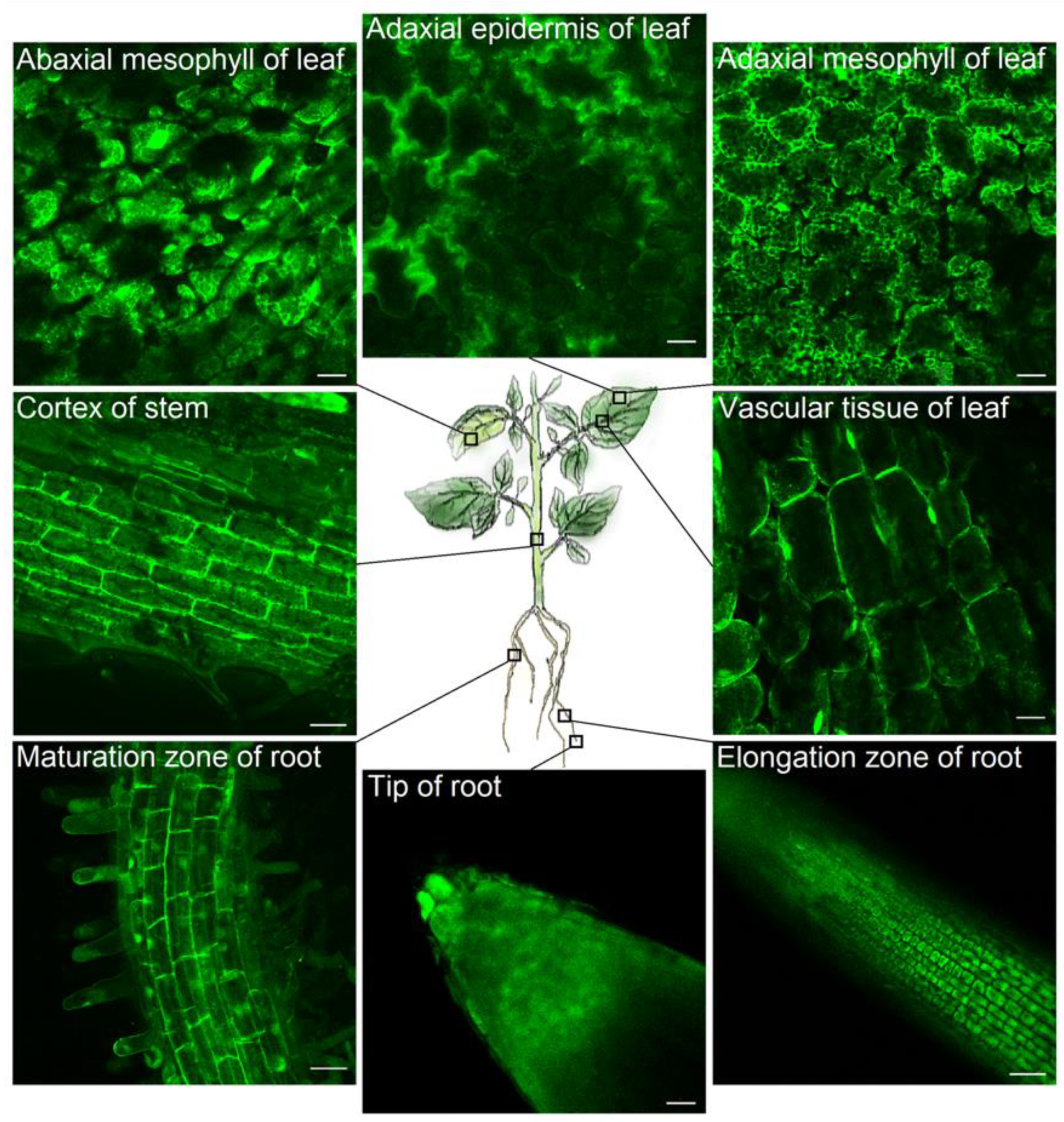
2.5. Fluorescence Imaging of Pt-GFP in the Leaf of a Whole Plant
- The abaxial side of the leaves was cooled or heated using Peltier device, the polarity of which can be changed to switch from cooling to heating and back. The temperature changed periodically. In total, four temperature effects were applied with increasing strength, lasting 2 min within a period of 5 min. The cooling strength was 16.4, 9.4, 4.9 and 3 °C. The heating was carried out with the same time intervals to 34.7, 42.9, 50.7 and 59.1 °C;
- The tips of the leaves were heated with a ceramic cement resistor for 4 min to 52 °C;
- The detached leaves treated with a H+-ATPase inhibitor (2.5 mM sodium orthovanadate) or standard solution (1 mM NaCl, 0.1 mM KCl, 0.1 mM CaCl2) were first cooled to 9.4 °C for 3 min, and then after 7 min heated to 42.9 °C for 3 min by the Peltier device. The treatment of the leaves with reagents was carried out by loading them through the petiole using a desiccator and vacuum pump at a pressure of 0.2 atmosphere for 5 min;
- H+-ATPase inhibitor or standard solution was added to 10 × 10 mm pieces cut out of the leaves and placed in Petri dishes (volume 15 mL) filled with standard solution.
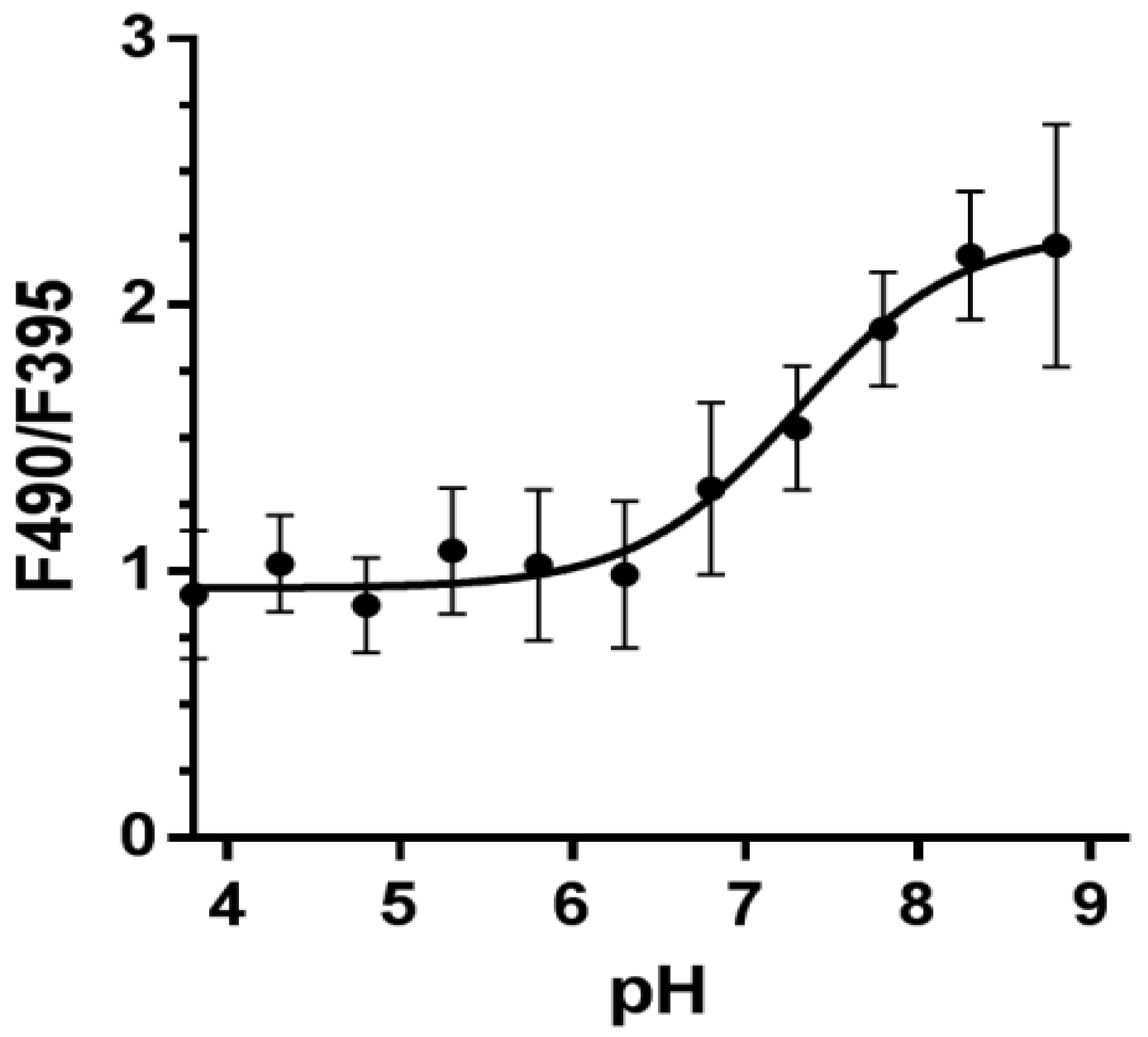
2.6. In Vivo Calibration of Pt-GFP Signal in the Potato Plants. Ratiometric Analysis
2.7. Testing the Temperature Dependence of Pt-GFP Fluorescence
2.8. Measurements of Electric Potential in Potato Plants Using Macroelectrodes
2.9. Temperature Changes of Potato Leaves
2.10. Statistical Analysis
3. Results
3.1. Characteristics of Potato Transformants
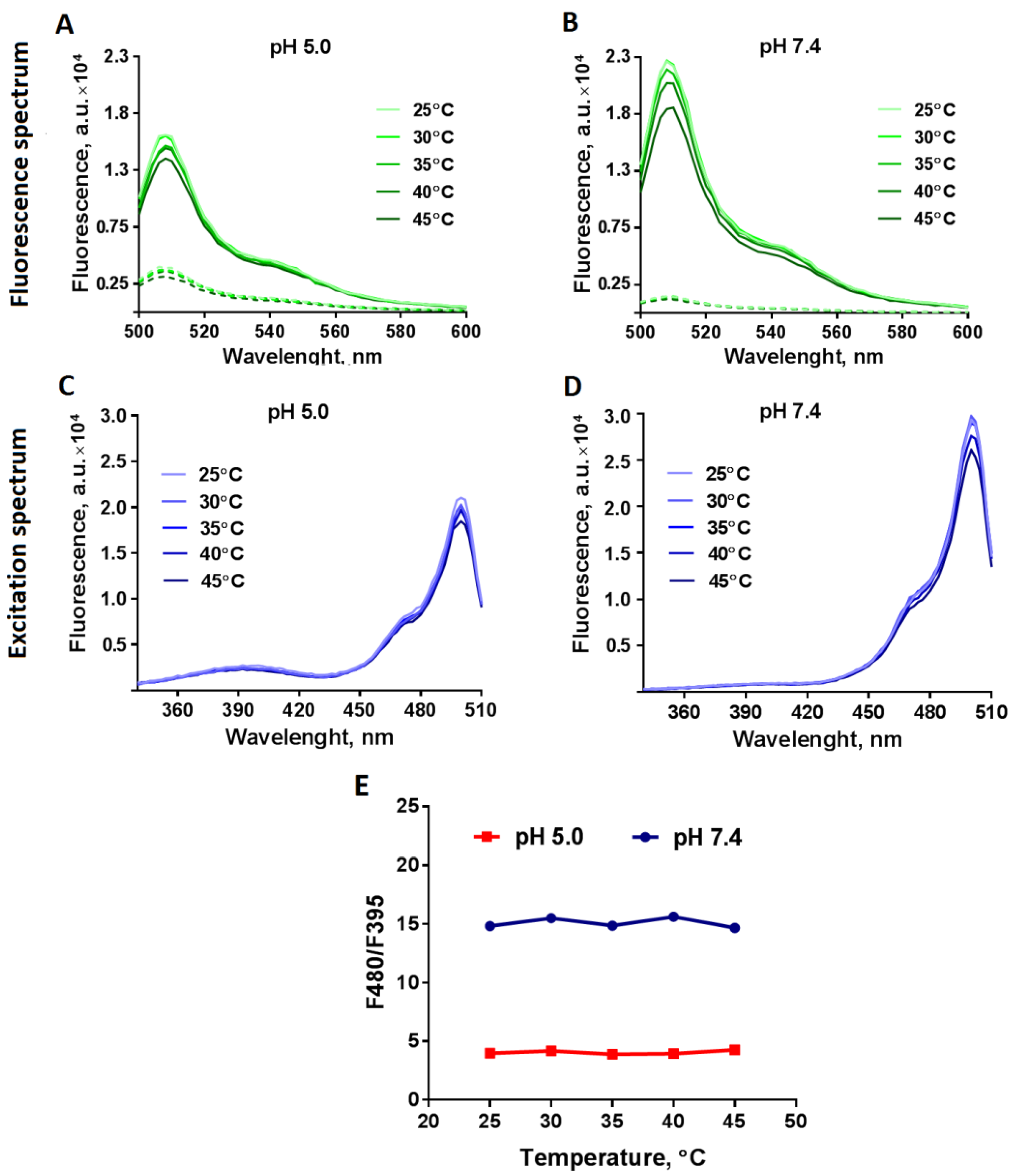
3.2. Testing the Temperature Dependence of Pt-GFP Fluorescence
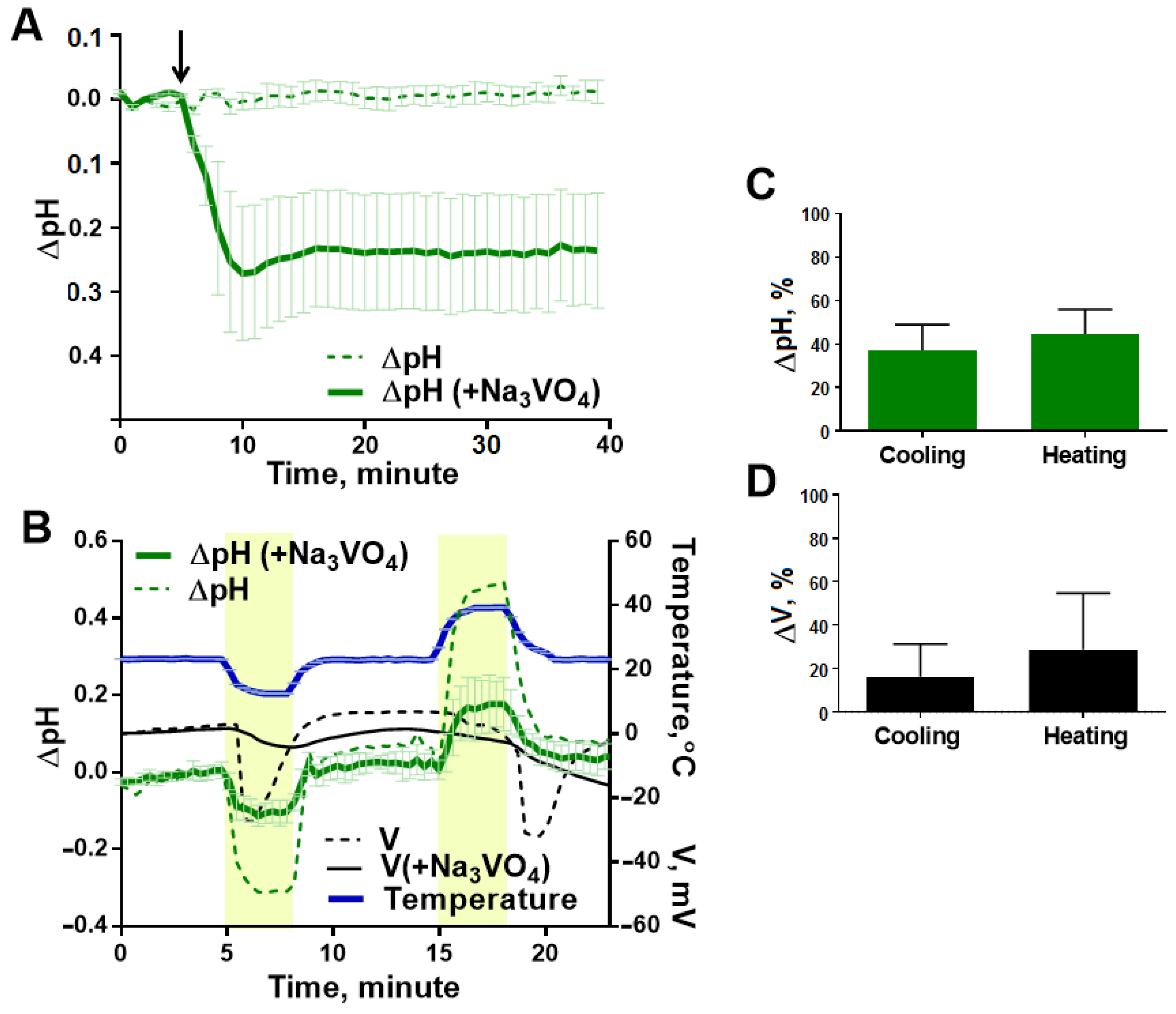
3.3. Temperature-Induced pH Shifts in the Potato Leaf
3.4. Registration of pH Changes at a Distance from the Zone of Local Stimulation
3.5. Analysis of the Mechanism of Temperature Induced Changes in pH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Kaur, L. Advances in Potato Chemistry and Technology, 1st ed.; Academic Press: Cambridge, MA, USA, 2009; p. 528. [Google Scholar]
- Halterman, D.; Guenthner, J.; Collinge, S.; Butler, N.; Douches, D.S. Biotech Potatoes in the 21st Century: 20 Years Since the First Biotech Potato. Am. J. Potato Res. 2016, 93, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Akpenpuun, T.D.; Mijinyawa, Y. Evaluation of a Greenhouse under Tropical Conditions Using Irish Potato (Solanum Tuberosum) as the Test Crop. Acta Technol. Agric. 2018, 21, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, A.; Huynh, H.D.; Endo, T.; Watanabe, K. Review of recent transgenic studies on abiotic stress tolerance and future molecular breeding in potato. Breed. Sci. 2015, 65, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Rykaczewska, K. The Effect of High Temperature Occurring in Subsequent Stages of Plant Development on Potato Yield and Tuber Physiological Defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Sang, W.-G.; Baek, J.-K.; Kim, J.-H.; Shin, P.; Seo, M.-C.; Cho, J.-I. The effect of concurrent elevation in CO2 and temperature on the growth, photosynthesis, and yield of potato crops. PLoS ONE 2020, 15, e0241081. [Google Scholar] [CrossRef]
- Struik, P.C. Responses of the Potato Plant to Temperature. In Potato Biology and Biotechnology: Advances and Perspectives, 1st ed.; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Taylor, M., MacKerron, D., Ross, H., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 367–393. [Google Scholar] [CrossRef]
- Orzechowski, S.; Sitnicka, D.; Grabowska, A.; Compart, J.; Fettke, J.; Zdunek-Zastocka, E. Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves. Int. J. Mol. Sci. 2021, 22, 7203. [Google Scholar] [CrossRef]
- Wei, S.; Liu, J.; Li, T.; Wang, X.; Peng, A.; Chen, C. Effect of High-Temperature Events When Heading into the Maturity Period on Summer Maize (Zea mays L.) Yield in the Huang-Huai-Hai Region, China. Atmosphere 2020, 11, 1291. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, T.; de Bruin, K.; Glomrød, S.; Shi, Q. Extreme Weather Impacts on Maize Yield: The Case of Shanxi Province in China. Sustainability 2017, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Tomás, D.; Rodrigues, J.C.; Viegas, W.; Silva, M. Assessment of High Temperature Effects on Grain Yield and Composition in Bread Wheat Commercial Varieties. Agronomy 2020, 10, 499. [Google Scholar] [CrossRef] [Green Version]
- Subedi, K.; Gregory, P.; Summerfield, R.; Gooding, M. Cold temperatures and boron deficiency caused grain set failure in spring wheat (Triticum aestivum L.). Field Crop. Res. 1998, 57, 277–288. [Google Scholar] [CrossRef]
- Chen, C.-T.; Setter, T. Role of Tuber Developmental Processes in Response of Potato to High Temperature and Elevated CO2. Plants 2021, 10, 871. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo-Rodríguez, A.; Rodríguez-Flores, M.S.; Meno, L.; Seijo, M.C. Changes in the Morphological Characteristics of Potato Plants Attributed to Seasonal Variability. Agriculture 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-U.; Seo, B.-S.; Choi, D.-H.; Ban, H.-Y.; Lee, B.-W. Impact of high temperatures on the marketable tuber yield and related traits of potato. Eur. J. Agron. 2017, 89, 46–52. [Google Scholar] [CrossRef]
- Vashisht, B.; Nigon, T.; Mulla, D.; Rosen, C.; Xu, H.; Twine, T.; Jalota, S. Adaptation of water and nitrogen management to future climates for sustaining potato yield in Minnesota: Field and simulation study. Agric. Water Manag. 2015, 152, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Felle, H.H. pH: Signal and Messenger in Plant Cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Kesten, C.; Gámez-Arjona, F.M.; Menna, A.; Scholl, S.; Dora, S.; Huerta, A.I.; Huang, H.Y.; Tintor, N.; Kinoshita, T.; Rep, M.; et al. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019, 38, e101822. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the Arabidopsis Endomembrane System. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, K.; Wingen, M.; Potzkei, J.; Gensch, T.; Jaeger, K.E.; Drepper, T. A novel FbFP-based biosensor toolbox for sensitive in vivo determination of intracellular pH. J. Biotechnol. 2017, 258, 25–32. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Förster, H.; Adaskaveg, J.E. Visualization of Localized Pathogen-Induced pH Modulation in Almond Tissues Infected by Colletotrichum acutatum Using Confocal Scanning Laser Microscopy. Phytopathology 2008, 98, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.C.; Allen, N.S. Changes in Cytosolic pH within Arabidopsis Root Columella Cells Play a Key Role in the Early Signaling Pathway for Root Gravitropism. Plant Physiol. 1999, 121, 1291–1298. [Google Scholar] [CrossRef] [Green Version]
- Gjetting, S.; Ytting, C.K.; Schulz, A.; Fuglsang, A.T. Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J. Exp. Bot. 2013, 63, 3207–3218. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Chirkova, T.V.; Shishova, M.F.; Lindberg, S.M. Potassium Efflux and Cytosol Acidification as Primary Anoxia-Induced Events in Wheat and Rice Seedlings. Plants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Barnes, A.C.; Benning, C.; Roston, R.L. Chloroplast Membrane Remodeling during Freezing Stress Is Accompanied by Cytoplasmic Acidification Activating Sensitive to Freezing2. Plant Physiol. 2016, 171, 2140–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S. Low temperature-induced cytoplasmic acidosis in cultured mung bean (Vigna radiate (L.) Wilczek) cells. Plant Physiol. 1994, 104, 1131–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Felle, H.H. pH Regulation in Anoxic Plants. Ann. Bot. 2005, 96, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, H.; Chanroj, S. Plant Endomembrane Dynamics: Studies of K+/H+ Antiporters Provide Insights on the Effects of pH and Ion Homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef] [Green Version]
- Wegner, L.H.; Shabala, S. Biochemical pH clamp: The forgotten resource in membrane bioenergetics. New Phytol. 2020, 225, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.-I.; Kinoshita, T. Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Polak, M.; Karcz, W. Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses. Int. J. Mol. Sci. 2021, 22, 5017. [Google Scholar] [CrossRef]
- Camoni, L.; Barbero, F.; Aducci, P.; Maffei, M.E. Spodoptera littoralis oral secretions inhibit the activity of Phaseolus lunatus plasma membrane H+-ATPase. PLoS ONE 2018, 13, e0202142. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Gray, W.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, H.; Chen, L.; Liu, L.; Wan, X. Maintenance of mesophyll potassium and regulation of plasma membrane H+-ATPase are associated with physiological responses of tea plants to drought and subsequent rehydration. Crop. J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Lindberg, S.; Banaś, A.; Stymne, S. Effects of different cultivation temperatures on plasma membrane ATPase activity and lipid composition of sugar beet roots. Plant Physiol. Biochem. 2005, 43, 261–268. [Google Scholar] [CrossRef]
- Muzi, C.; Camoni, L.; Visconti, S.; Aducci, P. Cold stress affects H+-ATPase and phospholipase D activity in Arabidopsis. Plant Physiol. Biochem. 2016, 108, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grams, T.; Lautner, S.; Felle, H.H.; Matyssek, R.; Fromm, J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 2009, 32, 319–326. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, Y.; Chen, L.; Sheng, F.; Zhou, H. Secondary Structure Changes and Thermal Stability of Plasma Membrane Proteins of Wheat Roots in Heat Stress. Am. J. Plant Sci. 2011, 2, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Mariamma, M.; Muthukumar, B.; Gnanam, A. Thermotolerance and effect of heat shock on the stability of the ATPase enzyme in rice. J. Plant Physiol. 1997, 150, 739–742. [Google Scholar] [CrossRef]
- Bobik, K.; Duby, G.; Nizet, Y.; Vandermeeren, C.; Stiernet, P.; Kanczewska, J.; Boutry, M. Two widely expressed plasma membrane H+-ATPase isoforms of Nicotiana tabacum are differentially regulated by phosphorylation of their penultimate threonine. Plant J. 2010, 62, 291–301. [Google Scholar] [CrossRef]
- Sze, H.; Li, X.; Palmgren, M.G. Energization of Plant Cell Membranes by H+-Pumping ATPases: Regulation and Biosynthesis. Plant Cell 1999, 11, 677–689. [Google Scholar] [PubMed] [Green Version]
- Szechynska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical Signaling, Photosynthesis and Systemic Acquired Acclimation. Front. Physiol. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2006, 30, 249–257. [Google Scholar] [CrossRef]
- Bowling, D. [23] Intracellular and intercellular pH measurement with microelectrodes. Methods Enzymol. 1989, 174, 331–338. [Google Scholar] [CrossRef]
- Kurkdjian, A.; Guern, J. Intracellular pH: Measurement and importance in cell activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 271–303. [Google Scholar] [CrossRef]
- Voipio, J.; Pasrernack, M.; Macleod, K. Ion-sensitive microelectrodes. In Microelectrode Techniques: The Plymouth Workshop Handbook, 2nd ed.; Ogden, D., Ed.; The Company of Biologists Limited: Cambridge, UK, 1994; pp. 275–316. [Google Scholar]
- Kunkel, J.G.; Cordeiro, S.; Xu, Y.J.; Shipley, A.M.; Feijó, J.A. Use of Non-Invasive Ion-Selective Microelectrode Techniques for the Study of Plant Development. In Plant Electrophysiology; Springer: New York, NY, USA, 2006; Volume 5, pp. 109–137. [Google Scholar]
- Diaspro, A.; Chirico, G.; Usai, C.; Ramoino, P.; Dobrucki, J. Photobleaching. In Handbook of Biological Confocal Microscopy, 3rd ed.; Pawley, J., Ed.; Springer: Boston, MA, USA, 2006; pp. 690–702. [Google Scholar] [CrossRef]
- Schulte, A.; Lorenzen, I.; Böttcher, M.; Plieth, C. A novel fluorescent pH probe for expression in plants. Plant Methods 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharnagl, C.; Raupp-Kossmann, R.; Fischer, S. Molecular Basis for pH Sensitivity and Proton Transfer in Green Fluorescent Protein: Protonation and Conformational Substates from Electrostatic Calculations. Biophys. J. 1999, 77, 1839–1857. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, M. Green Fluorescent Protein (GFP): Applications, Structure, and Related Photophysical Behavior. Chem. Rev. 2002, 102, 759–782. [Google Scholar] [CrossRef]
- Martinière, A.; Desbrosses, G.; Sentenac, H.; Paris, N. Development and properties of genetically encoded pH sensors in plants. Front. Plant Sci. 2013, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Peele, B.; Gururaja, T.L.; Anderson, D.C. Characterization and use of green fluorescent proteins from Renilla mulleri and Ptilosarcus gurnyei for the human cell display of functional peptides. J. Protein Chem. 2001, 20, 507–519. [Google Scholar] [CrossRef]
- Behera, S.; Xu, Z.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzländer, M.; Costa, A. Cellular Ca2+ Signals Generate Defined pH Signatures in Plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Fulton, T.M.; Chunwongse, J.; Tanksley, S.D. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 1995, 13, 207–209. [Google Scholar] [CrossRef]
- ZEISS ZEN. Digital Imaging for Light Microscopy. Downloads. Available online: https://www.zeiss.com/microscopy/int/products/microscope-software/zen.html#downloads (accessed on 10 September 2021).
- ImageJ. 2011. Available online: https://imagej.nih.gov/ij/ (accessed on 15 September 2021).
- Micro-Manager. Available online: https://micro-manager.org/ (accessed on 10 September 2021).
- GraphPad Curve Fitting Guide. Available online: https://static1.squarespace.com/static/564884dfe4b0734a6c40a22a/t/568a924c1115e06a4a3059bf/1451921996551/Prism-6-Curve-Fitting-Guide.pdf (accessed on 10 September 2021).
- IRSoft. Available online: https://www.testo.ru/ru-RU/funkcii_i_tekhnologii/po_testo_irsoft# (accessed on 10 September 2021).
- Geilfus, C.-M.; Mühling, K.H.; Kaiser, H.; Plieth, C. Bacterially produced Pt-GFP as ratiometric dual-excitation sensor for in planta mapping of leaf apoplastic pH in intact Avena sativa and Vicia faba. Plant Methods 2014, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafta, A.M.; Lorenzen, J.H. Effect of High Temperature on Plant Growth and Carbohydrate Metabolism in Potato. Plant Physiol. 1995, 109, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Krauss, A.; Marschner, H. Growth rate and carbohydrate metabolism of potato tubers exposed to high temperatures. Potato Res. 1984, 27, 297–303. [Google Scholar] [CrossRef]
- Siemering, K.R.; Golbik, R.; Sever, R.; Haseloff, J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr. Biol. 1996, 6, 1653–1663. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.R.; Kimata, Y.; Oka, M.; Nomaguchi, K.; Kohno, K. Thermosensitivity of Green Fluorescent Protein Fluorescence Utilized to Reveal Novel Nuclear-Like Compartments in a Mutant Nucleoporin NSP11. J. Biochem. 1995, 118, 13–17. [Google Scholar] [CrossRef]
- Yoshida, S.; Hotsubo, K.; Kawamura, Y.; Murai, M.; Arakawa, K.; Takezawa, D. Alterations of Intracellular pH in Response to Low Temperature Stresses. J. Plant Res. 1999, 112, 225–236. [Google Scholar] [CrossRef]
- Kadohama, N.; Goh, T.; Ohnishi, M.; Fukaki, H.; Mimura, T.; Suzuki, Y. Sudden Collapse of Vacuoles in Saintpaulia sp. Palisade Cells Induced by a Rapid Temperature Decrease. PLoS ONE 2013, 8, e57259. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y. Chilling induces a decrease in pyrophosphate-dependent H+-accumulation associated with a DeltapH(vac)-stat in mung bean, a chill-sensitive plant. Plant Cell Environ. 2008, 3, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, T.; Ruiz-Cabello, J.; Molina-García, A.D.; Escribano, M.I.; Merodio, C. Chilling Temperature Storage Changes the Inorganic Phosphate Pool Distribution in Cherimoya (Annona cherimola) Fruit. J. Am. Soc. Hortic. Sci. 2001, 126, 122–127. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nishimura, M.; Shimazaki, K. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of Fava Bean. Plant Cell 1995, 7, 1333–1342. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Wang, Z.; Huang, L.; Wang, C.; Hou, R.; Xu, Z.; Qiao, X. Research progress on electrical signals in higher plants. Prog. Nat. Sci. 2009, 19, 531–541. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Cold Signal Transduction and its Interplay with Phytohormones during Cold Acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janicka-Russak, M.; Kabała, K. Abscisic acid and hydrogen peroxide induce modification of plasma membrane H+-ATPase from Cucumis sativus L. roots under heat shock. J. Plant Physiol. 2012, 169, 1607–1614. [Google Scholar] [CrossRef]
- Yudina, L.; Sherstneva, O.; Sukhova, E.; Grinberg, M.; Mysyagin, S.; Vodeneev, V.; Sukhov, V. Inactivation of H+-ATPase Participates in the Influence of Variation Potential on Photosynthesis and Respiration in Peas. Plants 2020, 9, 1585. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Liu, Y.-P.; Pan, Q.-H.; Zhan, J.-C.; Wang, X.-Q.; Huang, W.-D. Changes in membrane-associated H+-ATPase activities and amounts in young grape plants during the cross adaptation to temperature stresses. Plant Sci. 2006, 170, 768–777. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef]
- Geilfus, K.M. The pH of the Apoplast: Dynamic Factor with Functional Impact under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef]
- Wegner, L.H.; Zimmermann, U. Bicarbonate-Induced Alkalinization of the Xylem Sap in Intact Maize Seedlings as Measured in Situ with a Novel Xylem pH Probe. Plant Physiol. 2004, 136, 3469–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.-J.; Chen, Y.; Wang, Z.-Y.; Xue, L.; Mao, T.-L.; Liu, Y.-M.; Wang, Z.-Y.; Huang, L. High-resolution non-contact measurement of the electrical activity of plants in situ using optical recording. Sci. Rep. 2015, 5, 13425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, S. PH as a stress signal. Plant Growth Regul. 1999, 29, 87–99. [Google Scholar] [CrossRef]
- Sukhov, V.; Surova, L.; Sherstneva, O.; Katicheva, L.; Vodeneev, V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front. Plant Sci. 2015, 5, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodeneev, V.A.; Akinchits, E.K.; Orlova, L.A.; Sukhov, V.S. The role of Ca2+, H+, and Cl− ions in generation of variation potential in pumpkin plants. Russ. J. Plant Physiol. 2011, 58, 974–981. [Google Scholar] [CrossRef]
- Sherstneva, O.N.; Vodeneev, V.; Katicheva, L.A.; Surova, L.M.; Sukhov, V. Participation of intracellular and extracellular pH changes in photosynthetic response development induced by variation potential in pumpkin seedlings. Biochemistry (Moscow) 2015, 80, 776–784. [Google Scholar] [CrossRef]
- Sukhov, V.; Surova, L.; Morozova, E.; Sherstneva, O.; Vodeneev, V. Changes in H+-ATP Synthase Activity, Proton Electrochemical Gradient, and pH in Pea Chloroplast Can Be Connected with Variation Potential. Front. Plant Sci. 2016, 7, 1092. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Westphal, L.; Strehmel, N.; Eschen-Lippold, L.; Bauer, N.; Westermann, B.; Rosahl, S.; Scheel, D.; Lee, J. pH effects on plant calcium fluxes: Lessons from acidification-mediated calcium elevation induced by the γ-glutamyl-leucine dipeptide identified from Phytophthora infestans. Sci. Rep. 2019, 9, 4733. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zdobnova, T.; Ladeynova, M.; Yudintsev, A.; Vodeneev, V.; Brilkina, A. Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP. Agriculture 2021, 11, 1131. https://doi.org/10.3390/agriculture11111131
Pecherina A, Grinberg M, Ageyeva M, Zdobnova T, Ladeynova M, Yudintsev A, Vodeneev V, Brilkina A. Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP. Agriculture. 2021; 11(11):1131. https://doi.org/10.3390/agriculture11111131
Chicago/Turabian StylePecherina, Anna, Marina Grinberg, Maria Ageyeva, Tatiana Zdobnova, Maria Ladeynova, Andrey Yudintsev, Vladimir Vodeneev, and Anna Brilkina. 2021. "Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP" Agriculture 11, no. 11: 1131. https://doi.org/10.3390/agriculture11111131
APA StylePecherina, A., Grinberg, M., Ageyeva, M., Zdobnova, T., Ladeynova, M., Yudintsev, A., Vodeneev, V., & Brilkina, A. (2021). Whole-Plant Measure of Temperature-Induced Changes in the Cytosolic pH of Potato Plants Using Genetically Encoded Fluorescent Sensor Pt-GFP. Agriculture, 11(11), 1131. https://doi.org/10.3390/agriculture11111131





