Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment and Agricultural Practices
2.2. Source and Plant Growth-Promoting Traits of Bacterial Strains
2.3. Bacterial and Mycorrhizal Inocula Preparation
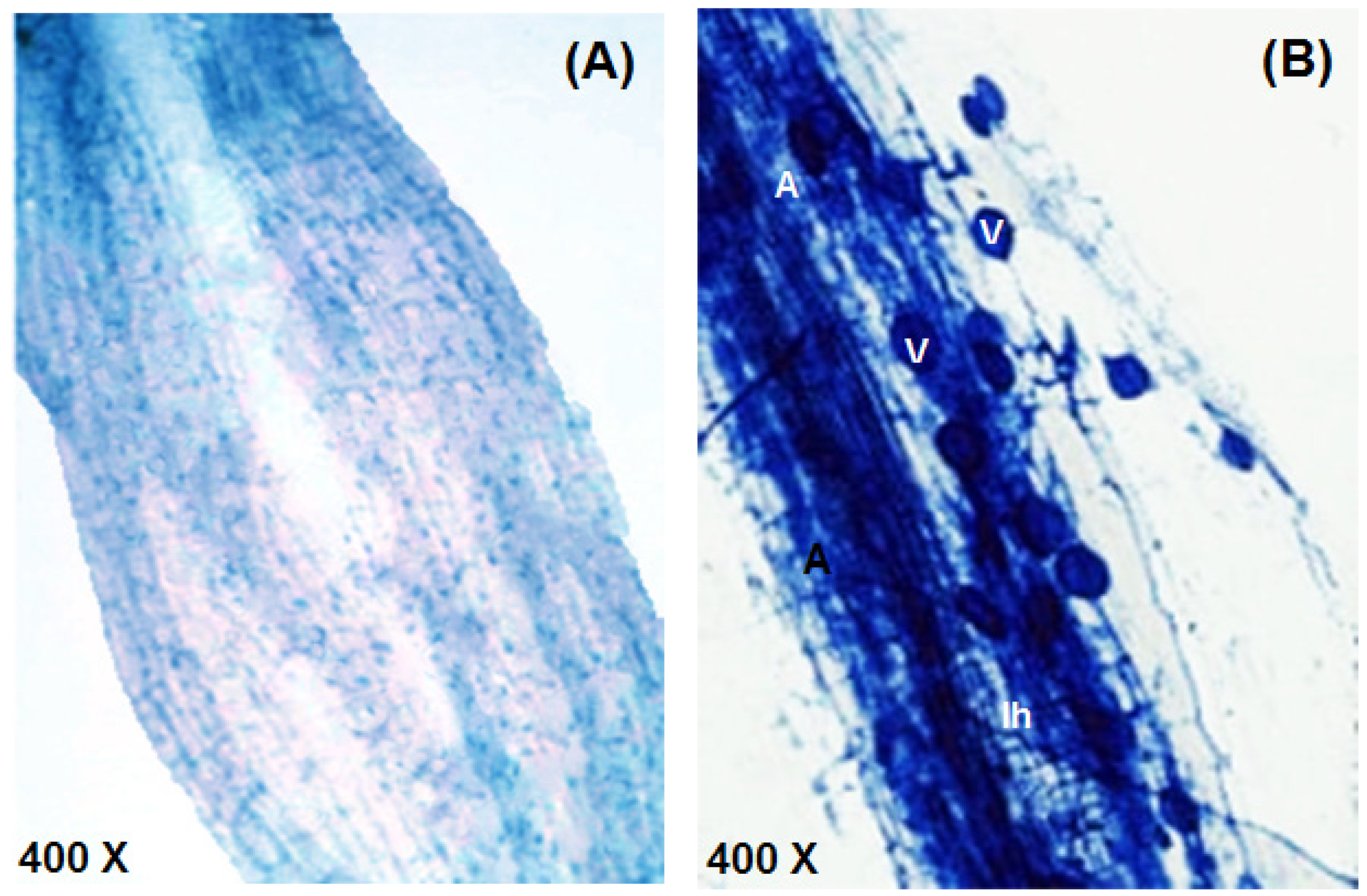
2.4. Staining and Detection of the Levels of Mycorrhizal Colonization
2.5. Morph-Physiological and Yield of Guar
2.6. Biochemical Analyses
2.7. Enzymatic Assays
2.8. Bacterial Count in the Rhizosphere
2.9. Statistical Analysis
3. Results
3.1. Plant Growth Promotion Traits
3.2. Morpho-Physiological Traits as Affected by Biofertilizer Treatments
3.3. Yield and Their Attributes as Affected by Biofertilizer Treatments
3.4. Effects of Biofertilizers on Nutrient Uptake
3.5. Effects of Biofertilizers on Seed Quality Properties
3.6. Effects of Biofertilizers on Enzyme Activities in the Rhizosphere of the Guar Plant
3.7. Bacterial Counts in the Rhizosphere and Mycorrhizal Colonization in Guar Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Meftahizadeh, H.; Ghorbanpour, M.; Asareh, M.H. Changes in phenological attributes, yield and phytochemical compositions of guar (Cyamopsis tetragonoloba L.) land araces under various irrigation regimes and planting dates. Sci. Hortic. 2019, 256, 108577. [Google Scholar] [CrossRef]
- Anuradha; Singh, R.K.; Pareek, B.; Kumar, D.; Meena, S.; Dubey, S.K. Different Levels of Fertilizers on Growth and Yield of Cluster Bean (Cyamopsis tetragonoloba L.) in Rainfed Area of Uttar Pradesh. India Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2029–2036. [Google Scholar]
- Kadian, N.; Yadav, K.; Aggarwal, A. Significance of bioinoculants in promoting growth, nutrient uptake and yield of Cyamopsis tetragonoloba (L.) “Taub”. Eur. J. Soil Biol. 2013, 58, 66–72. [Google Scholar] [CrossRef]
- Mathur, N.K. Industrial Galactomannan Polysaccharides; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Krikum, J.; Haas, J.H.; Dodd, J.; Kinsbursky, R. Mycorrhizal dependence of four crops in a P-absorbing soil. Plant Soil. 1990, 122, 213–217. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- Tarnabi, Z.M.; Iranbakhsh, A.; Mehregan, I.; Ahmadvand, R. Impact of arbuscular mycorrhizal fungi (AMF) on gene expression of some cell wall and membrane elements of wheat (Triticum aestivum L.) under water deficit using transcriptome analysis. Physiol. Mol. Biol. Plants. 2019, 26, 143–162. [Google Scholar] [CrossRef]
- El-Zehery, T.M.; Ghazi, A.A. Impact of Biochar and Biofertilizer on Guar Plant (Cyamopsis tetragonoloba L. Taub) Growth and Sandy Soil Fertility. J. Soil Sci. Agric. Eng. 2019, 10, 679–684. [Google Scholar] [CrossRef]
- Manohar, C.V.S.; Sharma, O.P.; Verma, H.P. Nutrient status and yield of clusterbean [Cyamopsis tetragonoloba (L.) Taub] as influenced by fertility levels and liquid biofertilizers. J. Pharmacogn. Phytochem. 2018, 7, 1840–1843. [Google Scholar]
- Elsheikh, E.A.E.; Ibrahim, K.A. The effect of Bradyrhizobium inoculation on yield and seed quality of guar (Cyamopsis tetragonoloba L.). Food Chem. 1999, 65, 183–187. [Google Scholar] [CrossRef]
- Miransari, M. Soil microbes and plant fertilization. Appl. Microbiol. Biotechnol. 2011, 92, 875–885. [Google Scholar] [CrossRef]
- Hauka, F.I.A.; Afify, A.H.; El-Sawah, A.M. Efficiency Evaluation of Some Rhizobacteria Isolated from Egyptian Soils, In Vitro as Biofertilizers. J. Agric. Chem. Biotechnol. 2017, 8, 231–235. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Hauka, F.I.A.; Afify, A.H. Dual inoculation with Azotobacter chroococcum MF135558 and Klebsiella oxytoca MF135559 enhance the growth and yield of wheat plant and reduce N-fertilizers usage. J. Food Dairy Sci. 2018, 10, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 2020, 10, 1513. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis Reduces Cadmium Accumulation and Improves Growth and Antioxidant Defense System in Two Wheat (Triticum aestivum L.) Varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Rathod, D.P.; Brestic, M.; Shao, H.B. Chlorophyll a fluorescence determines the drought resistance capabilities in two varieties of mycorhized and non-mycorrhized Glycine max Linn. Afr. J. Microbiol. Res. 2011, 17, 4197–4206. [Google Scholar]
- Ahammed, G.J.; Mao, Q.; Yan, Y.; Wu, M.; Wang, Y.; Ren, J.; Guo, P.; Liu, A.; Chen, S. Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology 2020, 110, 999–1009. [Google Scholar] [CrossRef]
- Alam, M.Z.; Hoque, M.A.; Ahammed, G.J.; Carpenter-Boggs, L. Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris. PLoS ONE 2019, 14, e0211441. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. Plant Growth-Promoting. Encyclopedia. Soils Environ. 2005, 1, 103–115. [Google Scholar]
- Afify, A.H.; Hauka, F.I.A.; El-Sawah, A.M. Plant Growth-Promoting Rhizobacteria enhance Onion (Allium cepa L.) productivity and minimize requisite chemical fertilization. Environ. Biodivers. Soil Secur. 2018, 2, 119–129. [Google Scholar] [CrossRef]
- Mand, S.; Dahiya, B.N.; Lakshminarayana, K. Nodulation, nitrogen fixation and biomass yield by slow and fast growing cow-pea rhizobia in guar under different environments. Ann. Biol. 1991, 7, 31–37. [Google Scholar]
- Abdel-Fattah, G.M.; Asrar, A. Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiol. Plant. 2012, 34, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, G.M. Functional activity of VA-mycorrhiza (Glomus mosseae) in the growth and productivity of soybean plants grown in sterilized soil. Folia Microbiol. 1997, 42, 495–502. [Google Scholar] [CrossRef]
- Xu, H.; Hongbo, S.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476. [Google Scholar] [CrossRef] [PubMed]
- Scheublin, T.R.; Van der Heijden, M.G.A. Arbuscular mycorrhizal fungi colonize non-fixing root nodules of several legume species. New Phytol. 2006, 172, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologiya 1948, 17, 362–370. [Google Scholar]
- Hemalatha, N.; Raja, N.; Jayachitra, A.; Rajalakshmi, A.; Valarmathi, N. Isolation and characterization of phosphate solubilizing bacteria and analyzing their effect on Capsicum annum L. Int. J. Biol. Pharm. Res. 2013, 4, 159–167. [Google Scholar]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Vincent, J.M.A. Manual for the Practical Study of the Root- Nodule Bacteria; IBP Handbook No. 15; Blackwell: Oxford, UK; Edinburgh, UK, 1970; pp. 54–58. [Google Scholar]
- Hafez, E.E.; Abdel-Fattah, G.M.; El-Haddad, S.A.; Rashad, Y.M. Molecular defense response of mycorrhizal bean plants infected with Rhizoctonia solani. Ann. Microbiol. 2013, 63, 1195–1203. [Google Scholar] [CrossRef]
- McGill, W.B.; Figueiredo, C.T. Total nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 201–211. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englwood Cliff, NY, USA, 1962. [Google Scholar]
- Ashraf, M.Y.; Khan, A.H.; Azmi, A.R. Cell membrane stability and its relation with some physiological process in wheat. Acta Agron. Hung. 1992, 41, 182–191. [Google Scholar]
- Chapman, D.H.; Parker, E.R. Determination of NPK Methods of Analysis for Soil, Plant and Waters; Public Division of Agricultural Sciences, University of California: Berkeley, CA, USA, 1961; pp. 150–179. [Google Scholar]
- Anderson, E. Endosperm mucilage of legumes. Occurrence and composition. Ind. Eng. Chem. 1949, 41, 2887–2890. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of A.O.A.C. International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Sene, M.; Thevenot, C.; Prioul, J.L. Simultaneous spectrophotometric determination of amylose and amylopectin in starch from maize kernel by multi wave length analysis. J. Cereal Sci. 1997, 26, 211–221. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Liu, T.; Xu, C.; Duan, D.; Peng, C. The variations in the soil enzyme activity, protein expression, microbial biomass, and community structure of soil contaminated by heavy metals. ISRN Soil Sci. 2013, 2013, 803150. [Google Scholar] [CrossRef]
- Abou-Aly, H.E.; Mady, M.A. Complemented effect of humic acid and biofertilizers on wheat (Triticum aestivum L.) productivity. Ann. Agric. Sci. Moshtohor 2009, 47, 1–12. [Google Scholar]
- Juge, C.; Prévosta, D.; Bertranda, A.; Bipfubusaa, M.; Chalifourb, F.P. Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium, Azospirillum and arbuscular mycorrhizae. Appl. Soil Ecol. 2012, 61, 147–157. [Google Scholar] [CrossRef]
- Tewfike, T.A. Effect of dual biofertilization, biogas manure and mineral fertilizer on guar plants {Cyamopsis tetragonoloba (L.) taub.]. J. Agric. Sci. Mansoura Univ. 2000, 25, 6481–6492. [Google Scholar]
- Ezawa, T.; Yoshida, T. Characterization of phosphatase in marigold roots infected with vesicular-arbuscular mycorrhizal fungi. Soil Sci. Plant Nutr. 1994, 40, 255–264. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Joshi, O.P.; Khan, I.R. Phytase, phosphatase activity and P-nutrition of soybean as influenced by inoculation of Bacillus. Indian J. Microbiol. 2011, 51, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Zai, X.M.; Hao, Z.P.; Zhao, H.; Qin, P. Rhizospheric niche of beach plum seedlings colonized by arbuscular mycorrhizal fungi. Sci. Silvae Sin. 2014, 50, 41–48. [Google Scholar]
- Beck, D.; Duc, G. Improving N2-fixation in faba bean: Rhizobium inoculation and N nutrition. In Present Status and Future Prospects of Faba Bean Production and Improvement in the Mediterranean Countries; Cunero, J.I., Saxena, M.C., Eds.; CIHEAM: Zaragoza, Ethiopia, 1991; pp. 97–103. [Google Scholar]
- Fox, S.L.; O’Hara, G.W.; Bräu, L. Enhanced nodulation and symbiotic effectiveness of Medicago truncatula when co-inoculated with Pseudomonas fluorescens WSM3457 and Ensifer (Sinorhizobium) medicae WSM419. Plant Soil 2011, 348, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Tena, W.; Wolde-Meskel, E.; Walley, F. Response of chickpea (Cicer arietinum L.) to inoculation with native and exotic Mesorhizobium strains in Southern Ethiopia. Afr. J. Biotechnol. 2016, 15, 1920–1929. [Google Scholar]
- Shi, Y.; Lou, K.; Li, C. Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth. Res. 2010, 105, 5–13. [Google Scholar] [CrossRef]
- Armada, E.; Portela, G.; Roldán, A.; Azcón, R. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 2014, 232, 640–648. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Pare, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in plant. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A soil bacterium regulates plant acquisition of iron via deficiency inducible mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef]
- Abd-Allah, W.H.; Khater, R.M. Effect of rock phosphate and mycorrhiza on vegetative growth and productivity of Ricinus communis var Red Arish under North Sinai conditions. Middle East J. Agric. 2016, 5, 412–421. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Tawaraya, K.; Naito, M.; Wagatsuma, T. Solubilization of insoluble inorganic phosphate by hyphal exudates of arbuscular mycorrhizal fungi. J. Plant Nutr. 2006, 29, 657–665. [Google Scholar] [CrossRef]
- Benedetto, A.; Magurno, F.; Bonfante, P.; Lanfranco, L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 2005, 15, 620–627. [Google Scholar] [CrossRef]
- Javot, H.; Pumplin, N.; Harrison, M.J. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ. 2007, 30, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Joner, E.J.; Johansen, A. Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol. Res. 2000, 104, 81–86. [Google Scholar] [CrossRef]
- Ezawa, T.; Smith, S.E.; Smith, F.A. P metabolism and transport in AM fungi. Plant Soil 2002, 244, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Singleton, P.W.; Abdel Magid, H.M.; Tavares, J.W. Effect of phosphorus on the effectiveness of strains of Rhizobium japonicum. Soil Sci. Soc. Am. J. 1985, 49, 613–616. [Google Scholar] [CrossRef]
- Barea, J.; Pozo, M.; Azco’n, R.; Azco´n-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Aly, H.E. Co-inoculation effect with Rhizobium and Azospirillum on growth, nodulation and yield of guar plant (Cyampsis tetragonoloba L.). Ann. Agric. Sci. Moshtohor. 2001, 39, 2171–2181. [Google Scholar]
- Geneca, M.; Zehirov, G.; Djonova, E.; Kaloyanova, N.; Georgiev, G.; Stancheva, I. The effect of inoculation of pea plants with mycorrhizal fungi and Rhizobium on nitrogen and phosphorus assimilation. Plant Soil Environ. 2006, 52, 435–440. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–110. [Google Scholar] [CrossRef]
- Stancheva, I.; Geneva, M.; Zehirov, G.; Tsvetkova, G.; Hristozkova, M.; Georgiev, G. Effects of combined inoculation of pea plants with arbuscular mycorrhizal fungi and rhizobium on nodule formation and nitrogen fixing activity. Gen. Appl. Plant Physiol. 2006, 4, 61–66. [Google Scholar]






| Property | 2018 | 2019 | |
|---|---|---|---|
| Chemical properities | OM% | 2.02 | 1.99 |
| pH | 7.93 | 8.61 | |
| EC | 2.04 | 2.09 | |
| Cations (meq L−1) | Ca2+ | 11.84 | 9.21 |
| Mg2+ | 6.09 | 4.92 | |
| Na+ | 5.39 | 2.93 | |
| K+ | 0.57 | 0.08 | |
| Anions (meq L−1) | CO32− | 0.00 | 0.00 |
| HCO3− | 3.07 | 1.42 | |
| Cl− | 10.17 | 10.17 | |
| SO42− | 10.66 | 5.56 | |
| Available nutrients (mg/kg) | N | 164.00 | 252.0 |
| P | 9.52 | 9.78 | |
| K | 314.56 | 526.50 | |
| Bacterial count | TBC | 6.17 | 6.28 |
| PSBC | 4.91 | 4.95 | |
| Year | Treatments | Shoot Length (cm) | Root Length (cm) | Branches/Plant | Dry Weight (g/plant) | LAI (cm2) | Chl. Content (mg L−1) |
|---|---|---|---|---|---|---|---|
| 2018 | Control | 183.6 (±1.4) e | 31.4 (±1.8) f | 4.3 (±1.5) c | 74.8 (±2.1) c | 43.1 (±2.0) e f | 31.3 (±1.0) e |
| Bradyrhizobium sp. | 209.6 (±4.2) b–d | 38.4 (±3.5) b–d | 6.3 (±1.1) bc | 96.1 (±4.4) b | 47.5 (±5.5) d–f | 48.7 (±4.1) ab | |
| B. subtilis | 201.7 (±4.6) d | 32.1 (±2.2) ef | 5.6 (±0.5) bc | 80.1 (±1.9) c | 41.4 (±1.8) f | 2.7 (±2.6) de | |
| Mycorrhiza | 214.5 (±7.0) a b | 40.2 (±1.8) a–c | 7.3 (±0.5) ab | 103.4 (±0.5) b | 53.6 (±2.8) c d | 41.2 (±3.3) c | |
| Mixture | 223.3 (±6.7) a | 43.6 (±3.3) a | 7.0 (±1.7) ab | 120.9 (±11.5) a | 61.8 (±6.9) a | 49.6 (±2.5) a | |
| 2019 | Control | 202.8 (±2.3) c d | 32.5 (±1.1) e f | 4.6 (±1.1) c | 78.9 (±3.5) c | 47.6 (±1.1) d–f | 38.4 (±2.3) cd |
| Bradyrhizobium sp. | 208.0 (±7.2) b–d | 36.9 (±2.9) b–e | 7.0 (±1.0) ab | 100.9 (±11.7) b | 49.0 (±1.0) c–e | 43.7 (±3.1) bc | |
| B. subtilis | 202.4 (±2.0) cd | 34.0 (±1.0) d–f | 7.3 (±1.5) ab | 84.5 (±5.5) c | 53.9 (±2.5) b–d | 33.5 (±0.9) de | |
| Mycorrhiza | 211.9 (±8.1) bc | 36.3 (±3.2) c–f | 8.6 (±0.5) a | 106.4 (±4.1) b | 55.0 (±4.1) bc | 42.2 (±6.6) c | |
| Mixture | 214.4 (±1.6) a b | 41.7 (±3.6) ab | 9.0 (±1.3) a | 128.6 (±6.5) a | 60.1 (±2.3) ab | 50.2 (±2.0) a |
| Year | Treatments | Number of Pods | 100 Seeds Weight (g) | Seeds Yield (ton/ha) |
|---|---|---|---|---|
| 2018 | Control | 92.0 (±7.0) de | 4.02 (±0.07) d | 1.93 (±0.2) c |
| Bradyrhizobium sp. | 95.0 (±2.0) c–e | 4.02 (±0.03) d | 2.29 (±0.2) b | |
| B. subtilis | 78.0 (±5.5) f | 4.06 (±0.16) d | 1.91 (±0.2) c | |
| Mycorrhiza | 101.3 (±4.1) bc | 4.11 (±0.08) cd | 2.22 (±0.02) bc | |
| Mixture | 106.3 (±3.0) b | 4.28 (±0.13) bc | 2.79 (±0.01) a | |
| 2019 | Control | 90.3 (±4.1) e | 3.92 (±0.02) d | 2.32 (±0.2) b |
| Bradyrhizobium sp. | 100.3 (±5.0) b–d | 4.05 (±0.07) d | 2.48 (±0.20 ab | |
| B. subtilis | 89.6 (±3.0) e | 4.03 (±0.13) d | 2.17 (±0.1) bc | |
| Mycorrhiza | 103.6 (±8.6) bc | 4.31 (±0.04) b | 2.47 (±0.1) ab | |
| Mixture | 124.3 (±3.0) a | 4.58 (±0.13) a | 2.82 (±0.02) a |
| Year | Treatments | Protein (%) | Carbohydrates (%) | Starch (%) | Fatty Acids (%) | Guaran (%) |
|---|---|---|---|---|---|---|
| 2018 | Control | 26.03 (±0.30) de | 59.13 (±0.23) f | 2.96 (±0.01) e | 1.68 (±0.02) c | 12.03 (±0.03) e |
| Bradyrhizobium sp. | 30.85 (±0.31) a | 60.63 (±0.51) d | 2.88 (±0.02) f | 1.62 (±0.02) d | 12.41 (±0.03) d | |
| B. subtilis | 27.63 (±0.53) bc | 61.63 (±0.32) bc | 3.23 (±0.030 c | 1.69 (±0.02) b c | 11.94 (±0.04) ef | |
| Mycorrhiza | 26.86 (±0.24) cd | 59.83 (±0.45) e | 3.13 (±0.06) d | 1.81 (±0.01) a | 11.84 (±0.03) f | |
| Mixture | 30.38 (±0.32) a | 62.73 (±0.37) a | 3.37 (±0.02) a | 1.69 (±0.01) bc | 12.27 (±0.02) d | |
| 2019 | Control | 25.83 (±0.25) e | 59.30 (±0.45) e f | 3.00 (±0.01) e | 1.57 (±0.020 e | 13.43 (±0.06) b |
| Bradyrhizobium sp. | 30.48 (±1.15) a | 61.33 (±0.47) c | 2.82 (±0.02) g | 1.60 (±0.03) de | 13.62 (±0.09) a | |
| B. subtilis | 28.00 (±0.24) b | 62.03 (±0.25) b | 3.20 (±0.03) c | 1.73 (±0.02) b | 12.57 (±0.15) c | |
| Mycorrhiza | 27.05 (±0.28) c | 60.53 (±0.45) d | 3.14 (±0.03) d | 1.84 (±0.04) a | 12.35 (±0.14) d | |
| Mixture | 30.72 (±0.43) a | 62.86 (±0.25) a | 3.31 (±0.04) b | 1.67 (±0.01) c | 13.52 (±0.07) ab |
| Year | Treatments | Bacterial Counts Log (cfu g−1 Dry Soil) | Mycorrhizal Colonization Levels (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 45 DAP | 45 DAP | 90 DAP | |||||||
| Total | P-Solubilizers | F | M | A | F | M | A | ||
| 2018 | Control | 8.036 f | 5.978 e | – | – | – | – | – | – |
| Bradyrhizobium sp. | 8.204b–d | 6.233 b c | – | – | – | – | – | – | |
| B. subtilis | 8.148d e | 6.207 c | – | – | – | – | – | – | |
| Mycorrhiza | 8.243 a–c | 6.276 a–c | 70.00 c | 32.17 c | 15.60 c | 83.33 c | 54.00 c | 37.00 b | |
| Mixture | 8.283 a b | 6.319 a b | 76.67 a | 37.83 a | 17.77 b | 90.00 a | 58.00 a | 39.60 a | |
| 2019 | Control | 8.110 e–f | 6.066 d | – | – | – | – | – | – |
| Bradyrhizobium sp. | 8.178 c–e | 6.246 b c | – | – | – | – | – | – | |
| B. subtilis | 8.297 a | 6.279 a–c | – | – | – | – | – | – | |
| Mycorrhiza | 8.303 a | 6.287 a–c | 70.00 c | 31.66 d | 16.00 c | 75.00 d | 52.00 d | 35.60 c | |
| Mixture | 8.306 a | 6.349 a | 75.00 b | 34.40 b | 18.66 a | 86.67 b | 56.60 b | 37.40 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sawah, A.M.; El-Keblawy, A.; Ali, D.F.I.; Ibrahim, H.M.; El-Sheikh, M.A.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 2021, 11, 194. https://doi.org/10.3390/agriculture11030194
El-Sawah AM, El-Keblawy A, Ali DFI, Ibrahim HM, El-Sheikh MA, Sharma A, Alhaj Hamoud Y, Shaghaleh H, Brestic M, Skalicky M, et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture. 2021; 11(3):194. https://doi.org/10.3390/agriculture11030194
Chicago/Turabian StyleEl-Sawah, Ahmed M., Ali El-Keblawy, Dina Fathi Ismail Ali, Heba M. Ibrahim, Mohamed A. El-Sheikh, Anket Sharma, Yousef Alhaj Hamoud, Hiba Shaghaleh, Marian Brestic, Milan Skalicky, and et al. 2021. "Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar" Agriculture 11, no. 3: 194. https://doi.org/10.3390/agriculture11030194










