The Crucial Role of Soil Organic Matter in Satisfying the Phosphorus Requirements of Olive Trees (Olea europaea L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Groves’ Management, Study Area and Experimental Design
2.2. Soil Chemical Analyses
2.3. Leaf Chemical Analyses
2.4. Determination of Acid, Alkaline and Pyrophosphatase Activity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Escobar, R.; Guerreiro, M.; Benlloch, M.; Benlloch-González, M. Symptoms of nutrient deficiencies in young olive trees and leaf nutrient concentration at which such symptoms appear. Sci. Hortic. 2016, 209, 279–285. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. Am. Soc. Hortic. Sci. 2010, 31, 155–229. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Olivera, M.; Tejera, N.; Iribarne, C.; Ocaña, A.; Lluch, C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): Effect of phosphorus. Physiol. Plant. 2004, 121, 498–505. [Google Scholar] [CrossRef]
- Meena, M.D.; Narjary, B.; Sheoran, P.; Jat, H.S.; Joshi, P.K.; Chinchmalatpure, A.R.; Yadav, G.; Yadav, R.K.; Meena, M.K. Changes of phosphorus fractions in saline soil amended with municipal solid waste compost and mineral fertilizers in a mustard-pearl millet cropping system. Catena 2018, 160, 32–40. [Google Scholar] [CrossRef]
- Holford, I.C.R. Soil phosphorus: Its measurement, and its uptake by plants. Aust. J. Soil Res. 1997, 35, 227–239. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.Â.; Moutinho-Pereira, J.M.; Correia, C.M.; Arrobas, M. Olive tree response to applied phosphorus in field and pot experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Haygarth, P.M.; Jarvie, H.P.; Powers, S.M.; Sharpley, A.N.; Elser, J.J.; Shen, J.; Peterson, H.M.; Chan, N.I.; Howden, N.J.K.; Burt, T.; et al. Sustainable phosphorus management and the need for a long-term perspective: The legacy hypothesis. Environ. Sci. Technol. 2014, 48, 8417–8419. [Google Scholar] [CrossRef]
- Robertson, G.P.; Gross, K.L.; Hamilton, S.K.; Landis, D.A.; Schmidt, T.M.; Snapp, S.S.; Swinton, S.M. Farming for ecosystem services: An ecological approach to production agriculture. Bioscience 2014, 64, 404–415. [Google Scholar] [CrossRef]
- Bechara, E.; Papafilippaki, A.; Doupis, G.; Sofo, A.; Koubouris, G. Nutrient dynamics, soil properties and microbiological aspects in an irrigated olive orchard managed with five different management systems involving soil tillage, cover crops and compost. J. Water Clim. Chang. 2018, 9, 736–747. [Google Scholar] [CrossRef]
- Moreno, B.; Garcia-Rodriguez, S.; Cañizares, R.; Castro, J.; Benítez, E. Rainfed olive farming in south-eastern Spain: Long-term effect of soil management on biological indicators of soil quality. Agric. Ecosyst. Environ. 2009, 131, 333–339. [Google Scholar] [CrossRef]
- Nieto, O.M.; Castro, J.; Fernández-Ondoño, E. Uso sustentável de solos de olival. Influência das técnicas de maneio nas suas propriedades. Spanish J. Soil Sci. 2012, 2, 70–77. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Turner, B.L.; Mahieu, N.; Condron, L.M. The phosphorus composition of temperate pasture soils determined by NaOH-EDTA extraction and solution 31P NMR spectroscopy. Org. Geochem. 2003, 34, 1199–1210. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Wang, J.B.; Chen, Z.H.; Chen, L.J.; Zhu, A.N.; Wu, Z.J. Surface soil phosphorus and phosphatase activities affected by tillage and crop residue input amounts. Plant Soil Environ. 2011, 57, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Dick, W.A.; Tabatabai, M.A. Inorganic pyrophosphatase activity of soils. Soil Biol. Biochem. 1978, 10, 58–65. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Smernik, R.J.; Marschner, P.; McNeill, A.M. Microbial synthesis of organic and condensed forms of phosphorus in acid and calcareous soils. Soil Biol. Biochem. 2008, 40, 932–946. [Google Scholar] [CrossRef]
- Jimenez-Moreno, M.J.; Fernandez-Escobar, R. Response of young olive plants (Olea europaea) to phosphorus application. Sci. Hortic. 2016, 51, 1167–1170. [Google Scholar] [CrossRef]
- Jimenez-Moreno, M.J.; Fernandez-Escobar, R. Influence of nutritional status of phosphorus on flowering in the olive (Olea europaea L.). Sci. Hortic. 2017, 223, 1–4. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Monokrousos, N.; Psoma, P.; Tziachris, P.; Metaxa, I.; Strikos, G.; Papadopoulos, F.H.; Papadopoulos, A.H. How fully productive olive trees (Olea europaea L., cv. ‘Chondrolia Chalkidikis’) manage to over-satisfy their P nutritional needs under low Olsen P availability in soils? Sci. Hortic. 2020, 265, 109251. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Delviniotis, A.; Panagakos, A.; Giannakoula, A.; Tranaka, V.; Molassiotis, A. Foliar manganese, zinc and boron application effects on mineral nutrition of an experimental olive grove (cv. “Chondrolia Chalkidikis”). J. Plant Nutr. 2017, 40, 1728–1742. [Google Scholar] [CrossRef]
- Stathis, D.; Mavromatis, T. Characteristics of precipitation in thessaloniki area, North Greece. Fresenius Environ. Bull. 2009, 18, 1270–1275. [Google Scholar]
- Allen, E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publishing: Oxford, UK, 1976. [Google Scholar]
- Chyla, M.A.; Zyrnicki, W. Determination of metal concentrations in animal hair by the ICP method: Comparison of various washing procedures. Biol. Trace Elem. Res. 2000, 75, 187–194. [Google Scholar] [CrossRef]
- Wolf, B. Communications in Soil Science and Plant Analysis Improvements in the azomethine—H method for the determination of boron. Commun. Soil Sci. Plant Anal. 1974, 5, 37–41. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Martorana, A.; Di Miceli, C.; Alfonzo, A.; Settanni, L.; Gaglio, R.; Caruso, T.; Moschetti, G.; Francesca, N. Effects of irrigation treatments on the quality of table olives produced with the Greek-style process. Ann. Microbiol. 2017, 67, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Kheir, A.M.S.; Ali, M.G.M.; Ali, O.A.M.; Abdelaal, A.I.N.; Lin, X.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kavvadias, V.; Papadopoulou, M.; Vavoulidou, E.; Theocharopoulos, S.; Koubouris, G.; Psarras, G.; Manolaraki, C.; Giakoumaki, G.; Vasiliadis, A. Effect of sustainable management of olive tree residues on soil fertility in irrigated and rain-fed olive orchards. J. Water Clim. Chang. 2018, 9, 764–774. [Google Scholar] [CrossRef]
- Kavvadias, V.; Papadopoulou, M.; Vavoulidou, E.; Theocharopoulos, S.; Repas, S.; Koubouris, G.; Psarras, G.; Kokkinos, G. Effect of addition of organic materials and irrigation practices on soil quality in olive groves. J. Water Clim. Chang. 2018, 9, 775–785. [Google Scholar] [CrossRef]
- Yusran, F.H. Existing versus added soil organic matter in relation to phosphorus availability on lateritic soils. J. Trop. Soils 2008, 13, 23–34. [Google Scholar] [CrossRef]
- Sui, Y.; Thompson, M.L. Phosphorus sorption, desorption, and buffering capacity in a biosolids-amended Mollisol. Soil Sci. Soc. Am. J. 2000, 64, 164–169. [Google Scholar] [CrossRef]
- Yusran, F.H. The relationship between phosphate adsorption and soil organic carbon from organic matter addition. J. Trop. Soils 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Monokrousos, N.; Papatheodorou, E.M.; Diamantopoulos, J.D.; Stamou, G.P. Soil quality variables in organically and conventionally cultivated field sites. Soil Biol. Biochem. 2006, 38. [Google Scholar] [CrossRef]
- Tang, H.M.; Xiao, X.P.; Tang, W.G.; Lin, Y.C.; Wang, K.; Yang, G.L. Effects of winter cover crops residue returning on soil enzyme activities and soil microbial community in double-cropping rice fields. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Yadav, B.; Sr, N.; Bl, Y.; Sharma, S.; Meena, S.K. Effect of different fertility levels and organic manures on biological properties of soil in Pearl millet. J. Pharmacogn. Phytochem. 2020, 9, 210–213. [Google Scholar]
- Reddy, S.B.; Nagaraja, M.S.; Mallesha, B.C.; Kadalli, G.G. Enzyme Activities at Varied Soil Organic Carbon Gradients under Different Land Use Systems of Hassan District in Karnataka. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1739–1745. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C. Application of vermicomposts and compost on tomato growth in Greenhouses. Compost Sci. Util. 2015, 23, 94–103. [Google Scholar] [CrossRef]
- Song, X.; Razavi, B.S.; Ludwig, B.; Zamanian, K.; Zang, H.; Kuzyakov, Y.; Dippold, M.A.; Gunina, A. Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci. Total Environ. 2020, 735, 139393. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Phosphorous cycle enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 161–183. [Google Scholar]
- Yadav, R.; Tarafdar, J. Influence of organic and inorganic phosphorus supply on the maximum secretion of acid phosphatase by plants. Biol. Fertil. Soils 2001, 34, 140–143. [Google Scholar] [CrossRef]
- Reitzel, K.; Turner, B.L. Quantification of pyrophosphate in soil solution by pyrophosphatase hydrolysis. Soil Biol. Biochem. 2014, 74, 95–97. [Google Scholar] [CrossRef]

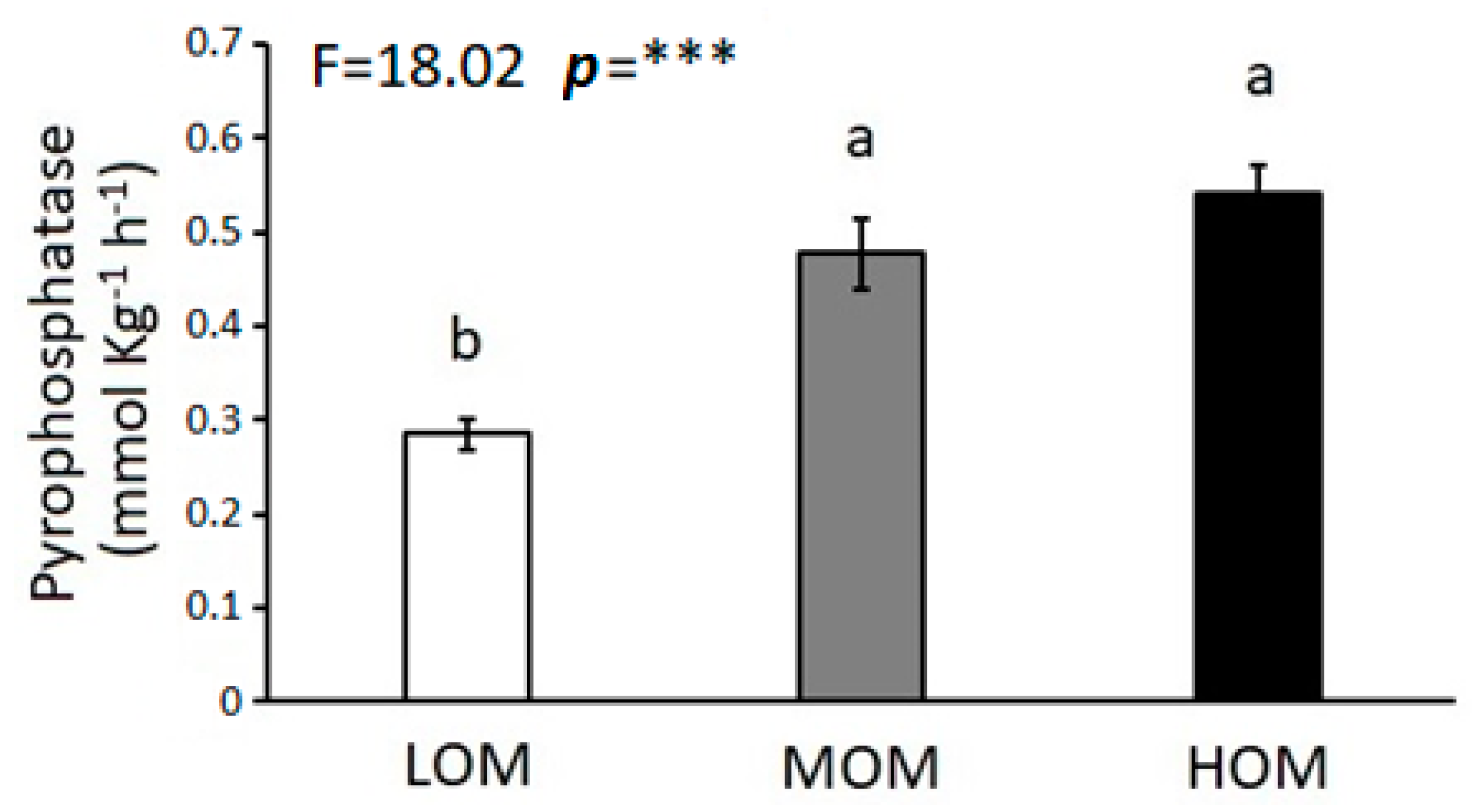
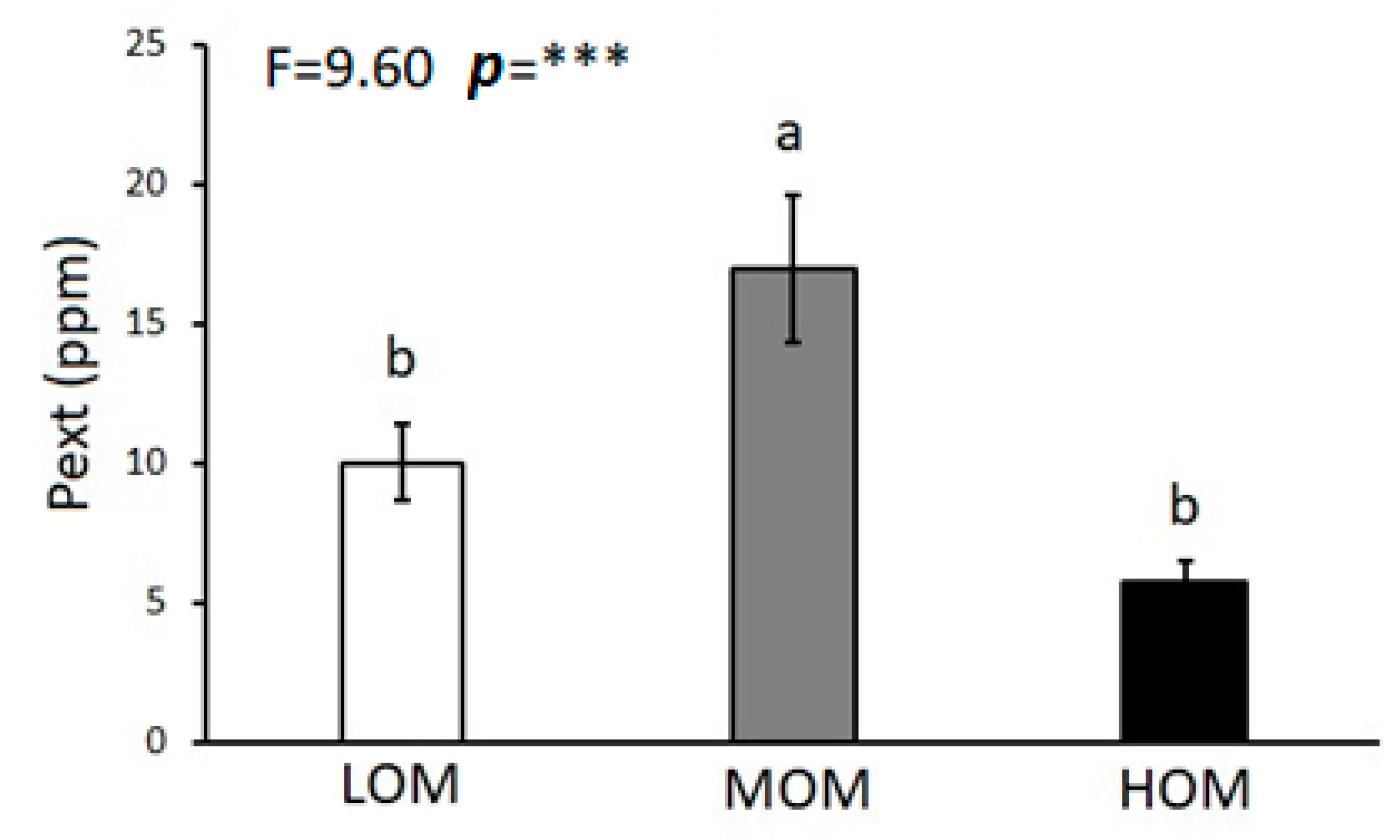



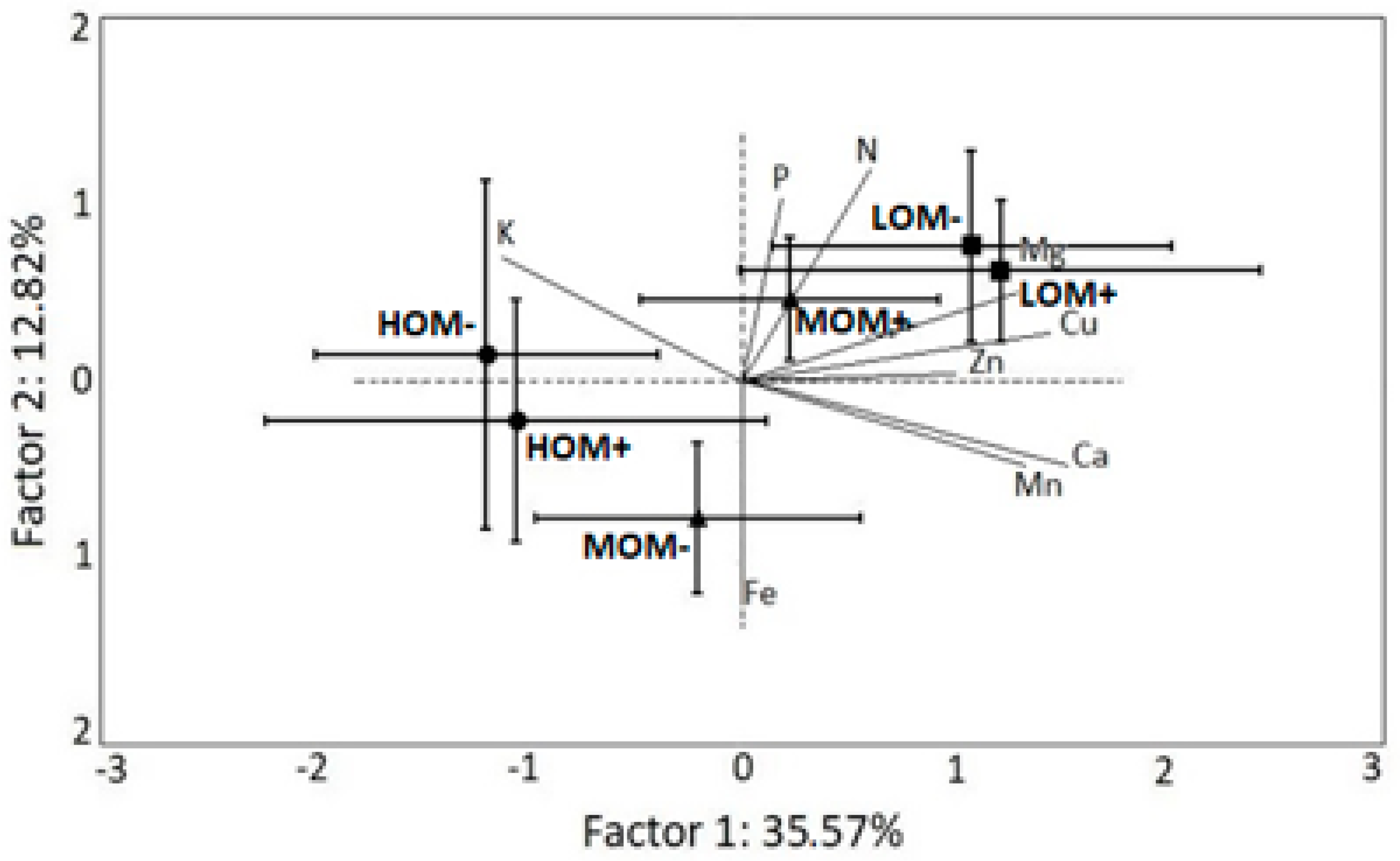
| LOM +I | LOM -I | MOM +I | MOM -I | HOM +I | HOM -I | OM | I | OM × I | |
|---|---|---|---|---|---|---|---|---|---|
| NO3− (ppm) | 132.60 (86.89) | 64.12 (21.04) | 203.91 (50.25) | 79.41 (25.65) | 47.92 (13.28) | 62.84 (7.34) | F = 1.14 p = ns | F = 1.61 p = ns | F = 0.73 p = ns |
| K (ppm) | 254.72 (39.46) | 272.50 (67.55) | 384.25 (74.33) | 416.12 (81.33) | 271.01 (125.11) | 343.57 (89.52) | F = 1.36 p = ns | F = 0.30 p = ns | F = 0.04 p = ns |
| Mg (ppm) | 512.27 (81.44) | 577.01 (114.52) | 620.81 (127.68) | 717.87 (258.70) | 371.50 (167.99) | 199.85 (24.59) | F = 3.67 p = * | F = 0.01 p = ns | F = 0.49 p = ns |
| Ca (ppm) | 101.10 (0.00) | 101.02 (0.00) | 299.40 (135.39) | 787.01 (268.98) | 316.75 (215.75) | 219.57 (118.57) | F = 4.43 p = ** | F = 0.99 p = ns | F = 2.09 p = ns |
| Fe (ppm) | 4.20 (0.49) | 5.45 (1.48) | 14.29 (4.09) | 22.03 (4.32) | 20.25 (5.45) | 21.20 (3.37) | F = 9.30 p = *** | F = 1.07 p = ns | F = 0.53 p = ns |
| Zn (ppm) | 0.84 (0.13) | 1.04 (0.20) | 1.02 (0.11) | 1.08 (0.09) | 2.16 (0.29) | 1.48 (0.20) | F = 12.21 p = *** | F = 0.57 p = ns | F = 3.06 p = ns |
| B (ppm) | 1.52 (0.44) | 1.71 (0.40) | 1.42 (0.27) | 2.71 (1.30) | 9.10 (7.18) | 2.01 (1.34) | F = 2.88 p = ns | F = 1.82 p = ns | F = 3.28 p = ns |
| Pext (ppm) | 10.45 (1.67) | 9.32 (2.37) | 12.56 (2.30) | 11.04 (2.17) | 5.94 (1.21) | 5.70 (0.91) | F = 4.29 p = * | F = 0.33 p = ns | F = 0.05 p = ns |
| ACP (mmol Kg−1h−1) | 0.25 (0.03) | 0.45 (0.04) | 0.33 (0.04) | 0.49 (0.06) | 0.71 (0.16) | 0.63 (0.04) | F = 17.50 p = *** | F = 4.48 p = * | F = 3.44 p = ns |
| ALP (mmol Kg−1h−1) | 0.32 (0.03) | 0.42 (0.04) | 0.33 (0.05) | 0.54 (0.10) | 0.54 (0.04) | 0.60 (0.05) | F = 4.89 p = ** | F = 5.98 p = * | F = 0.77 p = ns |
| PYP (mmol Kg−1h−1) | 0.27 (0.02) | 0.30 (0.03) | 0.39 (0.04) | 0.60 (0.05) | 0.55 (0.09) | 0.53 (0.03) | F = 20.18 p = *** | F = 4.56 p = * | F = 4.35 p = ns |
| LOM +I | LOM -I | MOM +I | MOM -I | HOM +I | HOM -I | OM | I | OM × I | |
|---|---|---|---|---|---|---|---|---|---|
| Νtotal % | 1.59 (0.06) | 1.55 (0.04) | 1.50 (0.07) | 1.17 (0.10) | 1.22 (0.18) | 1.29 (0.08) | F = 6.24 p = * | F = 1.92 p = ns | F = 2.79 p = ns |
| P % | 0.36 (0.03) | 0.34 (0.04) | 0.38 (0.03) | 0.38 (0.03) | 0.37 (0.07) | 0.29 (0.03) | F = 1.07 p = ns | F = 1.22 p = ns | F = 0.81 p = ns |
| Κ % | 0.72 (0.04) | 0.73 (0.05) | 0.77 (0.05) | 0.95 (0.06) | 0.89 (0.06) | 0.89 (0.05) | F = 3.93 p = * | F = 1.25 p = ns | F = 2.02 p = ns |
| Ca % | 2.07 (0.12) | 1.94 (0.22) | 2.03 (0.21) | 1.40 (0.22) | 1.59 (0.17) | 1.55 (0.11) | F = 2.46 p = ns | F = 2.80 p = ns | F = 1.49 p = ns |
| Mg % | 0.25 (0.02) | 0.25 (0.02) | 0.24 (0.03) | 0.22 (0.03) | 0.17 (0.01) | 0.16 (0.01) | F = 5.18 p = ** | F = 0.29 p = ns | F = 0.08 p = ns |
| B (ppm) | 28.66 (3.10) | 28.15 (1.95) | 26.68 (2.72) | 22.08 (1.40) | 20.04 (0.80) | 23.26 (1.41) | F = 3.04 p = ns | F = 0.08 p = ns | F = 1.09 p = ns |
| Zn (ppm) | 13.77 (1.40) | 15.79 (1.05) | 13.06 (0.88) | 11.59 (1.09) | 12.22 (0.96) | 13.53 (0.96) | F = 2.23 p = ns | F = 0.36 p = ns | F = 1.21 p = ns |
| Fe (ppm) | 67.90 (7.55) | 61.82 (2.11) | 68.64 (4.47) | 85.88 (11.13) | 65.29 (11.27) | 92.28 (9.58) | F = 1.27 p = ns | F = 2.65 p = ns | F = 1.51 p = ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, N.; Chatzistathis, T.; Papatheodorou, E.M.; Aschonitis, V.; Monokrousos, N. The Crucial Role of Soil Organic Matter in Satisfying the Phosphorus Requirements of Olive Trees (Olea europaea L.). Agriculture 2021, 11, 111. https://doi.org/10.3390/agriculture11020111
Christopoulou N, Chatzistathis T, Papatheodorou EM, Aschonitis V, Monokrousos N. The Crucial Role of Soil Organic Matter in Satisfying the Phosphorus Requirements of Olive Trees (Olea europaea L.). Agriculture. 2021; 11(2):111. https://doi.org/10.3390/agriculture11020111
Chicago/Turabian StyleChristopoulou, Niki, Theocharis Chatzistathis, Efimia M. Papatheodorou, Vassilis Aschonitis, and Nikolaos Monokrousos. 2021. "The Crucial Role of Soil Organic Matter in Satisfying the Phosphorus Requirements of Olive Trees (Olea europaea L.)" Agriculture 11, no. 2: 111. https://doi.org/10.3390/agriculture11020111









