The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants, Soil Preparation and Experiment Design
2.2. Growth and Flower Production
2.3. Measurements of Root Growth
2.4. Measurement of P and K Accumulation
2.5. Statistical Analyses
3. Results
3.1. Plant Growth Response to MKP Concentration
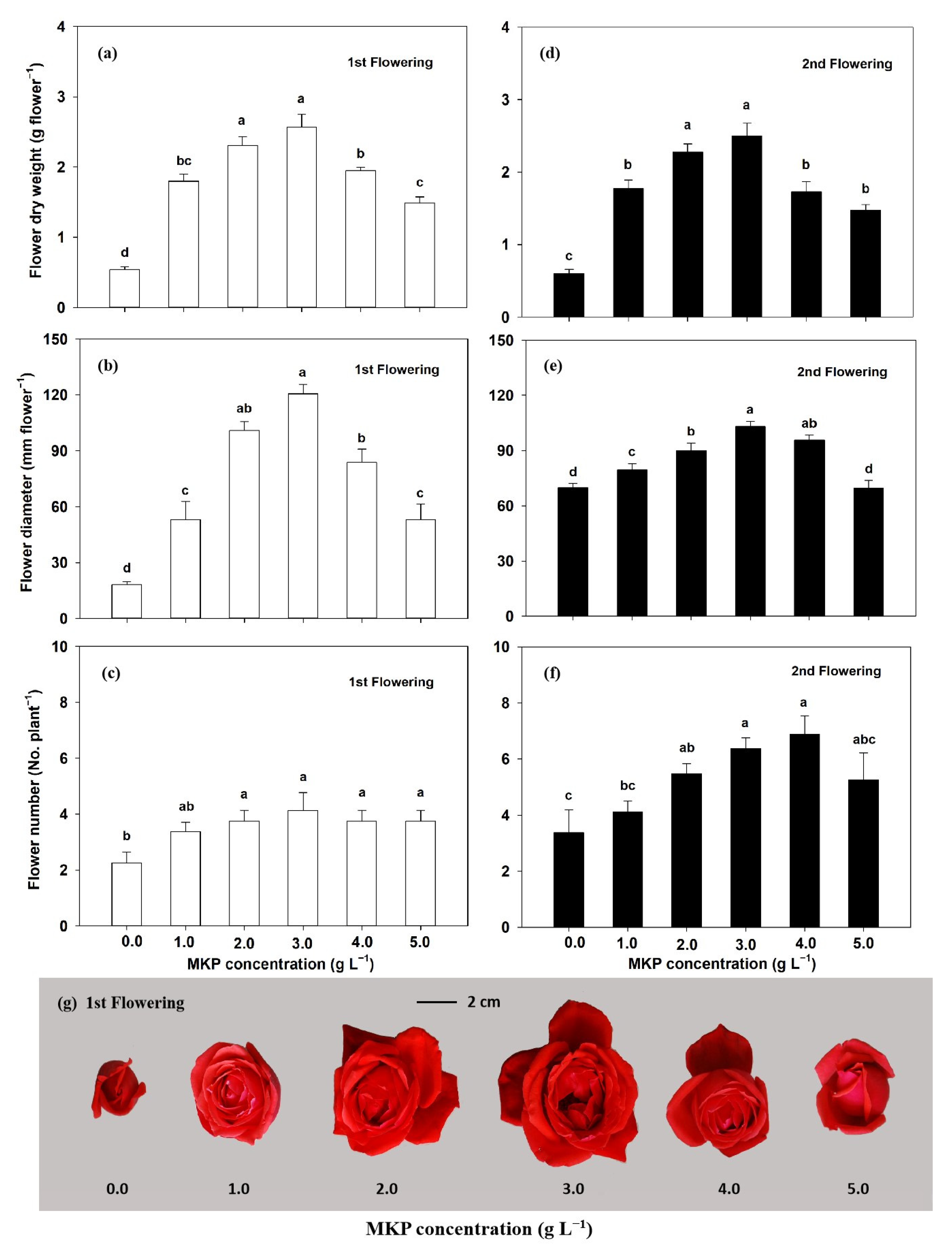
3.2. Flower Growth Response to MKP Concentration
3.3. Root Growth Response to MKP Concentration
3.4. Plant P and K Accumulation Response to MKP Concentration
3.5. Relationship between Flower Characteristics, Nutrient Accumulation, Root Length and MKP Concentrations
4. Discussion
4.1. Effects of MKP Concentration on Plant Growth and Flower Production
4.2. Effects of MKP Concentration on Root Growth and Nutrient Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Yang, Y.; Li, M.; Liu, J.; Jin, W. Residents’ preferences for roses, features of rose plantings and the relations between them in built-up areas of beijing, china. Urban For. Urban Green. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Hitter, T.; Szekely-Varga, Z.; Csiki, I.L.; Buta, E.; Cantor, M. Evaluation of some rosa cultivars under Transylvania climatic conditions. Curr. Trends Nat. Sci. 2020, 9, 153–159. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.; Cheng, B.; Han, Y.; Luo, L.; Pan, H.; Zhang, Q. Studies on the volatile compounds in flower extracts of Rosa odorata and R. chinensis. Ind. Crop. Prod. 2020, 146, 112143. [Google Scholar] [CrossRef]
- Wang, X.; Guo, T.; Wang, Y.; Xing, Y.; Wang, Y.; He, X. Exploring the optimization of water and fertilizer management practices for potato production in the sandy loam soils of Northwest China based on PCA. Agric. Water Manag. 2020, 237, 106180. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2011. [Google Scholar]
- Penpillo, R.L.; Ballano, M.L. Effect of different organic and inorganic fertilizers on the flower yield of roses (Rosa cv.). WVSU Res. J. 2012, 12, 71–78. [Google Scholar]
- Tariq, M.; Afzal, M.N.; Muhammad, D.; Ahmad, S.; Shahzad, A.N.; Kiran, A.; Wakeel, A. Relationship of tissue potassium content with yield and fiber quality components of Bt cotton as influenced by potassium application methods. Field Crop. Res. 2018, 229, 37–43. [Google Scholar] [CrossRef]
- Garad, B.V.; Jogdand, S.M.; More, V.; Kulkarni, S.S. Effect of chemicals on flowering and fruiting in mango (Mangifera indica L.) cv. Keshar. Ecol. Environ. Conserv. 2013, 19, 835–838. [Google Scholar]
- Kumar, A.; Singh, S.K.; Pandey, S.D.; Patel, R.K.; Nath, V. Effect of foliar spray of chemicals on flowering and fruiting in Litchi. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zambrosi, F.C.B. Foliar phosphorus applications in the forms of phosphate and phosphite have contrasting effects on wheat performance under field conditions. J. Crop. Sci. Biotechnol. 2019, 22, 395–401. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimãres, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Hur, R.G.; Ahmad, A.; Mahmood, N. Response of foliar application of KNO3 on yield, yield components and lint quality of cotton (Gossypium hirsutum L.). Afr. J. Agric. Res. 2011, 6, 5457–5463. [Google Scholar]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.O. Phosphate availability regulates root system architecture in arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Chen, L.; Du, M.; Zhang, Y.; Zhang, Y. Localized and moderate phosphorus application improves plant growth and phosphorus accumulation in Rosa multiflora Thunb. ex Murr. via efficient root system development. Forests 2020, 11, 570. [Google Scholar] [CrossRef]
- Nerson, H.; Edelstein, M.; Berdugo, R.; Ankorion, Y. Monopotassium phosphate as a phosphorus and potassium source for greenhouse-winter-grown cucumber and muskmelon. J. Plant Nutr. 1997, 20, 335–344. [Google Scholar] [CrossRef]
- Elwan, M.W.M. Ameliorative effects of di-potassium hydrogen orthophosphate on salt-stressed eggplant. J. Plant Nutr. 2010, 33, 1593–1604. [Google Scholar] [CrossRef]
- Meng, D.; Xu, P.; Dong, Q.; Wang, S.; Wang, Z. Comparison of foliar and root application of potassium dihydrogen phosphate in regulating cadmium translocation and accumulation in tall fescue (Festuca arundinacea). Water Air Soil Pollut. 2017, 228, 118. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of NH4 +-N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant Soil 2013, 372, 65–80. [Google Scholar] [CrossRef]
- Pinno, B.D.; Wilson, S.D. Fine root response to soil resource heterogeneity differs between grassland and forest. Plant Ecol. 2013, 214, 821–829. [Google Scholar] [CrossRef]
- Johnson, C.M.; Ulrich, A. Analytical Methods for Use in Plant Analysis; University of California, Agricultural Experiment Station: Berkeley, CA, USA, 1959. [Google Scholar]
- Reuveni, R.; Agapov, M.; Raviv, M. Effects of foliar sprays of phosphates on powdery mildew (Sphaerotheca pannosa) of roses. J. Phytopathol. 1994, 142, 331–337. [Google Scholar]
- Mudau, F.N.; Theron, K.I.; Rabe, E. Rind texture and juice acid content of Citrus spp. as affected by foliar sprays of mono-potassium phosphate (MKP), urea ammonium phosphate (UAP) and mono-ammonium phosphate (MAP). S. Afr. J. Plant Soil 2005, 22, 269–273. [Google Scholar] [CrossRef]
- Baiea, M.H.M.; El-Sharony, T.F.; Abd El-Moneim, E.A. Effect of different forms of potassium on growth, yield and fruit quality of mango cv. Hindi. Int. J. ChemTech Res. 2015, 8, 1582–1587. [Google Scholar]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and fruit set of olive trees in response to nitrogen, phosphorus, and potassium. J. Am. Soc. Hortic. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ince, F.; Amador, B.M.; Cakir, A.; Sakar, E. Ameliorative effects of potassium phosphate on salt-stressed pepper and cucumber. J. Plant Nutr. 2003, 26, 807–820. [Google Scholar] [CrossRef]
- Pal, P.K.; Mahajan, M.; Agnihotri, V.K. Foliar application of plant nutrients and kinetin modifies growth and essential oil profile in Rosa damascena under acidic conditions. Acta Physiol. Plant. 2016, 38, 1–14. [Google Scholar] [CrossRef]
- Cabrera, R.I.; Evans, R.Y.; Paul, J.L. Nitrogen partitioning in rose plants over a flowering cycle. Sci. Hortic. 1995, 63, 67–76. [Google Scholar] [CrossRef]
- Kannan, S. Foliar fertilization for sustainable crop production. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer: Dordrecht, The Netherlands, 2010; pp. 371–402. [Google Scholar]
- Ahmad, I.; Dole, J.M.; Nelson, P. Nitrogen application rate, leaf position and age affect leaf nutrient status of five specialty cut flowers. Sci. Hortic. 2012, 142, 14–22. [Google Scholar] [CrossRef]
- Hu, B.; Chu, C. Nitrogen–phosphorus interplay: Old story with molecular tale. New Phytol. 2020, 225, 1455–1460. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; von Wirén, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by NPF7.3/NRT1.5 and SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, L.; Tian, H.; Rengel, Z.; Shen, J. Deep banding of phosphorus and nitrogen enhances Rosa multiflora growth and nutrient accumulation by improving root spatial distribution. Sci. Hortic. 2021, 277, 109800. [Google Scholar] [CrossRef]
- Wei, H.; Hauer, R.J.; Chen, G.; Chen, G.; Chen, X.; He, X. Growth, nutrient assimilation, and carbohydrate metabolism in Korean pine (Pinus koraiensis) seedlings in response to light spectra. Forests 2020, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Attaullah, K.; Wang, J.; Yu, D.; Yang, Y.; Yang, L.; Lu, Z. Root traits determine variation in nonstructural carbohydrates (NSCs) under different drought intensities and soil substrates in three temperate tree species. Forests 2020, 11, 415. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.-N.; Li, C.-L.; Wu, F.; Li, S.-P.; Han, S.-Q.; Li, M.-F. Desuckering effect of KH2PO4 mixed with paclobutrazol and its influence on banana (Musa paradisiaca AA) mother plant growth. Sci. Hortic. 2018, 240, 484–491. [Google Scholar] [CrossRef]
- Barranco, D.; Ercan, H.; Muñoz-Díez, C.; Belaj, A.; Arquero, O. Factors influencing the efficiency of foliar sprays of monopotassium phosphate in the olive. Int. J. Plant Prod. 2010, 4, 235–240. [Google Scholar]
- Amaral, J.; Ribeyre, Z.; Vigneaud, J.; Sow, M.D.; Fichot, R.; Messier, C.; Pinto, G.; Nolet, P.; Maury, S. Advances and promises of epigenetics for forest trees. Forests 2020, 11, 976. [Google Scholar] [CrossRef]




| Sampling Time | Parameters | MKP Concentration (g·L−1) | |||||
|---|---|---|---|---|---|---|---|
| 0.0 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | ||
| 1st Flowering | Shoot dry weight (g·plant−1) | 7.9 (0.26) d | 16 (0.40) bc | 17 (0.40) 4b | 20 (0.79) a | 19 (0.64) a | 15 (0.42) c |
| Root dry weight (g·plant−1) | 3.6 (0.16) d | 5.3 (0.23) c | 6.8 (0.40) b | 9.9 (0.54) a | 6.4 (0.26) bc | 5.3 (0.53) c | |
| Shoot:root ratio | 2.2 (0.13) b | 3.1 (0.18) a | 2.6 (0.21) ab | 2.1 (0.16) b | 3.1 (0.23) a | 2.9 (0.26) a | |
| Leaf SPAD | 36 (1.7) d | 44 (1.2) bc | 46 (1.8) b | 53 (0.83) a | 51 (0.77) a | 41 (1.1) c | |
| 2nd Flowering | Shoot dry weight (g·plant−1) | 30 (1.1) d | 46 (2.1) bc | 54 (2.8) ab | 60 (3.4) a | 48 (3.2) bc | 41 (4.6) c |
| Root dry weight (g·plant−1) | 6.7 (0.31) b | 7.8 (0.71) ab | 10.9 (2.2) a | 10.2 (1.2) ab | 8.1 (0.80) ab | 7.2 (0.85) b | |
| Shoot:root ratio | 4.5 (0.36) a | 6.0 (0.45) a | 5.3 (0.97) a | 6.1 (1.0) a | 6.0 (0.30) a | 6.0 (1.2) a | |
| Leaf SPAD | 43 (1.9) c | 50 (0.75) b | 51 (0.69) b | 55 (0.70) a | 56 (0.47) a | 49 (0.83) b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Wang, X.; Yuan, W.; Tang, H.; Luan, M. The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth. Agriculture 2021, 11, 1210. https://doi.org/10.3390/agriculture11121210
Ma Q, Wang X, Yuan W, Tang H, Luan M. The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth. Agriculture. 2021; 11(12):1210. https://doi.org/10.3390/agriculture11121210
Chicago/Turabian StyleMa, Qinghua, Xinghong Wang, Weijie Yuan, Hongliang Tang, and Mingbao Luan. 2021. "The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth" Agriculture 11, no. 12: 1210. https://doi.org/10.3390/agriculture11121210
APA StyleMa, Q., Wang, X., Yuan, W., Tang, H., & Luan, M. (2021). The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth. Agriculture, 11(12), 1210. https://doi.org/10.3390/agriculture11121210






