Richness of Rhizosphere Organisms Affects Plant P Nutrition According to P Source and Mobility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Experimental Device and Nutrient Media
2.3. Experimental Design
2.4. Plant and Medium Measurements
2.5. Data Analyses
3. Results
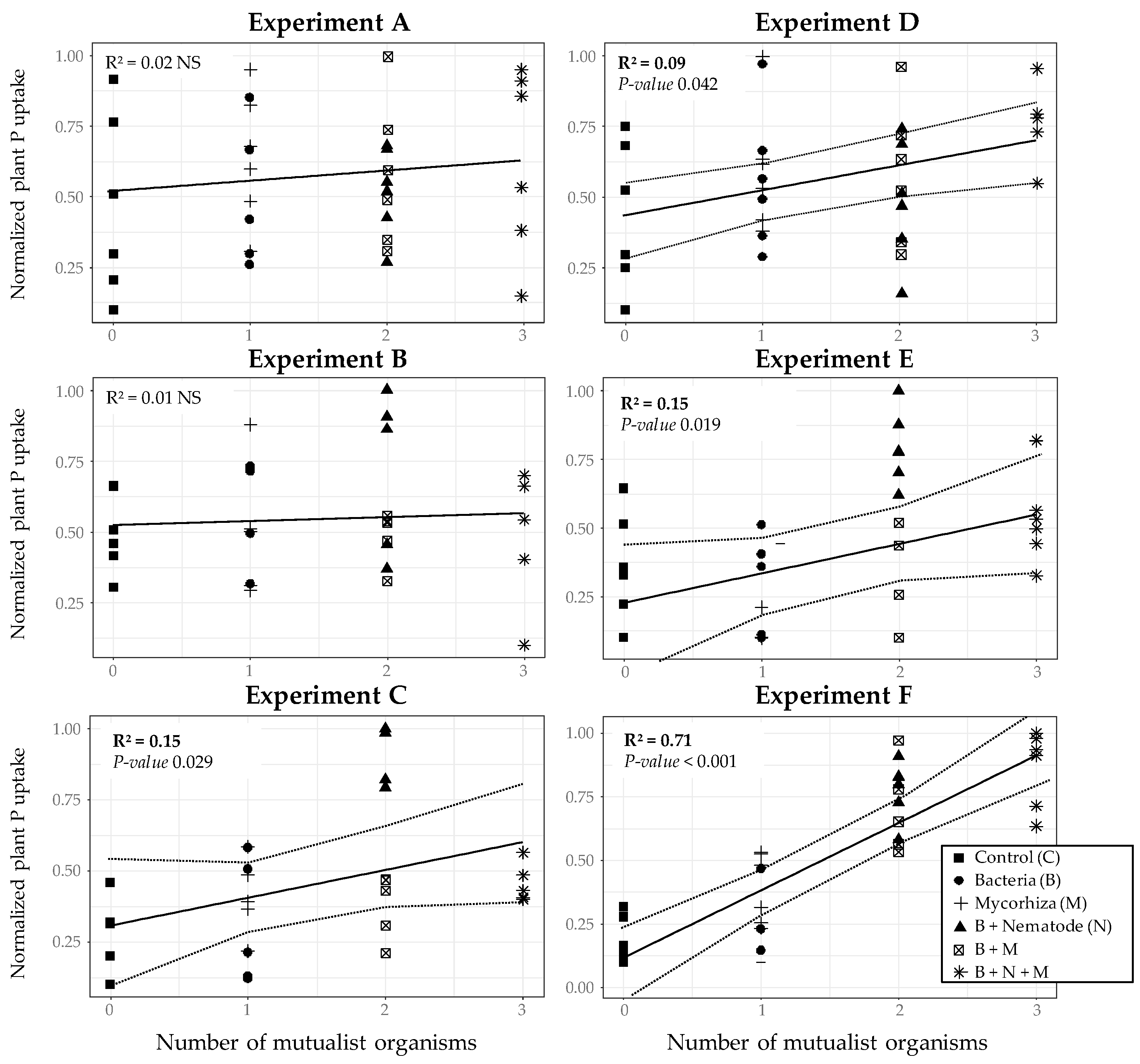
3.1. Plant P Response to Enriched Biological Assemblages in Low-P Sorbing Medium
3.2. Plant P Response to Enriched Biological Assemblages in High-P Sorbing Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Becquer, A.; Trap, J.; Irshad, U.; Ali, M.A.; Claude, P. From soil to plant, the journey of P through trophic relationships and ectomycorrhizal association. Front. Plant Sci. 2014, 5, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plassard, C.; Dell, B. Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 2010, 30, 1129–1139. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Plassard, C.; Louche, J.; Ali, M.A.; Duchemin, M.; Legname, E.; Cloutier-Hurteau, B. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]
- Clarholm, M. Interactions of bacteria, protozoa and plants leading to mineralization of soil-nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Irshad, U.; Villenave, C.; Brauman, A.; Plassard, C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 2011, 43, 2121–2126. [Google Scholar] [CrossRef]
- Ranoarisoa, M.P.; Trap, J.; Pablo, A.L.; Dezette, D.; Plassard, C. Micro-food web interactions involving bacteria, nematodes, and mycorrhiza enhance tree P nutrition in a high P-sorbing soil amended with phytate. Soil Biol. Biochem. 2020, 143, 107728. [Google Scholar] [CrossRef]
- Irshad, U.; Brauman, A.; Villenave, C.; Plassard, C. Phosphorus acquisition from phytate depends on efficient bacterial grazing, irrespective of the mycorrhizal status of Pinus pinaster. Plant Soil 2012, 358, 148–161. [Google Scholar] [CrossRef]
- Mezeli, M.M.; Page, S.; George, T.S.; Neilson, R.; Mead, A.; Blackwell, M.S.; Haygarth, P.M. Using a meta-analysis approach to understand complexity in soil biodiversity and phosphorus acquisition in plants. Soil Biol. Biochem. 2020, 142, 107695. [Google Scholar] [CrossRef]
- Johnson, N.C. Can fertilization of soil select less mutualistic mycorrhizae? Bull. Ecol. Soc. Am. 1993, 3, 749–757. [Google Scholar] [CrossRef]
- Marmeisse, R.; Guidot, A.; Gay, G.; Lambilliotte, R.; Sentenac, H.; Combier, J.P.; Melayah, D.; Fraissinet-Tachet, L.; Debaud, J.C. Hebeloma cylindrosporum-a model species to study ectomycorrhizal symbiosis from gene to ecosystem. New Phytol. 2004, 163, 481–498. [Google Scholar] [CrossRef]
- Becquer, A.; Torres-Aquino, M.; Le Guernevé, C.; Amenc, L.K.; Trives-Segura, C.; Staunton, S.; Quiquampoix, H.; Plassard, C. Establishing a symbiotic interface between cultured ectomycorrhizal fungi and plants to follow fungal phosphate metabolism. Bio-Protocol 2017, 7, e2577. [Google Scholar] [CrossRef]
- Morizet, J.; Mingeau, M. Influence des facteurs de milieu sur l’absorption hydrique. Etude effectuee sur tomate decapitee en exsudation. i. facteurs nutritionnels. Ann. Agron. 1976, 27, 183–205. [Google Scholar]
- Casarin, V.; Plassard, C.; Souche, G.; Arvieu, J.C. Quantification of oxalate ions and protons released by ectomycorrhizal fungi in rhizosphere soil. Agronomie 2003, 23, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Aquino, M.; Plassard, C. Dynamics of ectomycorrhizal mycelial growth and P transfer to the host plant in response to low and high soil P availability. FEMS Microbiol. Ecol. 2004, 48, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, T.; Zibilske, L.M. Determination of low concentration of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Team, R.C. RStudio: Integrated Development for R. 2016. Available online: https://rstudio.com/products/rstudio (accessed on 20 November 2015).
- Velásquez, E.; Lavelle, P.; Andrade, M. GISQ, a multifunctional indicator of soil quality. Soil Biol. Biochem. 2007, 39, 3066–3080. [Google Scholar] [CrossRef]
- Naeem, S.; Loreau, M.; Inchausti, P. Biodiversity and ecosytsem functioning: The emergence of a synthetic ecological framework. In Biodiversity and Ecosystem Functioning: Synthesis and Perspectives; Loreau, S.M., Naeem, P., Eds.; Inchausti. Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Louche, J.; Ali, M.A.; Cloutier-Hurteau, B.; Sauvage, F.X.; Quiquampoix, H.; Plassard, C. Efficiency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol. Ecol. 2010, 73, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Raffaelli, D.; Schmid, B.; Tilman, D.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Experiments * | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Medium | ||||||
| Type | Agarose | Agarose | Agarose | Soil # | Soil # | Soil # |
| Volume | 70 mL | 70 mL | 70 mL | 30 g | 30 g | 30 g |
| Phosphorus | ||||||
| Source | Absent | NaH2PO4 | Phytate | Absent | NaH2PO4 | Phytate |
| Rate (per dish) | / | 6.5 mg-P | 9.3 mg-P | / | 6.5 mg-P | 9.3 mg-P |
| Duration (d) | 45 | 45 | 45 | 100 | 100 | 100 |
| Sample size | 6 | 5 | 5 | 6 | 6 | 6 |
| Terms | Coefficients | |||
|---|---|---|---|---|
| Estimate | Std. Error | t-Value | Pr(>|t|) | |
| Experiment B | −0.03 | 0.174 | −0.16 | 0.871 |
| Experiment C | −0.12 | 0.102 | −1.17 | 0.243 |
| Experiment D | −0.07 | 0.097 | −0.67 | 0.504 |
| Experiment E | −0.18 | 0.098 | −1.80 | 0.074 |
| Experiment F | −0.33 | 0.097 | −3.37 | 0.001 |
| Mycorhiza (M) | 0.11 | 0.069 | 1.56 | 0.120 |
| Nematode (N) | 0.02 | 0.084 | 0.25 | 0.807 |
| Bacteria (Ba) | 0.01 | 0.084 | 0.13 | 0.900 |
| Experiment B:M | −0.20 | 0.110 | −1.79 | 0.076 |
| Experiment C:M | −0.18 | 0.102 | −1.80 | 0.074 |
| Experiment D:M | 0.04 | 0.097 | 0.43 | 0.669 |
| Experiment E:M | −0.28 | 0.099 | −2.81 | 0.005 |
| Experiment F:M | 0.12 | 0.096 | 1.23 | 0.219 |
| Experiment B:N | 0.03 | 0.147 | 0.23 | 0.816 |
| Experiment C:N | 0.31 | 0.125 | 2.45 | 0.015 |
| Experiment D:N | 0.03 | 0.119 | 0.29 | 0.776 |
| Experiment E:N | 0.38 | 0.122 | 3.13 | 0.002 |
| Experiment F:N | 0.31 | 0.117 | 2.68 | 0.008 |
| Experiment B:Ba | 0.09 | 0.120 | 0.75 | 0.452 |
| Experiment C:Ba | −0.01 | 0.125 | −0.09 | 0.930 |
| Experiment D:Ba | 0.05 | 0.119 | 0.39 | 0.699 |
| Experiment E:Ba | 0.01 | 0.119 | 0.07 | 0.945 |
| Experiment F:Ba | 0.14 | 0.119 | 1.15 | 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trap, J.; Ranoarisoa, P.M.; Irshad, U.; Plassard, C. Richness of Rhizosphere Organisms Affects Plant P Nutrition According to P Source and Mobility. Agriculture 2021, 11, 157. https://doi.org/10.3390/agriculture11020157
Trap J, Ranoarisoa PM, Irshad U, Plassard C. Richness of Rhizosphere Organisms Affects Plant P Nutrition According to P Source and Mobility. Agriculture. 2021; 11(2):157. https://doi.org/10.3390/agriculture11020157
Chicago/Turabian StyleTrap, Jean, Patricia Mahafaka Ranoarisoa, Usman Irshad, and Claude Plassard. 2021. "Richness of Rhizosphere Organisms Affects Plant P Nutrition According to P Source and Mobility" Agriculture 11, no. 2: 157. https://doi.org/10.3390/agriculture11020157
APA StyleTrap, J., Ranoarisoa, P. M., Irshad, U., & Plassard, C. (2021). Richness of Rhizosphere Organisms Affects Plant P Nutrition According to P Source and Mobility. Agriculture, 11(2), 157. https://doi.org/10.3390/agriculture11020157






