Prediction of Reproductive Success in Multiparous First Service Dairy Cows by Parameters from In-Line Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Animals
2.2. Measurements
2.3. Data Analysis and Statistics
3. Results
3.1. Relation of Cows’ Pregnancy Success with Automatic Milking System (AMS) Parameters
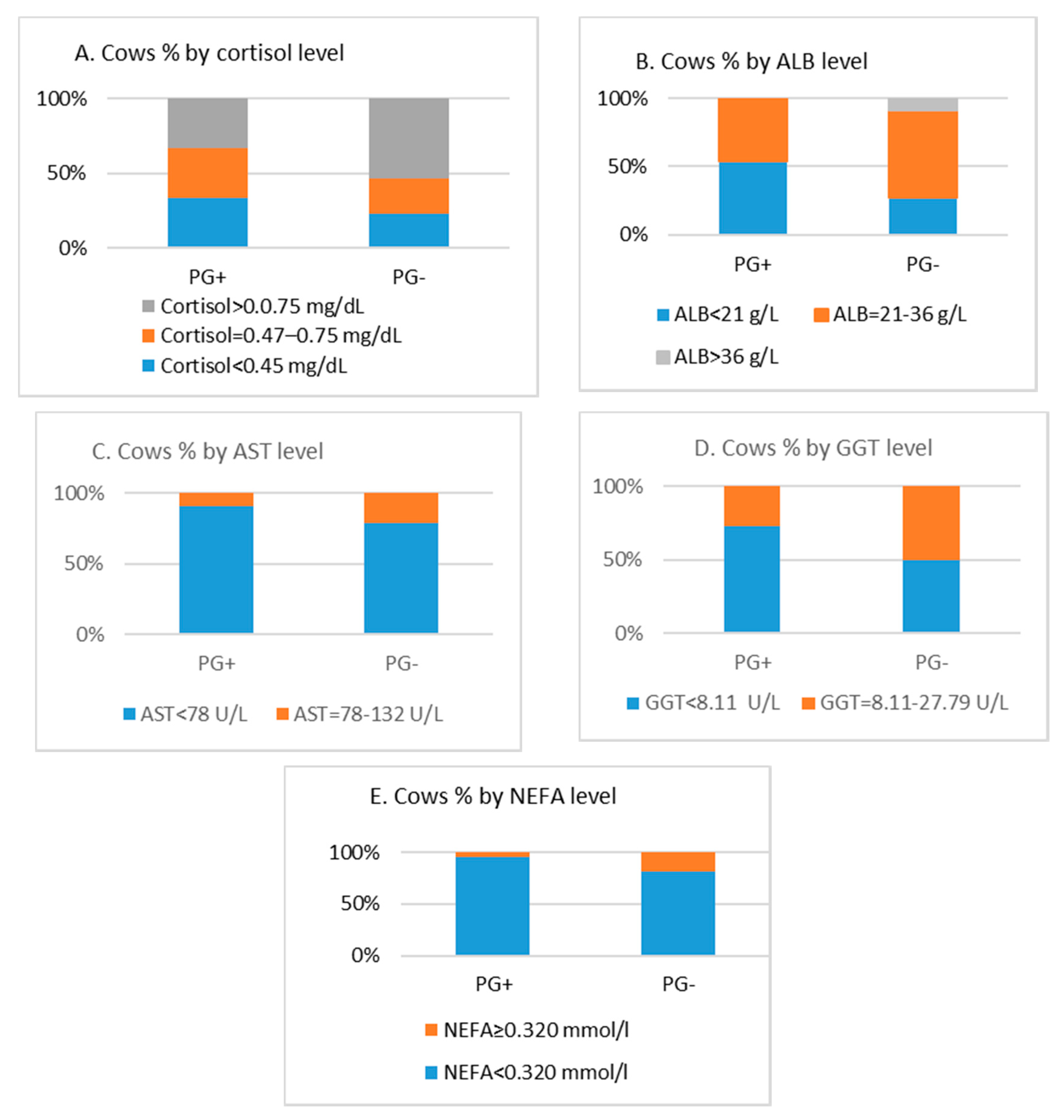
3.2. Relation Between Cows’ Pregnancy Success and Biochemical Parameters in Serum
3.3. The Relationship of Cow Pregnancy with Milk and Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Animal Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Barkema, H.W.; Von Keyserlingk, M.A.; Kastelic, J.P.; Lam, T.J.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited review: Changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Maeda, T. Inline progesterone monitoring in the dairy industry. Trends Biotechnol. 2017, 35, 579–582. [Google Scholar] [CrossRef]
- Larson, S.F.; Butler, W.R.; Currie, W.B. Reduced fertility associated with low progesterone postbreeding and increased milk urea nitrogen in lactating cows. J. Dairy Sci. 1997, 80, 1288–1295. [Google Scholar] [CrossRef]
- Blom, J.Y.; Christensen, J.M.; Ridder, C. Real-time analyses of BHB in milk can monitor ketosis and its impact on reproduction in dairy cows. In Precision Livestock Farming Applications: Making Sense of Sensors to Support Farm Management; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 2788–2796. [Google Scholar]
- Nyman, S.; Johansson, K.; de Koning, D.J.; Berry, D.P.; Veerkamp, R.F.; Wall, E.; Berglund, B. Genetic analysis of atypical progesterone profiles in Holstein-Friesian cows from experimental research herds. J. Dairy Sci. 2014, 97, 7230–7239. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Chastant-Maillard, S. Methods and on-farm devices to predict calving time in cattle. Vet. J. 2015, 205, 349–356. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Televičius, M.; Malašauskienė, D. Changes in the real-time registration of milk β-hydroxybutyrate according to stage and lactation number, milk yield, and status of reproduction in dairy cows. Pol. J. Vet. Sci. 2018, 21, 763–768. [Google Scholar]
- Antanaitis, R.; Malašauskienė, D.; Televičius, M.; Juozaitienė, V.; Rutkauskas, A.; Palubinskas, G. Inline changes in lactate dehydrogenase, milk concentration according to the stage and number of lactation periods, including the status of reproduction and milk yield in dairy cows. Pol. J. Vet. Sci. 2020, 23, 153–156. [Google Scholar]
- Jackson, P.G.G.; Cockcroft, P.D. Clinical Examination of Farm Animals; Copyright© Blackwell Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Stojević, Z.; Piršljin, J.; Milinković-Tur, S.; Zdelar-Tuk, M.; Ljubić, B.B. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Veterinarski Arhiv 2005, 75, 67–73. [Google Scholar]
- Petrera, F.; Napolitano, F.; Dal Prà, A.; Abeni, F. Plasma parameters related to energy and lipid metabolism in periparturient M odenese and Italian Friesian cows. J. Animal Physiol. Animal Nutr. 2015, 99, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Hillerton, J.E.; Walton, A.W. Identification of subclinical mastitis with a hand-held electrical conductivity meter. Vet. Record 1991, 128, 513–515. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef]
- Rutherford, A.J.; Oikonomou, G.; Smith, R.F. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. J. Dairy Sci. 2016, 99, 4808–4815. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 3595–3601. [Google Scholar] [CrossRef]
- Wathes, D.C.; Fenwick, M.; Cheng, Z.; Bourne, N.; Lewellyn, S.; Morris, D.G.; Fitzpatrick, R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology 2007, 68, S232–S241. [Google Scholar] [CrossRef] [PubMed]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.C.; Sloth, K.N.; Løvendahl, P. Electrical conductivity of milk: Ability to predict mastitis status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef]
- Televičius, M.; Juozaitiene, V.; Malašauskienė, D.; Antanaitis, R.; Rutkauskas, A.; Urbutis, M.; Baumgartner, W. Inline Milk Lactose Concentration as Biomarker of the Health Status and Reproductive Success in Dairy Cows. Agriculture 2021, 11, 38. [Google Scholar] [CrossRef]
- Costa, A.; Egger-Danner, C.; Mészáros, G.; Fuerst, C.; Penasa, M.; Sölkner, J.; Fuerst-Waltl, B. Genetic associations of lactose and its ratios to other milk solids with health traits in Austrian Fleckvieh cows. J. Dairy Sci. 2019, 102, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Cincović, M.R.; Nemec, M.; Belić, B.; Djoković, R.; Klinkon, M.; Starič, J. Beta-hydroxybutyrate in milk as screening test for subclinical ketosis in dairy cows. Pol. J. Vet. Sci. 2017, 20, 507–512. [Google Scholar] [CrossRef]
- Bastin, C.; Abdelsayed, M.; Gengler, N.; Bradley, A.J. Invited review: Opportunities for genetic improvement of metabolic diseases. J. Dairy Sci. 2016, 99, 6855–6873. [Google Scholar]
- Tatone, E.H.; Duffield, T.F.; LeBlanc, S.J.; DeVries, T.J.; Gordon, J.L. Investigating the within-herd prevalence and risk factors for ketosis in dairy cattle in Ontario as diagnosed by the test-day concentration of β-hydroxybutyrate in milk. J. Dairy Sci. 2017, 100, 1308–1318. [Google Scholar] [CrossRef]
- Yang, D.A.; Johnson, W.O.; Müller, K.R.; Gates, M.C.; Laven, R.A. Estimating the herd and cow level prevalence of bovine digital dermatitis on New Zealand dairy farms: A Bayesian superpopulation approach. Prev. Vet. Med. 2019, 165, 76–84. [Google Scholar] [CrossRef]
- Fleck, A. Clinical and nutritional aspects of changes in acutephase proteins during inflammation. Proc. Nutr. Soc. 1989, 48, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi-Fani, M.; Omidi, A.; Mirzaei, A.; Nazifi, S.; Nowroozi, K.H. A field study on glucose, non-esterified fatty acids, betahydroxybutyrate and thyroid hormones in dairy cows during the breeding period in Fars province, Iran. Iran J. Vet. Res. 2018, 1, 55. [Google Scholar]
- Farid, A.S.; Honkawa, K.; Fath, E.M.; Nonaka, N.; Horii, Y. Serum paraoxonase-1 as biomarker for improved diagnosis of fatty liver in dairy cows. BMC Vet. Res. 2013, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Lu, Y.; Nafikov, R.; Sonon, R.N.; Young, J.W.; Beitz, D.C. Acute phase response indicates inflammatory conditions may play a role in the pathogenesis of fatty liver in dairy cows. J. Dairy Sci. 2002, 85, 189. [Google Scholar]
- Mordak, R.; Kupczyński, R.; Kuczaj, M.; Niżański, W. Analysis of correlations between selected blood markers of liver function and milk composition in cows during late lactation period. Ann. Anim. Sci. 2020, 20, 871–886. [Google Scholar] [CrossRef]
- Burnett, T.A.; Madureira, A.M.; Silper, B.F.; Tahmasbi, A.; Nadalin, A.; Veira, D.M.; Cerri, R.L. Relationship of concentrations of cortisol in hair with health, biomarkers in blood, and reproductive status in dairy cows. J. Dairy Sci. 2015, 98, 4414–4426. [Google Scholar] [CrossRef]
- Walker, S.L.; Smith, R.F.; Jones, D.N.; Routly, J.E.; Dobson, H. Chronic stress, hormone profiles and estrus intensity in dairy cattle. Horm. Behav. 2008, 53, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare 1; University of California: Davis, CA, USA, 2000; p. 21. [Google Scholar]
- Khatun, M.; Bruckmaier, R.M.; Thomson, P.C.; House, J.; García, S.C. Suitability of somatic cell count, electrical conductivity, and lactate dehydrogenase activity in foremilk before versus after alveolar milk ejection for mastitis detection. J. Dairy Sci. 2019, 102, 9200–9212. [Google Scholar] [CrossRef] [PubMed]
- Mollenhorst, H.; Rijkaart, L.J.; Hogeveen, H. Mastitis alert preferences of farmers milking with automatic milking systems. J. Dairy Sci. 2012, 95, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Chapinal, N.; LeBlanc, S.J.; Kelton, D.F.; Dubuc, J.; Duffield, T.F. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J. Dairy Sci. 2012, 95, 3057–3063. [Google Scholar] [CrossRef] [PubMed]

| Measured Parameters | Source |
|---|---|
| Milk yield (MY) | Herd Navigator |
| Electric milk conductivity (EC) front right (FR), front left (FL), back left (BL), back right (BR) | Herd Navigator |
| Lactate dehydrogenase (LDH) | Herd Navigator |
| β-hydroxybutyric acid (BHB) | Herd Navigator |
| Albumin (ALB) | Hitachi 705 analyzer |
| Aspartate aminotransferase (AST) | Hitachi 705 analyzer |
| Gamma glutamyltransferase (GGT) | Hitachi 705 analyzer |
| Cortisol | Analyzer AIA-360 |
| Non-esterified fatty acids (NEFA) | Rx Daytona, Randox Laboratories |
| Parameter | PG− (n = 48) | PG+ (n = 44) | p (Between Days) | p (Between Groups | ||
|---|---|---|---|---|---|---|
| M | SEM | M | SEM | |||
| MY (kg/d) | 44.56 | 2.252 | 43.99 | 2.203 | 0.009 | 0.231 |
| FL (mS/cm) | 4.41 | 0.06 | 4.17 | 0.048 | 0.423 | 0.002 |
| FR (mS/cm) | 4.45 | 0.05 | 4.17 | 0.043 | 0.192 | <0.001 |
| BL (mS/cm) | 4.37 | 0.043 | 4.15 | 0.05 | 0.521 | 0.002 |
| BR (mS/cm) | 4.42 | 0.047 | 4.14 | 0.044 | 0.458 | <0.001 |
| LDH (µmol/min) | 29.23 | 3.288 | 22.81 | 2.310 | 0.560 | 0.366 |
| BHB (mmol/L) | 0.06 | 0.001 | 0.05 | 0.001 | 0.078 | 0.003 |
| Parameter | PG− | PG+ | p (Between Days) | p (Between Groups) | ||
|---|---|---|---|---|---|---|
| M | SEM | M | SEM | |||
| Cortisol (mmol/L) | 1.37 | 0.358 | 0.83 | 0.119 | 0.612 | 0.227 |
| ALB (g/L) | 20.85 | 1.257 | 24.85 | 1.046 | 0.201 | 0.017 |
| AST (IU/L) | 49.51 | 2.666 | 57.37 | 2.755 | 0.543 | 0.048 |
| GGT (IU/L) | 10.46 | 1.555 | 6.50 | 0.519 | 0.487 | 0.017 |
| NEFA (mmol/L) | 0.16 | 0.008 | 0.14 | 0.006 | 0.199 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Zamokas, G.; Baumgartner, W. Prediction of Reproductive Success in Multiparous First Service Dairy Cows by Parameters from In-Line Sensors. Agriculture 2021, 11, 334. https://doi.org/10.3390/agriculture11040334
Antanaitis R, Juozaitienė V, Malašauskienė D, Televičius M, Urbutis M, Zamokas G, Baumgartner W. Prediction of Reproductive Success in Multiparous First Service Dairy Cows by Parameters from In-Line Sensors. Agriculture. 2021; 11(4):334. https://doi.org/10.3390/agriculture11040334
Chicago/Turabian StyleAntanaitis, Ramūnas, Vida Juozaitienė, Dovilė Malašauskienė, Mindaugas Televičius, Mingaudas Urbutis, Gintaras Zamokas, and Walter Baumgartner. 2021. "Prediction of Reproductive Success in Multiparous First Service Dairy Cows by Parameters from In-Line Sensors" Agriculture 11, no. 4: 334. https://doi.org/10.3390/agriculture11040334
APA StyleAntanaitis, R., Juozaitienė, V., Malašauskienė, D., Televičius, M., Urbutis, M., Zamokas, G., & Baumgartner, W. (2021). Prediction of Reproductive Success in Multiparous First Service Dairy Cows by Parameters from In-Line Sensors. Agriculture, 11(4), 334. https://doi.org/10.3390/agriculture11040334







