Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Upstream and Downstream Fermentation Process
Cultivation Conditions
Pilot Fermentation
Post-Fermentation Processing
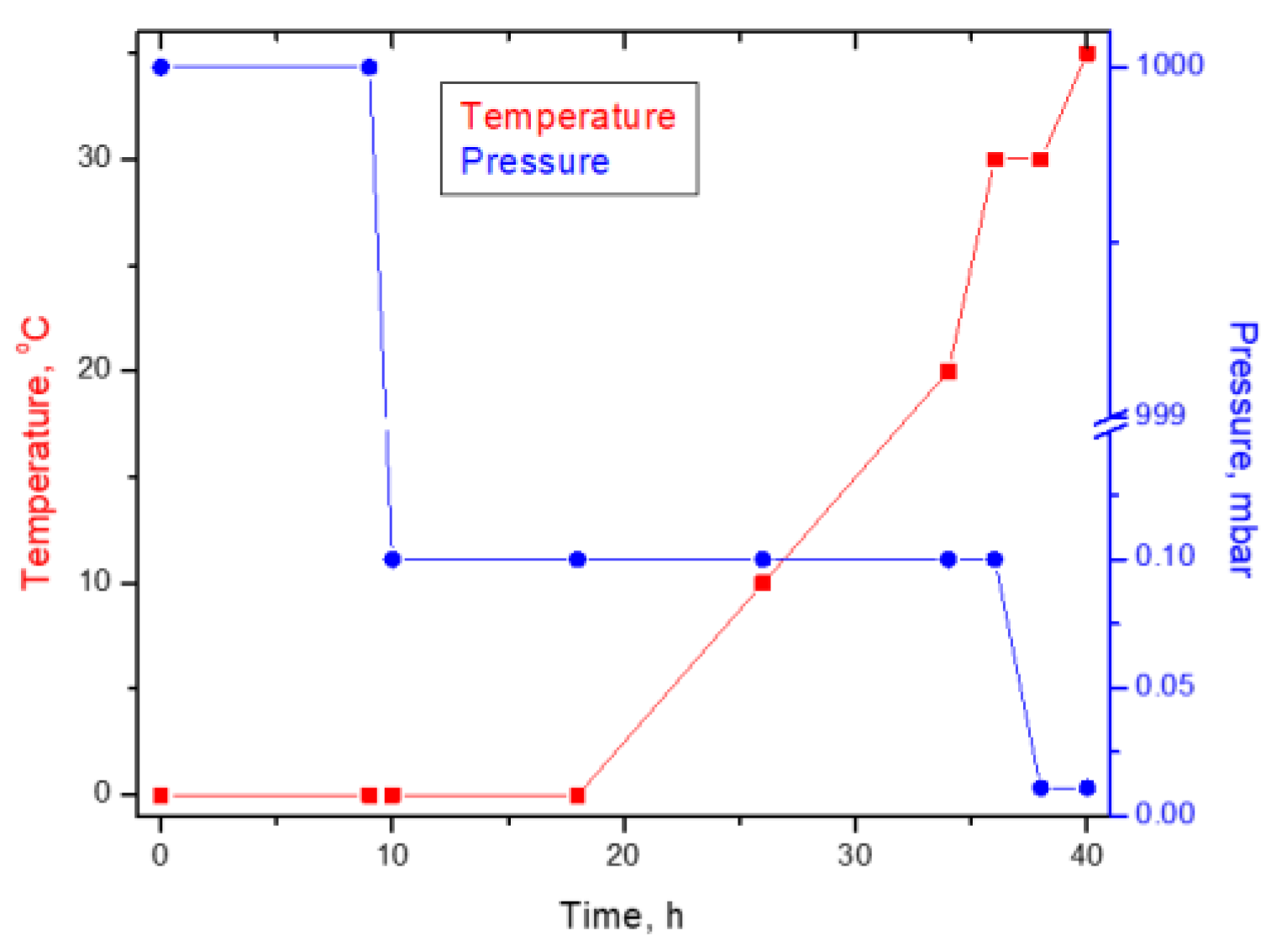
2.2.2. Biomass Drying by Freeze Drying
2.2.3. Determination of the Moisture and the Crude Protein Content for the Yeast Biomass
2.2.4. Determination of the Dry Cell Weight and the Wet Cell Weight for the Yeast Biomass
2.2.5. Dried Yeast Biomass Wettability
2.2.6. Design of Experiments and Data Statistical Analysis
3. Results and Discussion
3.1. Responses of Brewing Yeast Biotechnological Processing
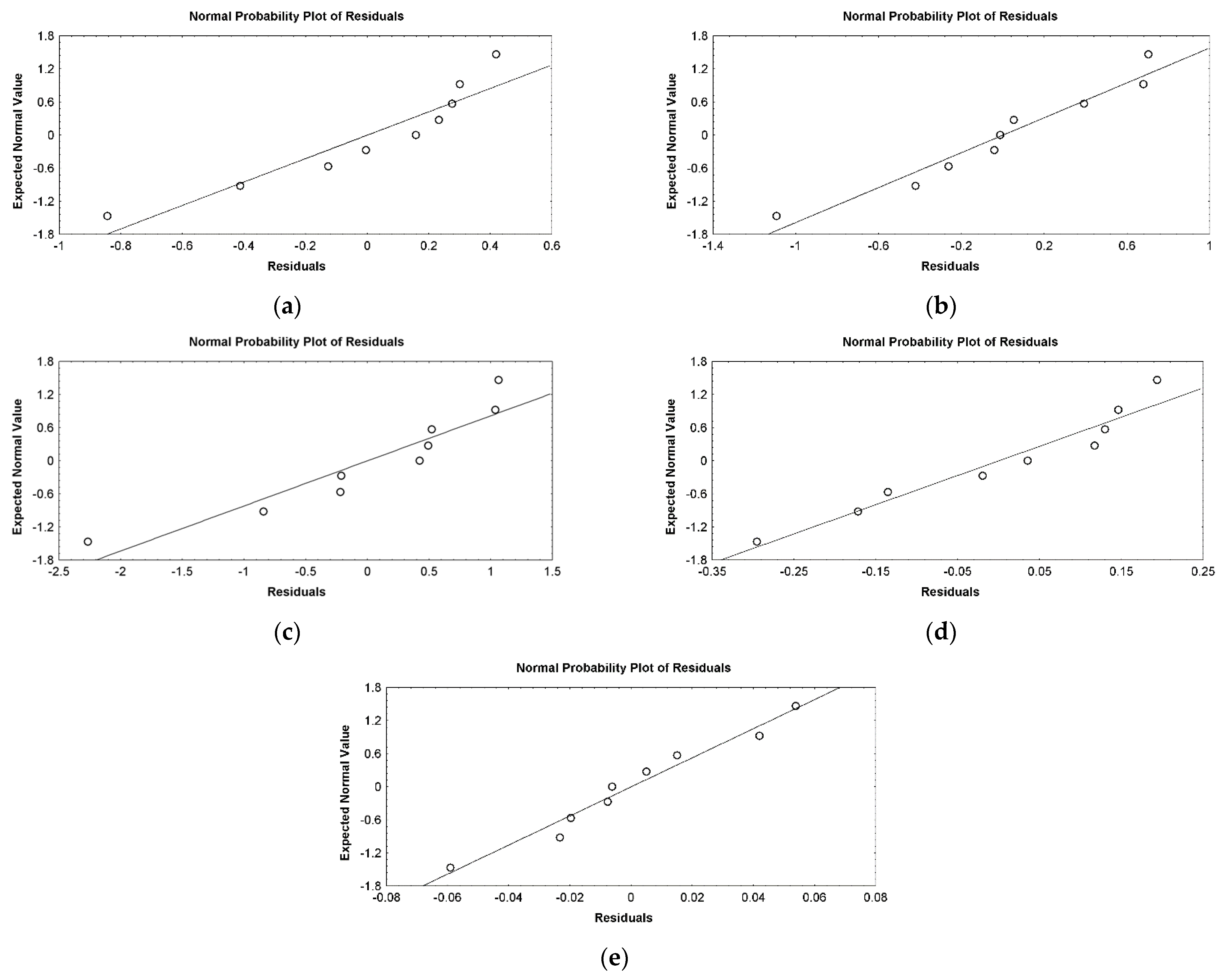
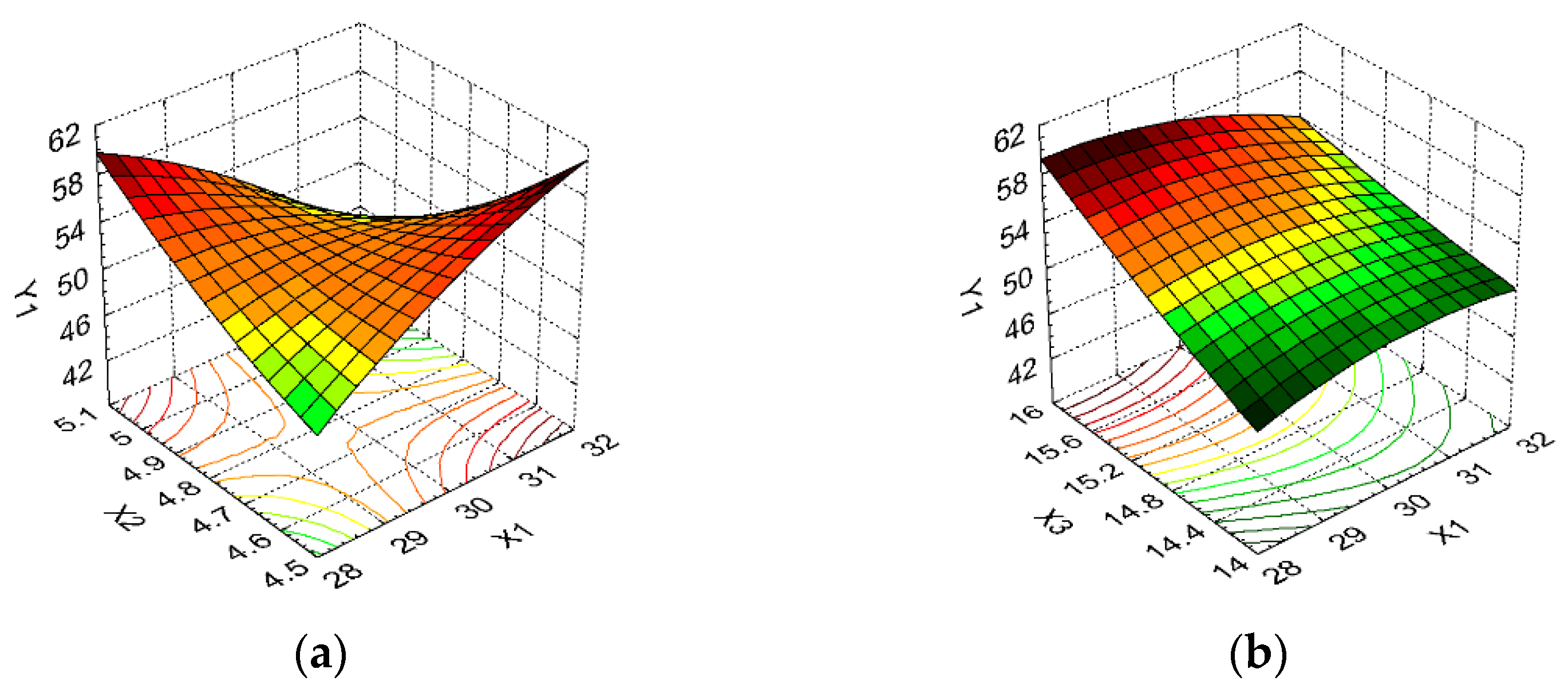
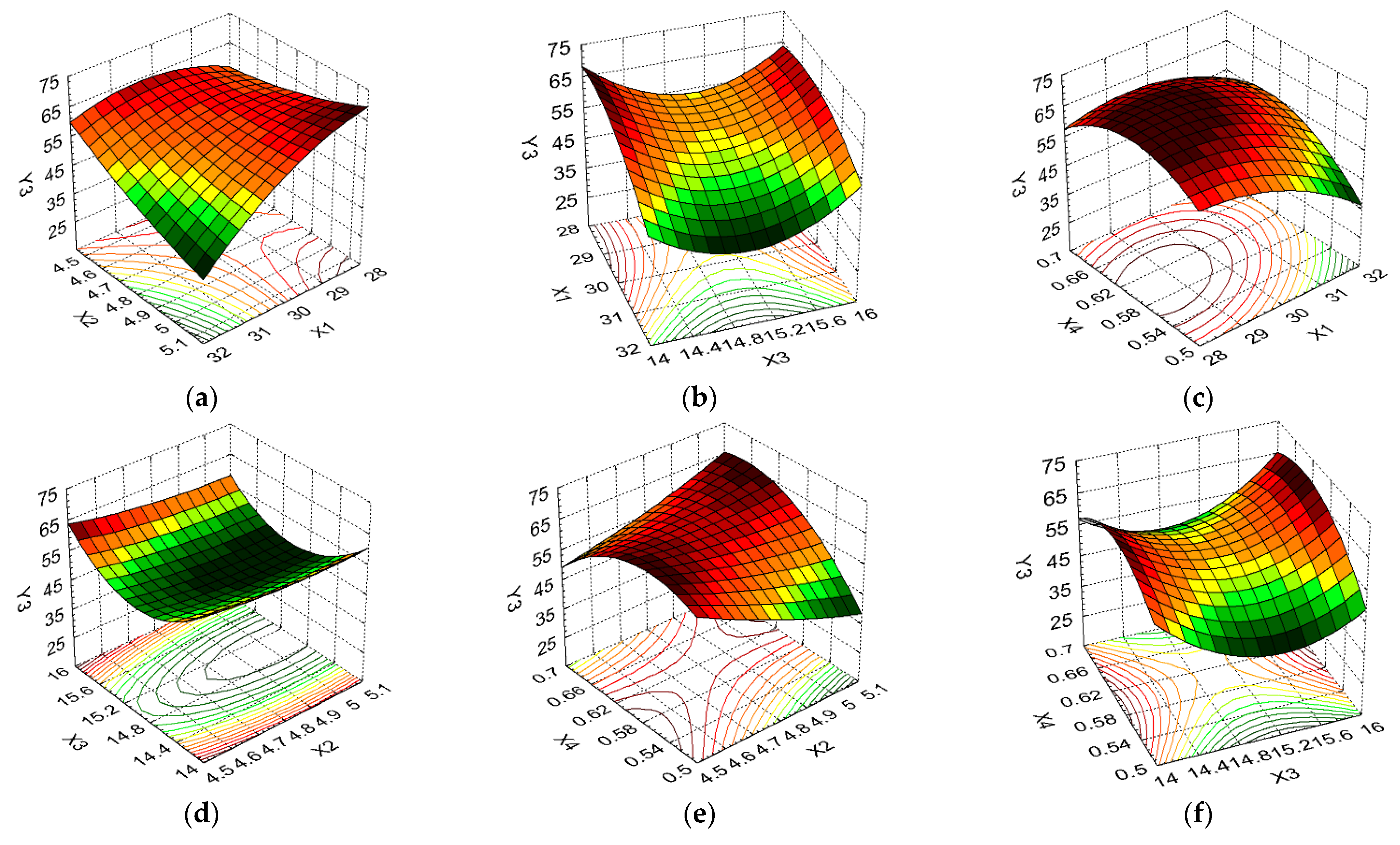
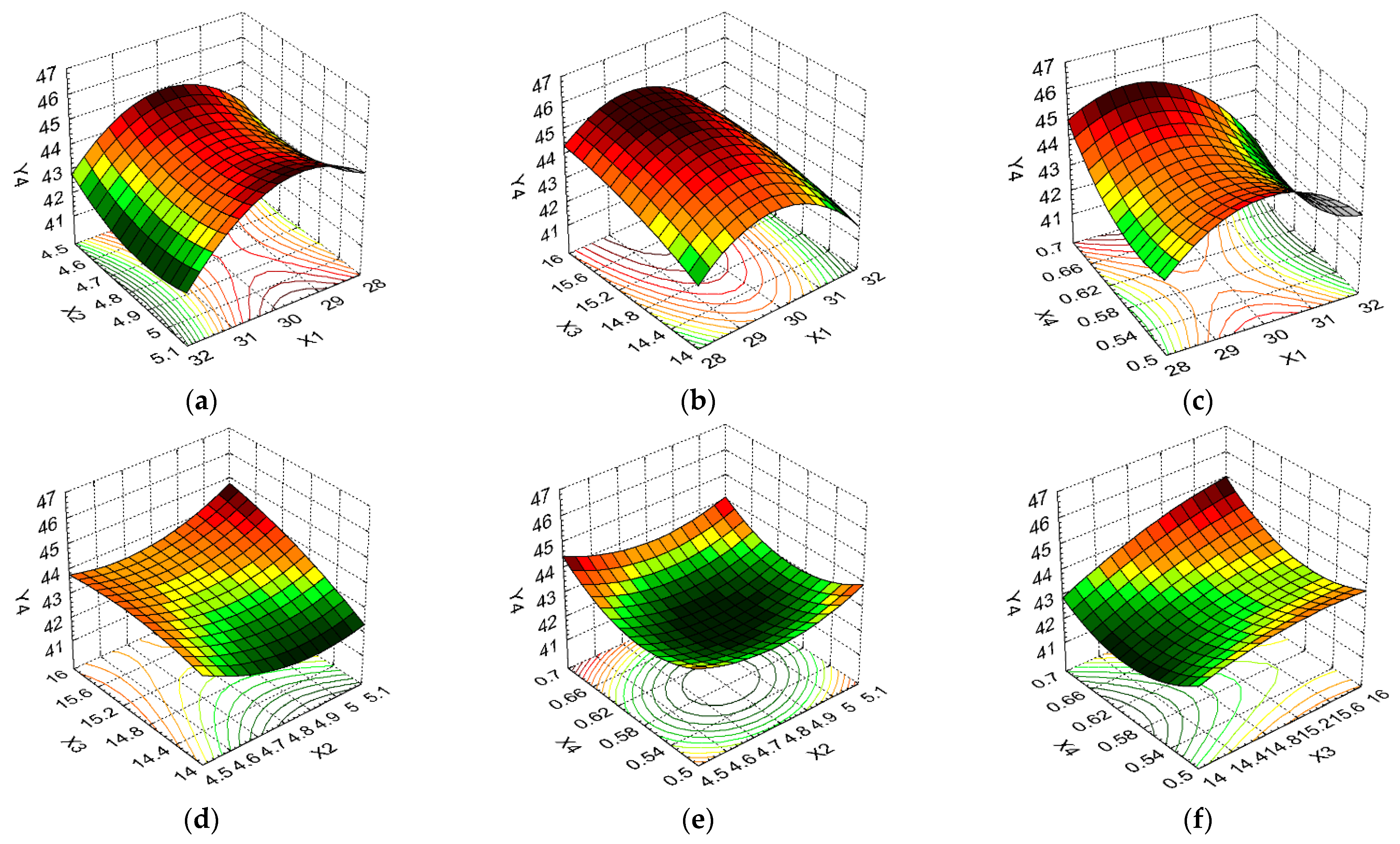
3.2. Design of Experiments and Data Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrera, O.R.; Salinas-Chavira, J.; Castillo, Y.C. Yeasts as Dietary Additives to Manipulate Ruminal Fermentation: Effect on Nutrient Utilization and Productive Performance of Ruminants. Yeasts Biotechnol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Shurson, G. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed. Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Retta, K.S. Role of probiotics in rumen fermentation and animal performance: A review. Int. J. Livest. Prod. 2016, 7, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhang, B.X.; Yao, K.; Yoon, I.; Chung, Y.H.; Wang, J.K.; Liu, J.X. Effects of Supplemental Levels of Saccharomyces cerevisiae Fermentation Product on Lactation Performance in Dairy Cows under Heat Stress. Asian Australas. J. Anim. Sci. 2016, 29, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippini, R.R.; Martiniano, S.E.; Ingle, A.P.; Marcelino, P.R.F.; Silva, G.M.; Barbosa, F.G.; Santos, J.; Da Silva, S.S. Agroindustrial Byproducts for the Generation of Biobased Products: Alternatives for Sustainable Biorefineries. Front. Energy Res. 2020, 8, 152. [Google Scholar] [CrossRef]
- Kennes, C. Bioconversion Processes. Fermentation 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.M.; Walker, R.S. Enhancing Yeast Alcoholic Fermentations. In Advances in Applied Microbiology; Gadd, G., Sariaslani, S., Eds.; Academic Press: London, UK, 2018; pp. 87–129. [Google Scholar] [CrossRef]
- Willaert, R.G. Yeast Biotechnology. Fermentation 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J. Production of biopharmaceutical proteins by yeast: Advances through metabolic engineering. Bioengineered 2013, 4, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciurzyńska, A.; Lenart, A. Freeze-Drying—Application in Food Processing and Biotechnology—A Review. Pol. J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Aberle, C.; Lewis, M.; Yu, G.; Lei, N.; Xu, J. Liquid marbles as thermally robust droplets: Coating-assisted Leidenfrost-like effect. Soft Matter 2011, 7, 11314–11318. [Google Scholar] [CrossRef] [Green Version]
- Ghica, M.V.; Moisescu, S.; Albu, M.G. Design and optimization of some collagen-minocycline based hydrogels potentially applicable for the treatment of cutaneous wound infections. Pharmazie 2011, 66, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Popa, L.; Saramet, G.; Leca, M.; Lupuliasa, D.; Moisescu, S. Optimization of the pharmaceutical products and process design applying Taguchi quality engineering principles. Farmacia 2011, 59, 321–328. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response surface methodology and Taguchi approach to assess the combined effect of formulation factors on minocycline delivery from collagen sponges. Pharmazie 2013, 68, 340–348. [Google Scholar] [CrossRef]
- Ghica, M.V.; Kaya, M.G.A.; Dinu-Pîrvu, C.-E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, S.; Türkmen, M.; Kaya, M.G.A.; Dinu-Pîrvu, C.-E. Selection of Optimal Operating Conditions for Extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.M.; Kaya, M.G.A.; Iovu, H.; Stavarache, C.E.; Chelaru, C.; Constantinescu, R.R.; Dinu-Pîrvu, C.-E.; Ghica, M.V. Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis. Coatings 2020, 10, 990. [Google Scholar] [CrossRef]
- Prisada, R.M.; Dinu-Pirvu, C.E.; Moldovan, H.; Ghica, M.V.; Avrămescu, R.E.; Popa, L. Perspectives to describe surface properties of raw pharmaceutical materials. A fractal approach on the wetting of powders. Farmacia 2020, 68, 354–361. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu, M.G.; Kaya, D.A.; Popa, L.; Öztürk, Ș.; Rusu, L.C.; Dinu-Pîrvu, C.; Chelaru, C.; Albu, L.; Meghea, A.; et al. The effect of lavandula essential oils on release of niflumic acid from collagen hydrolysates. Korean J. Chem. Eng. 2016, 33, 1325–1330. [Google Scholar] [CrossRef]
- Nowak, E.; Combes, G.; Stitt, E.H.; Pacek, A.W. A comparison of contact angle measurement techniques applied to highly porous catalyst supports. Powder Technol. 2013, 233, 52–64. [Google Scholar] [CrossRef]
- Liu, T.; Hao, J.; Yang, B.; Hu, B.; Cui, Z.; Li, S. Contact angle measurements: An alternative approach towards understanding the mechanism of increased drug dissolution from ethylcellulose tablets containing surfactant and exploring the relationship between their contact angles and dissolution behaviors. AAPS PharmSciTech 2018, 19, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wei, C.; Qian, F.; Li, S. Surface wettability modulated by surfactant and its effects on the drug release and absorption of fenofibrate solid dispersions. AAPS PharmSciTech 2019, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, C.; Gong, S.; Mei, Y.; Li, H.; Ma, W. Investigation on contact angle measurement methods and wettability transition of porous surfaces. Surf. Coat. Technol. 2016, 292, 72–77. [Google Scholar] [CrossRef]
- Avrămescu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Prisada, R.; Popa, L. Superhydrophobic natural and artificial surfaces—A structural approach. Materials 2018, 11, 866. [Google Scholar] [CrossRef] [Green Version]
- Lazghab, M.; Saleh, K.; Pezron, I.; Guigon, P.; Komunjer, L. Wettability assessment of finely divided solids. Powder Technol. 2005, 157, 79–91. [Google Scholar] [CrossRef]
- Buckton, G. Assessment of the wettability of pharmaceutical powders. J. Adhes. Sci. Technol. 1993, 7, 205–219. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Zhang Newby, B. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Bormashenko, E. General equation describing wetting of rough surfaces. J. Colloid Interface Sci. 2011, 360, 317–319. [Google Scholar] [CrossRef]






| Independent Variables | Coded Symbol | Coded and Uncoded Variation Levels | ||
|---|---|---|---|---|
| Low (1) | Middle (2) | High (3) | ||
| Temperature, T (C°) | X1 | 28 | 30 | 32 |
| pH | X2 | 4.5 | 4.8 | 5.1 |
| Carbon source, CS (g/100 mL) | X3 | 14 | 15 | 16 |
| Nitrogen source, NS (g/100 mL) | X4 | 0.5 | 0.6 | 0.7 |
| Dependent Variables | Coded Symbol | Constraints | ||
| WCW (g/L) | Y1 | Maximize | ||
| DCW (g) | Y2 | Maximize | ||
| CA (°) | Y3 | Minimize | ||
| PC (%) | Y4 | Maximize | ||
| DS (%) | Y5 | Maximize | ||
| Exp. No. | Independent Variables (Coded Level) | |||
|---|---|---|---|---|
| X1 Temperature | X2 pH | X3 CS | X4 NS | |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 2 | 1 | 2 | 3 |
| 5 | 2 | 2 | 3 | 1 |
| 6 | 2 | 3 | 1 | 2 |
| 7 | 3 | 1 | 3 | 2 |
| 8 | 3 | 2 | 1 | 3 |
| 9 | 3 | 3 | 2 | 1 |
| Exp. No. | Independent Variables (Coded Level/Physical Level) | Responses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1–T (°C) | X2–pH | X3–CS (g%) | X4–NS (g%) | Y1–WCW (g/L) | Y2–DCW (g) | Y3–CA (°) | Y4–PC (%) | Y5–DS (%) | ||||||
| Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | |||||
| 1 | 1 (28) | 1 (4.5) | 1 (14) | 1 (0.5) | 46.250 | 45.949 | 11.640 | 11.681 | 62.44 | 61.92 | 42.99 | 42.86 | 91.83 | 91.84 |
| 2 | 1 (28) | 2 (4.8) | 2 (15) | 2 (0.6) | 54.500 | 55.344 | 15.272 | 14.568 | 59.08 | 61.34 | 42.48 | 42.77 | 92.16 | 92.10 |
| 3 | 1 (28) | 3 (5.1) | 3 (16) | 3 (0.7) | 60.500 | 60.224 | 17.650 | 17.662 | 64.05 | 63.56 | 44.83 | 44.71 | 91.60 | 91.61 |
| 4 | 2 (30) | 1 (4.5) | 2 (15) | 3 (0.7) | 60.000 | 59.767 | 15.897 | 15.843 | 54.97 | 53.91 | 45.74 | 45.87 | 92.02 | 92.01 |
| 5 | 2 (30) | 2 (4.8) | 3 (16) | 1 (0.5) | 51.500 | 51.626 | 13.032 | 14.125 | 56.52 | 55.48 | 44.89 | 44.69 | 92.15 | 92.20 |
| 6 | 2 (30) | 3 (5.1) | 1 (14) | 2 (0.6) | 51.250 | 50.831 | 11.935 | 12.196 | 70.01 | 69.59 | 43.41 | 43.37 | 92.71 | 92.74 |
| 7 | 3 (32) | 1 (4.5) | 3 (16) | 2 (0.6) | 59.250 | 59.092 | 15.690 | 15.297 | 60.02 | 60.86 | 42.30 | 42.32 | 91.67 | 91.65 |
| 8 | 3 (32) | 2 (4.8) | 1 (14) | 3 (0.7) | 51.925 | 52.337 | 13.260 | 13.681 | 51.13 | 51.34 | 41.45 | 41.30 | 90.89 | 90.91 |
| 9 | 3 (32) | 3 (5.1) | 2 (15) | 1 (0.5) | 44.725 | 44.729 | 12.427 | 11.748 | 31.05 | 31.27 | 42.63 | 42.81 | 92.13 | 92.08 |
| Responses | Sources of Variation | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y1 | Regression | 264.814 | 5 | 52.962 0.440 | 120.346 | 0.00181 <0.05 |
| Residual | 1.320 | 3 | ||||
| Total | 266.134 | 8 | ||||
| Y1 | Regression | 32.562 | 4 | 8.140 0.638 | 12.746 | 0.01511 <0.05 |
| Residual | 2.554 | 4 | ||||
| Total | 35.116 | 8 | ||||
| Y2 | Regression | 963.834 | 6 | 160.639 4.416 | 36.369 | 0.02699 <0.05 |
| Residual | 8.833 | 2 | ||||
| Total | 972.667 | 8 | ||||
| Y4 | Regression | 16.132 | 6 | 2.688 0.113 | 23.768 | 0.04092 <0.05 |
| Residual | 0.226 | 2 | ||||
| Total | 16.358 | 8 | ||||
| Y5 | Regression | 2.011 | 6 | 0.335 0.004 | 71.104 | 0.01393 <0.05 |
| Residual | 0.009 | 2 | ||||
| Total | 2.020 | 8 |
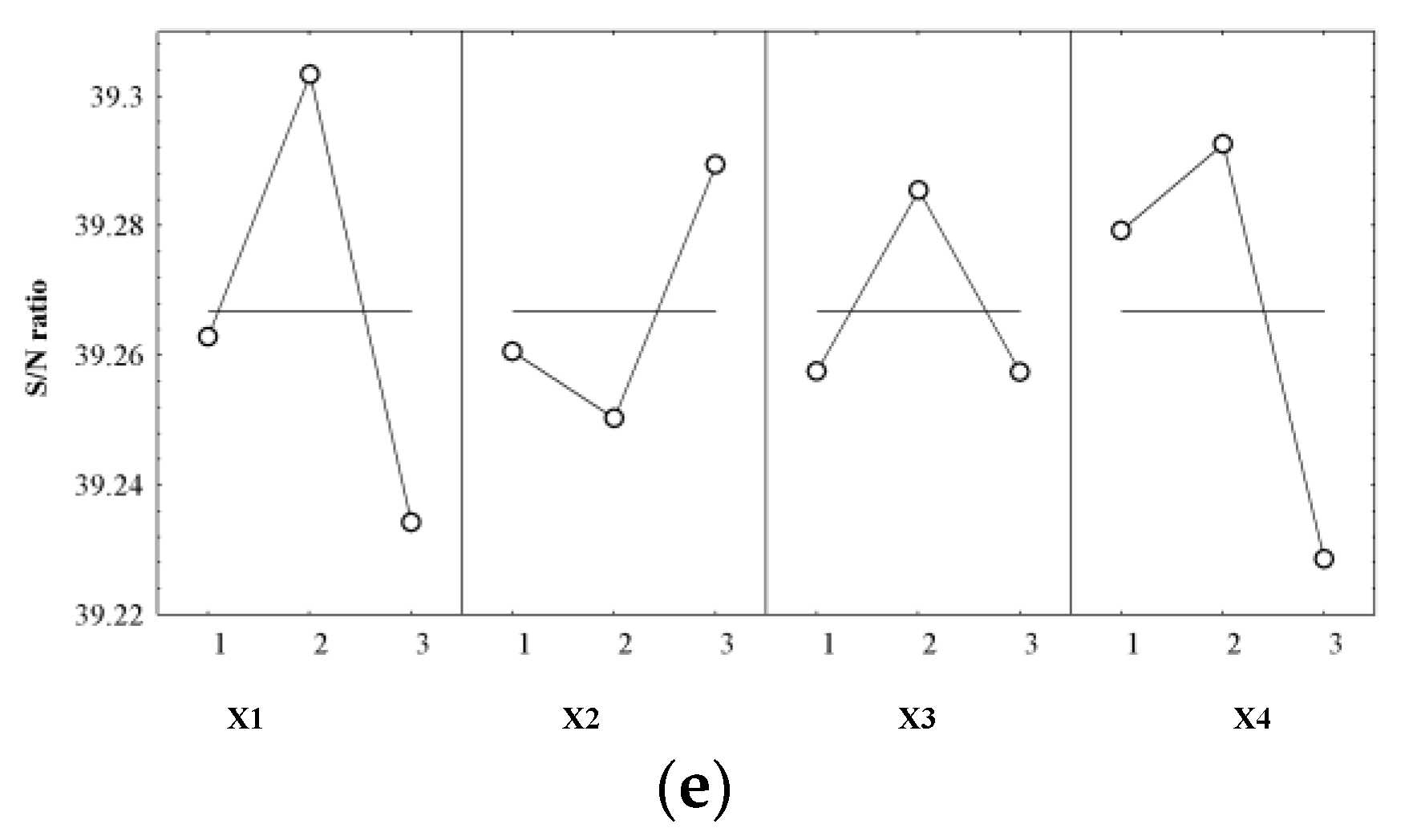
| Control Factors (Input Variables) | Y1 | Y2 | Y3 | Y4 | Y5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Larger-the-Better” | Effect Size | “Larger-the-Better” | Effect Size | “Smaller-the-Better” | Effect Size | “Larger-the-Better” | Effect Size | “Larger-the-Better” | Effect Size | |
| X1 | 2 | 0.172 | 1 | 0.416 | 3 | 1.673 | 2 | 0.251 | 2 | 0.036 |
| X2 | 1 | 0.281 | 1 | 0.191 | 3 | 0.575 | 1 | 0.052 | 3 | 0.023 |
| X3 | 3 | 0.616 | 3 | 0.821 | 2 | 1.509 | 3 | 0.119 | 2 | 0.019 |
| X4 | 3 | 0.676 | 3 | 0.910 | 1 | 1.268 | 3 | 0.114 | 2 | 0.026 |
| S/N ratio expected (dB) | 36.237 | 25.235 | −29.841 | 33.285 | 39.371 | |||||
| Exp. No. | Combination of Optimal Cultivation Conditions | Response Variable | Observed Value | Predictive Value | Predicted Error (%) |
|---|---|---|---|---|---|
| X1:X2:X3:X4 | |||||
| Exp. 10 | 30:4.5:16:0.7 | Y1 (g/L) | 62.89 | 66.24 | −5.06 |
| Y2 (g) | 16.25 | 17.38 | −6.50 | ||
| Y3 (°) | 63.87 | 65.95 | −3.15 | ||
| Y4 (%) | 47.05 | 50.27 | −6.40 | ||
| Y5 (%) | 91.92 | 92.30 | −0.41 | ||
| Exp. 11 | 28:4.5:16:0.7 | Y1 (g/L) | 65.00 | 68.14 | −4.61 |
| Y2 (g) | 17.28 | 17.75 | −2.65 | ||
| Y3 (°) | 75.29 | 79.72 | −5.56 | ||
| Y4 (%) | 49.88 | 54.55 | −8.56 | ||
| Y5 (%) | 93.45 | 92.05 | +1.52 | ||
| Exp. 9 | 32:5.1:15:0.5 | Y1 (g/L) | 44.72 | 44.73 | −0.02 |
| Y2 (g) | 12.43 | 11.75 | +5.78 | ||
| Y3 (°) | 31.05 | 31.27 | −0.70 | ||
| Y4 (%) | 42.63 | 42.81 | −0.42 | ||
| Y5 (%) | 92.13 | 92.08 | +0.06 | ||
| Exp. 12 | 30:5.1:15:0.6 | Y1 (g/L) | 53.85 | 51.80 | +3.95 |
| Y2 (g) | 14.80 | 13.94 | +6.17 | ||
| Y3 (°) | 48.09 | 50.38 | −4.54 | ||
| Y4 (%) | 42.85 | 41.42 | +3.45 | ||
| Y5 (%) | 93.09 | 92.10 | +1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bărbulescu, I.D.; Ghica, M.V.; Begea, M.; Albu Kaya, M.G.; Teodorescu, R.I.; Popa, L.; Mărculescu, S.I.; Cîrîc, A.I.; Dumitrache, C.; Lupuliasa, D.; et al. Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique. Agriculture 2021, 11, 1237. https://doi.org/10.3390/agriculture11121237
Bărbulescu ID, Ghica MV, Begea M, Albu Kaya MG, Teodorescu RI, Popa L, Mărculescu SI, Cîrîc AI, Dumitrache C, Lupuliasa D, et al. Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique. Agriculture. 2021; 11(12):1237. https://doi.org/10.3390/agriculture11121237
Chicago/Turabian StyleBărbulescu, Iuliana Diana, Mihaela Violeta Ghica, Mihaela Begea, Mădălina Georgiana Albu Kaya, Răzvan Ionuț Teodorescu, Lăcrămioara Popa, Simona Ioana Mărculescu, Alexandru Ionuț Cîrîc, Corina Dumitrache, Dumitru Lupuliasa, and et al. 2021. "Optimization of the Fermentation Conditions for Brewing Yeast Biomass Production Using the Response Surface Methodology and Taguchi Technique" Agriculture 11, no. 12: 1237. https://doi.org/10.3390/agriculture11121237







