Sheep Excrement Increases Mass of Greenhouse Gases Emissions from Soil Growing Two Forage Crop and Multi-Cutting Reduces Intensity
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sampling and Analysis
2.4. Statistical Analyses
3. Results
3.1. Biomass of Forage Crops and Soil Physicochemical Properties
3.1.1. Aboveground Biomass and Root Biomass of Forage Crops
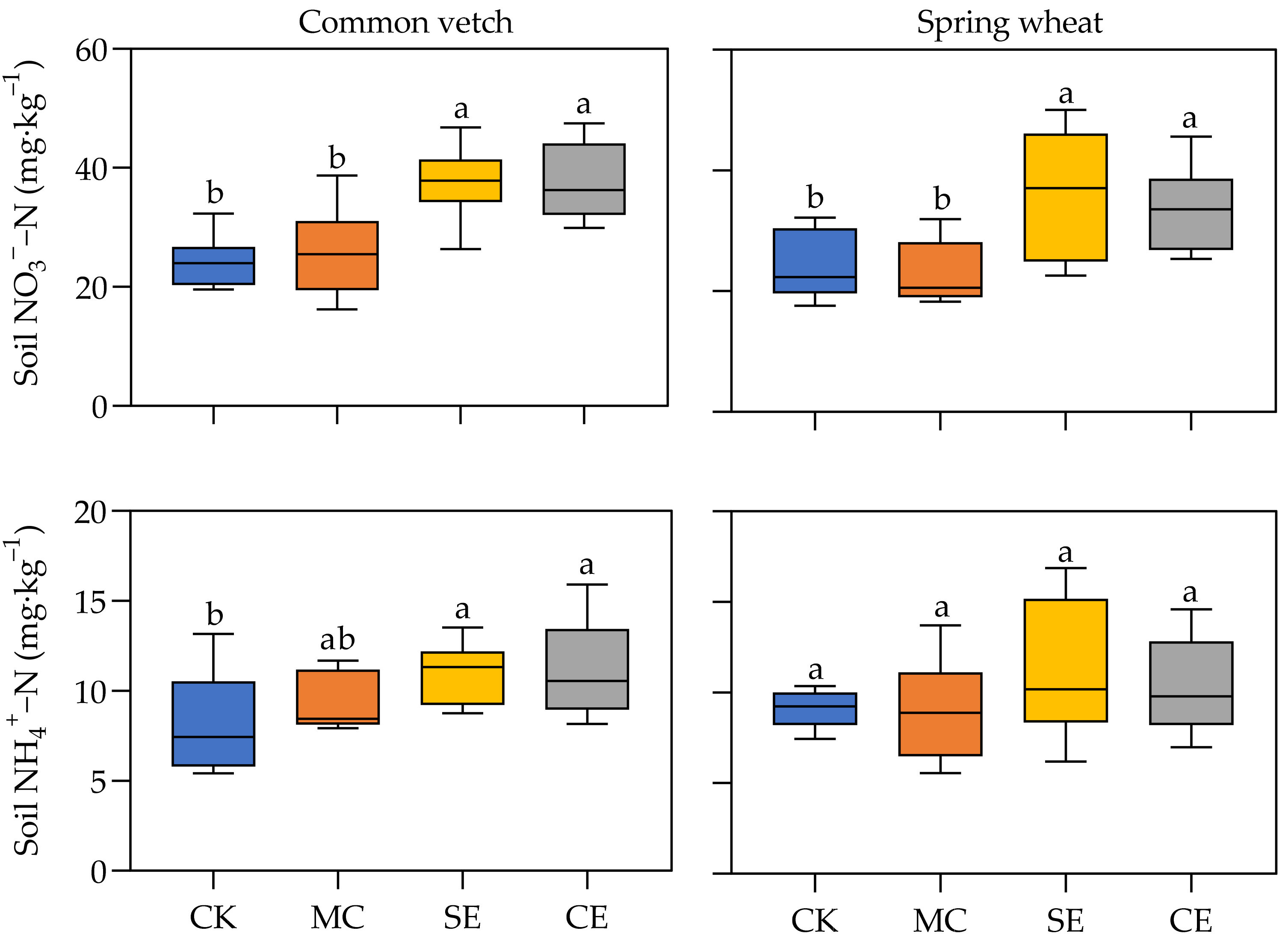
3.1.2. Soil Physicochemical Properties
3.2. Greenhouse Gas (GHG) Emissions
3.2.1. CO2 Emissions
CO2 Emission from Common Vetch
CO2 Emission from Spring Wheat
3.2.2. N2O Emissions
N2O Emission from Common Vetch
N2O Emission from Spring Wheat
3.2.3. CH4 Emissions
CH4 Emission from Common Vetch
CH4 Emission from Spring Wheat
3.3. Total Seasonal Global Warming Potential (GWP)
3.4. GHG Emission Intensity (GHGI)
3.5. Structural Equation Model (SEM)
4. Discussion
4.1. Effects of Multi-Cutting and Sheep Excrement on Forage Biomass and Dry Matter
4.2. Effects of Multi-Cutting and Sheep Excrement on Physicochemical Properties of Soil
4.3. Effects of Multi-Cutting and Sheep Excrement on GHG Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ning, J.; He, X.Z.; Hou, F.J. C3 and C4 Grass Species: Who Can Reduce Soil Nitrous Oxide Emissions in a Continental Arid Region? Atmosphere 2020, 11, 958. [Google Scholar] [CrossRef]
- Hansen, J.E.; Lacis, A.A. Sun and Dust Versus Greenhouse Gases—An Assessment of Their Relative Roles in Global Climate Change. Nature 1990, 346, 713–719. [Google Scholar] [CrossRef]
- Tan, L.S.; Ge, Z.M.; Zhou, X.H.; Li, S.H.; Li, X.Z.; Tang, J.W. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef]
- Peichl, M.; Leava, N.A.; Kiely, G. Above- and belowground ecosystem biomass, carbon and nitrogen allocation in recently afforested grassland and adjacent intensively managed grassland. Plant Soil 2012, 350, 281–296. [Google Scholar] [CrossRef]
- Cardoso, A.D.; Brito, L.D.; Janusckiewicz, E.R.; Morgado, E.D.; Barbero, R.P.; Koscheck, J.F.W.; Reis, R.A.; Ruggieri, A.C. Impact of Grazing Intensity and Seasons on Greenhouse Gas Emissions in Tropical Grassland. Ecosystems 2017, 20, 845–859. [Google Scholar] [CrossRef]
- Belanger, G.; Tremblay, G.F.; Papadopoulos, Y.A.; Duynisveld, J.; Lajeunesse, J.; Lafreniere, C.; Fillmore, S.A.E. Yield and nutritive value of binary legume-grass mixtures under grazing or frequent cutting. Can. J. Plant Sci. 2018, 98, 395–407. [Google Scholar] [CrossRef]
- Banik, B.K.; Durmic, Z.; Erskine, W.; Revell, C. Anti-methanogenic advantage of biserrula (Biserrula pelecinus) over subterranean clover (Trifolium subterraneum) from in vitro fermentation is maintained across growth stages and cutting treatments. Crop. Pasture Sci. 2019, 70, 263–272. [Google Scholar] [CrossRef]
- Jones, S.K.; Helfter, C.; Anderson, M.; Coyle, M.; Campbell, C.; Famulari, D.; Di Marco, C.; van Dijk, N.; Tang, Y.S.; Topp, C.F.E.; et al. The nitrogen, carbon and greenhouse gas budget of a grazed, cut and fertilised temperate grassland. Biogeosciences 2017, 14, 2069–2088. [Google Scholar] [CrossRef]
- Li, C.S.; Salas, W.; Zhang, R.H.; Krauter, C.; Rotz, A.; Mitloehner, F. Manure-DNDC: A biogeochemical process model for quantifying greenhouse gas and ammonia emissions from livestock manure systems. Nutr. Cycl. Agroecosyst. 2012, 93, 163–200. [Google Scholar] [CrossRef]
- Shi, H.Q.; Hou, L.Y.; Yang, L.Y.; Wu, D.X.; Zhang, L.H.; Li, L.H. Effects of grazing on CO2, CH4, and N2O fluxes in three temperate steppe ecosystems. Ecosphere 2017, 8, e01760. [Google Scholar] [CrossRef]
- Tang, S.M.; Wang, K.; Xiang, Y.Z.; Tian, D.S.; Wang, J.S.; Liu, Y.S.; Cao, B.; Guo, D.; Niu, S.L. Heavy grazing reduces grassland soil greenhouse gas fluxes: A global meta-analysis. Sci. Total Environ. 2019, 654, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.B.; Liebig, M.A.; Hanson, J.D. Soil carbon dioxide fluxes in northern semiarid grasslands. Soil Biol. Biochem. 2002, 34, 1235–1241. [Google Scholar] [CrossRef]
- Li, C.S. Modeling trace gas emissions from agricultural ecosystems. Nutr. Cycl. Agroecosyst. 2000, 58, 259–276. [Google Scholar] [CrossRef]
- Zhu, X.X.; Luo, C.Y.; Wang, S.P.; Zhang, Z.H.; Cui, S.J.; Bao, X.Y.; Jiang, L.L.; Li, Y.M.; Li, X.N.; Wang, Q.; et al. Effects of warming, grazing/cutting and nitrogen fertilization on greenhouse gas fluxes during growing seasons in an alpine meadow on the Tibetan Plateau. Agric. For. Meteorol. 2015, 214, 506–514. [Google Scholar] [CrossRef]
- van der Weerden, T.J.; Sherlock, R.R.; Williams, P.H.; Cameron, K.C. Nitrous oxide emissions and methane oxidation by soil following cultivation of two different leguminous pastures. Biol. Fert. Soils 1999, 30, 52–60. [Google Scholar] [CrossRef]
- Roman, M.; Roman, M.; Roman, K.K. Spatial differentiation of particulates emission resulting from agricultural production in Poland. Agric. Econ. Czech. 2019, 65, 375–384. [Google Scholar] [CrossRef]
- Roman, M.; Roman, K.K.; Roman, M. Methods of Estimating Particulates Emission in Agriculture Exemplified by Animal Husbandry. In Hradec Economic Days 2019 Part II; Jedlicka, P., Maresova, P., Soukal, I., Eds.; University of Hradec Králové: Hradec Králové, Czech Republic, 2019; Volume 9, pp. 260–268. [Google Scholar] [CrossRef]
- Wiesner, S.; Duff, A.J.; Desai, A.R.; Panke-Buisse, K. Increasing Dairy Sustainability with Integrated Crop-Livestock Farming. Sustainability 2020, 12, 765. [Google Scholar] [CrossRef]
- Donnelly, M.E.; Ominski, K.; McGeough, E.J.; Wittenberg, K.; Legesse, G. Effect of age at calving on greenhouse gas emissions from simulated beef farms grazing four stockpiled forage species in late fall/early winter. J. Anim. Sci. 2020, 98, 131–132. [Google Scholar] [CrossRef]
- Liu, J.S.; Chen, C.; Pan, Y.; Zhang, Y.; Gao, Y. The Intensity of Simulated Grazing Modifies Costs and Benefits of Physiological Integration in a Rhizomatous Clonal Plant. Int. J. Env. Res. Pub. Health 2020, 17, 2724. [Google Scholar] [CrossRef]
- Yuan, J.H.; Li, H.Y.; Yang, Y.F. The Compensatory Tillering in the Forage GrassHordeum brevisubulatumAfter Simulated Grazing of Different Severity. Front. Plant Sci. 2020, 11, 792. [Google Scholar] [CrossRef]
- Pan, Y.T.; Wang, Y.J.; Lou, S.N.; Wanapat, M.; Wang, Z.F.; Zhu, W.H.; Hou, F.J. Selenium supplementation improves nutrient intake and digestibility, and mitigates CH4 emissions from sheep grazed on the mixed pasture of alfalfa and tall fescue. J. Anim. Physiol. An. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Liu, N.; Kan, H.M.; Yang, G.W.; Zhang, Y.J. Changes in plant, soil, and microbes in a typical steppe from simulated grazing: Explaining potential change in soil C. Ecol. Monogr. 2015, 85, 269–286. [Google Scholar] [CrossRef]
- Mikola, J.; Setala, H.; Virkajarvi, P.; Saarijarvi, K.; Ilmarinen, K.; Voigt, W.; Vestberg, M. Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol. Monogr. 2009, 79, 221–244. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.Y.; Matthew, C.; Wood, B.; Hou, F.J. Key sources and seasonal dynamics of greenhouse gas fluxes from yak grazing systems on the Qinghai-Tibetan Plateau. Sci. Rep. 2017, 7, 40857. [Google Scholar] [CrossRef]
- Ginting, D.; Kessavalou, A.; Eghball, B.; Doran, J.W. Greenhouse gas emissions and soil indicators four years after manure and compost applications. J. Environ. Qual. 2003, 32, 23–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Y.J.; Wang, X.D.; Ding, W.X.; Tian, L.L.; Zhao, H.; Lu, X.Y. Potential short-term effects of yak and Tibetan sheep dung on greenhouse gas emissions in two alpine grassland soils under laboratory conditions. Biol. Fert. Soils. 2013, 49, 1215–1226. [Google Scholar] [CrossRef]
- Schonbach, P.; Wolf, B.; Dickhofer, U.; Wiesmeier, M.; Chen, W.W.; Wan, H.W.; Gierus, M.; Butterbach-Bahl, K.; Kogel-Knabner, I.; Susenbeth, A.; et al. Grazing effects on the greenhouse gas balance of a temperate steppe ecosystem. Nutr. Cycl. Agroecosyst. 2012, 93, 357–371. [Google Scholar] [CrossRef]
- Zhang, A.F.; Bian, R.J.; Pan, G.X.; Cui, L.Q.; Hussain, Q.; Li, L.Q.; Zheng, J.W.; Zheng, J.F.; Zhang, X.H.; Han, X.J.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Meuriot, F.; Avice, J.C.; Simon, J.C.; Laine, P.; Decau, M.L.; Ourry, A. Influence of initial organic N reserves and residual leaf area on growth, N uptake, N partitioning and N storage in Alfalfa (Medicago sativa) during post-cutting regrowth. Ann. Bot. 2004, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Orodho, A.B.; Trlica, M.J. Clipping and Long-Term Grazing Effects on Biomass and Carbohydrate Reserves of Indian Ricegrass. J. Range Manag. 1990, 43, 52–57. [Google Scholar] [CrossRef]
- Wherley, B.G.; Sinclair, T.R.; Dukes, M.D.; Schreffler, A.K. Nitrogen and Cutting Height Influence Root Development during Warm-Season Turfgrass Sod Establishment. Agron. J. 2011, 103, 1629–1634. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.A.; Percival, H.J.; Sparling, G.P. An approach for estimating when soils will reach maximum nitrogen storage. Soil Use Manag. 2004, 20, 281–286. [Google Scholar] [CrossRef]
- Frank, D.A.; Groffman, P.M.; Evans, R.D.; Tracy, B.F. Ungulate stimulation of nitrogen cycling and retention in Yellowstone Park grasslands. Oecologia 2000, 123, 116–121. [Google Scholar] [CrossRef]
- Aarons, S.R.; O’Connor, C.R.; Hosseini, H.M.; Gourley, C.J.P. Dung pads increase pasture production, soil nutrients and microbial biomass carbon in grazed dairy systems. Nutr. Cycl. Agroecosyst. 2009, 84, 81–92. [Google Scholar] [CrossRef]
- Cegarra, J.; Alburquerque, J.A.; Gonzalvez, J.; Tortosa, G.; Chaw, D. Effects of the forced ventilation on composting of a solid olive-mill by-product (“alperujo”) managed by mechanical turning. Waste Manag. 2006, 26, 1377–1383. [Google Scholar] [CrossRef]
- Gutierrez, O.; Oramas, A.; Cairo, J. A note on the chemical composition of faeces and urine of grazing cows. Cuban J. Agric. Sci. 1998, 32, 71–73. [Google Scholar]
- Ma, X.Z.; Wang, S.P.; Jiang, G.M.; Haneklaus, S.; Schnug, E.; Nyren, P. Short-term effect of targeted placements of sheep excrement on grassland in inner mongolia on soil and plant parameters. Commun. Soil Sci. Plan. 2007, 38, 1589–1604. [Google Scholar] [CrossRef]
- Lin, X.W.; Wang, S.P.; Ma, X.Z.; Xu, G.P.; Luo, C.Y.; Li, Y.N.; Jiang, G.M.; Xie, Z.B. Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods. Soil Biol. Biochem. 2009, 41, 718–725. [Google Scholar] [CrossRef]
- Taumer, J.; Kolb, S.; Boeddinghaus, R.S.; Wang, H.T.; Schoning, I.; Schrumpf, M.; Urich, T.; Marhan, S. Divergent drivers of the microbial methane sink in temperate forest and grassland soils. Glob. Chang. Biol. 2020, 27, 940. [Google Scholar] [CrossRef]
- Liebig, M.A.; Kronberg, S.L.; Gross, J.R. Effects of normal and altered cattle urine on short-term greenhouse gas flux from mixed-grass prairie in the Northern Great plains. Agric. Ecosyst. Environ. 2008, 125, 57–64. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, D.; Zhang, Y.J.; Chen, W.Q.; Wang, C.J.; Yang, X.M.; Luo, W. Dynamic changes of CH4 and CO2 emission from grazing sheep urine and dung patches in typical steppe. Atmos. Environ. 2013, 79, 576–581. [Google Scholar] [CrossRef]
- Petersen, S.O.; Sommer, S.G.; Aaes, O.; Soegaard, K. Ammonia losses from urine and dung of grazing cattle: Effect of N intake. Atmos. Environ. 1998, 32, 295–300. [Google Scholar] [CrossRef]
- Pan, J.; Qi, S.Y.; Sun, Y.F.; Jiang, Y.Y.; Zhao, N.; Huang, L.L.; Sun, Y.N. Nitrogen removal and nitrogen functional gene abundances in three subsurface wastewater infiltration systems under different modes of aeration and influent C/N ratios. Bioresour. Technol. 2017, 241, 1162–1167. [Google Scholar] [CrossRef]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Biol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Monaghan, R.M.; Barraclough, D. Nitrous-Oxide and Dinitrogen Emissions from Urine-Affected Soil under Controlled Conditions. Plant Soil 1993, 151, 127–138. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Webb, J.; Anthony, S.G.; Brown, L.; Lyons-Visser, H.; Ross, C.; Cottrill, B.; Johnson, P.; Scholefield, D. The impact of increasing the length of the cattle grazing season on emissions of ammonia and nitrous oxide and on nitrate leaching in England and Wales. Agric. Ecosyst. Environ. 2005, 105, 307–321. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]






| V | C | E | V × C | V × E | C × E | V × C × E | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| RB | 172.986 | <0.001 | 0.270 | 0.611 | 0.326 | 0.576 | 2.215 | 0.156 | 2.215 | 0.156 | 0.108 | 0.747 | 0.051 | 0.825 |
| SB | 37.989 | <0.001 | 118.373 | <0.001 | 3.208 | 0.092 | 0.567 | 0.462 | 0.567 | 0.462 | 0.057 | 0.814 | 0.272 | 0.609 |
| RB/SB | 135.819 | <0.001 | 73.642 | <0.001 | 2.567 | 0.129 | 10.694 | <0.01 | 10.694 | <0.01 | 1.064 | 0.318 | 0.000 | 0.983 |
| tDM | 3.447 | 0.082 | 121.917 | <0.001 | 3.923 | 0.065 | 0.075 | 0.788 | 0.075 | 0.788 | 0.116 | 0.738 | 0.360 | 0.557 |
| SM | 0.176 | 0.680 | 1.038 | 0.323 | 1.189 | 0.292 | 0.211 | 0.652 | 0.211 | 0.652 | 0.011 | 0.918 | 0.055 | 0.818 |
| ST | 2.260 | 0.152 | 0.202 | 0.659 | 3.711 | 0.072 | 0.230 | 0.638 | 0.230 | 0.638 | 0.647 | 0.433 | 0.123 | 0.730 |
| NO3−-N | 1.300 | 0.271 | 0.085 | 0.775 | 38.503 | <0.001 | 0.705 | 0.413 | 0.705 | 0.413 | 0.102 | 0.754 | 0.006 | 0.942 |
| NH4+-N | 0.027 | 0.871 | 0.010 | 0.921 | 12.102 | <0.01 | 0.959 | 0.342 | 0.959 | 0.342 | 0.435 | 0.519 | 0.034 | 0.855 |
| CO2 | 5.098 | <0.05 | 0.020 | 0.889 | 33.622 | <0.001 | 0.138 | 0.715 | 13.276 | <0.01 | 0.014 | 0.909 | 0.003 | 0.959 |
| N2O | 19.282 | <0.001 | 0.005 | 0.947 | 1406.045 | <0.001 | 1.618 | 0.222 | 6.851 | <0.05 | 1.306 | 0.270 | 0.005 | 0.946 |
| CH4 | 17.342 | <0.001 | 0.009 | 0.927 | 588.222 | <0.001 | 0.054 | 0.819 | 1.558 | 0.230 | 0.132 | 0.721 | 0.399 | 0.536 |
| GWPt | 11.348 | <0.01 | 0.000 | 0.989 | 80.768 | <0.001 | 0.064 | 0.804 | 9.375 | <0.01 | 0.072 | 0.792 | 0.054 | 0.819 |
| GHGI | 3.626 | 0.075 | 1.152 | 0.299 | 43.585 | <0.001 | 0.291 | 0.597 | 7.535 | <0.05 | 37.820 | <0.001 | 0.314 | 0.583 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Shi, L.; Lou, S.; Ning, J.; Guo, Y.; Jia, Q.; Hou, F. Sheep Excrement Increases Mass of Greenhouse Gases Emissions from Soil Growing Two Forage Crop and Multi-Cutting Reduces Intensity. Agriculture 2021, 11, 238. https://doi.org/10.3390/agriculture11030238
Zhao X, Shi L, Lou S, Ning J, Guo Y, Jia Q, Hou F. Sheep Excrement Increases Mass of Greenhouse Gases Emissions from Soil Growing Two Forage Crop and Multi-Cutting Reduces Intensity. Agriculture. 2021; 11(3):238. https://doi.org/10.3390/agriculture11030238
Chicago/Turabian StyleZhao, Xinzhou, Lina Shi, Shanning Lou, Jiao Ning, Yarong Guo, Qianmin Jia, and Fujiang Hou. 2021. "Sheep Excrement Increases Mass of Greenhouse Gases Emissions from Soil Growing Two Forage Crop and Multi-Cutting Reduces Intensity" Agriculture 11, no. 3: 238. https://doi.org/10.3390/agriculture11030238
APA StyleZhao, X., Shi, L., Lou, S., Ning, J., Guo, Y., Jia, Q., & Hou, F. (2021). Sheep Excrement Increases Mass of Greenhouse Gases Emissions from Soil Growing Two Forage Crop and Multi-Cutting Reduces Intensity. Agriculture, 11(3), 238. https://doi.org/10.3390/agriculture11030238






