Chemical Removal of Cu and Zn from Swine Feces before Soil Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Swine Feces
2.2. Removal of Cu and Zn
2.2.1. Selection of a Chemical Reagent
2.2.2. Impact of Solid-to-Liquid Ratios, Stirring Time, and Reagent Concentrations
2.2.3. Addition of the Ultrasound Process
2.3. Analyses of Cu and Zn
2.4. Analysis of Total Nitrogen
3. Results and Discussion
3.1. Selection of Method
3.2. Optimal Solid to-Liquid Ratio
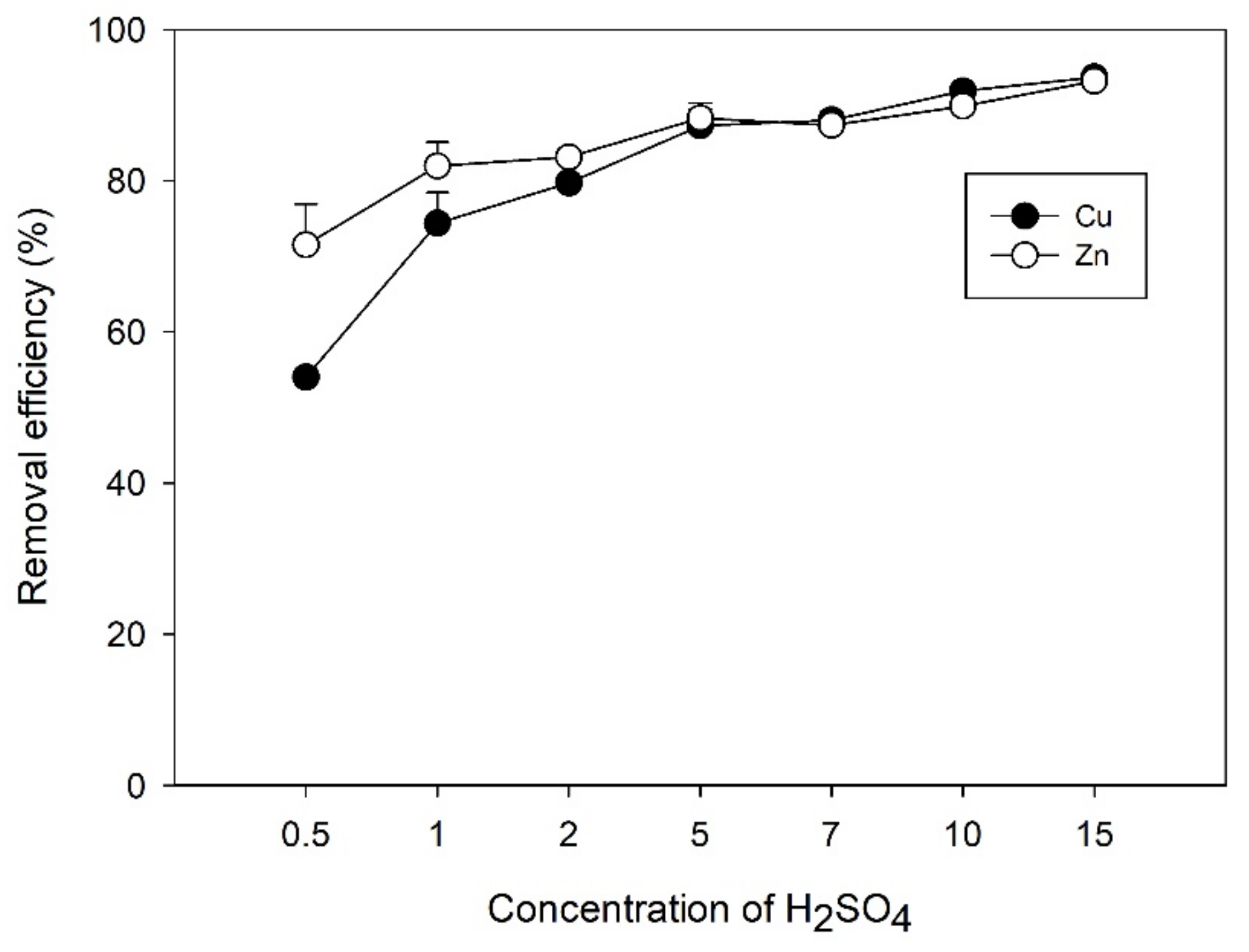
3.3. Effect of H2SO4 Concentrations
3.4. Effect of Reaction Time
3.5. Effect of the Addition of the Ultrasound Process
3.6. Effect of H2SO4 on TN Concentrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Cu | Zn | |||||
| Conc. | SD | Conc. | SD | |||
| Before treatment | 198 | 7.38 | 474 | 15.8 | ||
| After treatment | Reagents | HNO3 | 38.6 | 3.91 | 77.7 | 10.2 |
| H2SO4 | 17.0 | 0.48 | 46.9 | 4.93 | ||
| Citric acid | 172 | 11.5 | 167 | 6.91 | ||
| Oxalic acid | 97.1 | 5.96 | 206 | 16.6 | ||
| H2SO4 concentration (%) | 0.5 | 90.9 | 1.90 | 135 | 25.7 | |
| 1 | 50.7 | 8.07 | 85.6 | 15.0 | ||
| 2 | 40.1 | 1.02 | 80.0 | 3.03 | ||
| 5 | 25.1 | 4.35 | 55.7 | 9.46 | ||
| 7 | 23.7 | 0.36 | 59.9 | 8.22 | ||
| 10 | 16.0 | 1.56 | 48.0 | 2.44 | ||
| 15 | 12.5 | 1.63 | 32.3 | 0.57 | ||
| solid-to-liquid ratio | 1:10 | 58.5 | 1.76 | 131 | 2.94 | |
| 1:25 | 41.2 | 0.31 | 71.4 | 4.83 | ||
| 1:37.5 | 25.2 | 0.00 | 50.5 | 0.00 | ||
| 1:50 | 16.0 | 1.56 | 48.0 | 2.44 | ||
| 1:100 | 28.6 | 0.00 | 26.3 | 0.00 | ||
| 1:200 | 26.6 | 2.21 | 26.4 | 0.53 | ||
| Reaction time (h) | 1 | 55.4 | 6.13 | 50.0 | 1.85 | |
| 2 | 24.6 | 1.93 | 57.6 | 4.49 | ||
| 3.5 | 25.4 | 2.67 | 48.6 | 5.40 | ||
| 5 | 16.0 | 1.56 | 48.0 | 2.44 | ||
| 8 | 12.3 | 2.59 | 44.1 | 3.87 | ||
| Ultrasound | 1 | 62.7 | 2.00 | 79.6 | 6.62 | |
| 1% H2SO4 | 2 | 47.9 | 1.15 | 78.9 | 9.16 | |
| Reaction time (h) | 4 | 59.8 | 0.23 | 98.6 | 20.6 | |
| Ultrasound | 1 | 28.5 | 5.73 | 38.0 | 1.00 | |
| 10% H2SO4 | 2 | 13.6 | 0.57 | 40.6 | 0.75 | |
| Reaction time (h) | 4 | 22.2 | 2.68 | 38.1 | 0.64 | |
References
- Bao, Q.; Lin, Q.; Tian, G.; Wang, G.; Yu, J.; Peng, G. Copper distribution in water-dispersible colloids of swine manure and its transport through quartz sand. J. Hazard. Mater. 2011, 186, 1660–1666. [Google Scholar] [CrossRef]
- Gao, F.-Z.; He, L.-Y.; He, L.-X.; Zou, H.-Y.; Zhang, M.; Wu, D.-L.; Liu, Y.-S.; Shi, Y.-J.; Bai, H.; Ying, G.-G. Untreated swine wastes changed antibiotic resistance and microbial community in the soils and impacted abundances of antibiotic resistance genes in the vegetables. Sci. Total Environ. 2020, 741, 140482. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.H.; Turner, C.H. Manure Management: Treatment Strategies for Sustainable Agriculture, 2nd ed.; Silsoe Research Institute: Wrest Park, Silsoe, UK, 2003. [Google Scholar]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.D. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J. Anim. Feed Sci. 1998, 7 (Suppl. S1), 135–142. [Google Scholar] [CrossRef] [Green Version]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef] [Green Version]
- Svane, S.; Karring, H. A comparison of the transition metal concentrations in the faeces, urine, and manure slurry from different livestock animals related to environmentally relevant microbial processes. Cogent Chem. 2019, 5, 1644702. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Fanning, S.; Wang, L.; Li, M.; Maheshwari, N.; Sun, J.; Li, F. Effects of metal and metalloid pollutants on the microbiota composition of feces obtained from twelve commercial pig farms across China. Sci. Total Environ. 2019, 647, 577–586. [Google Scholar] [CrossRef]
- Ding, F.; He, Z.; Liu, S.; Zhang, S.; Zhao, F.; Li, Q.; Stoffella, P.J. Heavy metals in composts of China: Historical changes, regional variation, and potential impact on soil quality. Environ. Sci. Pollut. Res. 2017, 24, 3194–3209. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, J.; Wang, X.; Song, W.; Zhang, K.; Sun, W.; Zhang, X.; Zhang, Y.; Li, H. Effects of Copper Addition on Copper Resistance, Antibiotic Resistance Genes, and intl1 during Swine Manure Composting. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Wu, F.; Ju, T.; Gong, Y.; Zhang, Y.; Wu, X.; Hou, H.; Zhao, L.; Shi, H. Mass balance-based inventory of heavy metals inputs to and outputs from agricultural soils in Zhejiang Province, China. Sci. Total Environ. 2019, 649, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Jakubus, M.; Dach, J.; Starmans, D. Biovailability of copper and zinc in pig and cattle slurries. Fresen. Environ. Bull. 2013, 22, 995–1002. [Google Scholar]
- Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Rufete, B.; Paredes, C. Characterisation of the organic matter pool in manures. Bioresour. Technol. 2005, 96, 153–158. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, B.; Ziadi, N.; Chantigny, M.H.; Bélanger, G.; Massé, D.I. Biosolids from Treated Swine Manure and Papermill Residues Affect Corn Fertilizer Value. Agron. J. 2012, 104, 483–492. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Cotta, M.A. Examination of the Aerobic Microflora of Swine Feces and Stored Swine Manure. J. Environ. Qual. 2016, 45, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, X.; He, C.; Chen, C.-L.; Bai, J.; Ren, N.; Wang, J.-Y. Transformation of dissolved organic matters in swine, cow and chicken manures during composting. Bioresour. Technol. 2014, 168, 222–228. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Li, X.; Ren, N. Spatial nitrifications of microbial processes during composting of swine, cow and chicken manure. Sci. Rep. 2015, 5, 14932. [Google Scholar] [CrossRef] [PubMed]
- Ziemer, C.J.; Bonner, J.M.; Cole, D.; Vinjé, J.; Constantini, V.; Goyal, S.; Gramer, M.; Mackie, R.; Meng, X.J.; Myers, G.; et al. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application1. J. Anim. Sci. 2010, 88 (Suppl. S13), E84–E94. [Google Scholar] [CrossRef] [Green Version]
- Moura, A.C.D.; Sampaio, S.C.; Remor, M.B.; Silva, A.P.D.; Pereira, P.A.M. Long-term effects of swine wastewater and mineral fertilizer association on soil microbiota. Eng. Agríc. 2016, 36, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Sui, Q.; Zhang, J.; Chen, M.; Wang, R.; Wang, Y.; Wei, Y. Fate of microbial pollutants and evolution of antibiotic resistance in three types of soil amended with swine slurry. Environ. Pollut. 2019, 245, 353–362. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Gonzatto, R.; Aita, C.; Lupatini, M.; Jacques, R.J.S.; Kuramae, E.E.; Antoniolli, A.I.; Roesch, L.F.W. Temporal variability of soil microbial communities after application of dicyandiamide-treated swine slurry and mineral fertilizers. Soil Biol. Biochem. 2016, 97, 71–82. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Zheng, L.; Gong, H.; Dai, L.; Dai, X. An overview of removing heavy metals from sewage sludge: Achievements and perspectives. Environ. Pollut. 2020, 266, 115375. [Google Scholar] [CrossRef]
- Ma, D.; Su, M.; Qian, J.; Wang, Q.; Meng, F.; Ge, X.; Ye, Y.; Song, C. Heavy metal removal from sewage sludge under citric acid and electroosmotic leaching processes. Sep. Purif. Technol. 2020, 242, 116822. [Google Scholar] [CrossRef]
- Shim, M.J.; Jung, T.Y.; Yoon, D.H.; Yang, Y.M.; Rumky, J.; Yoon, Y.Y. HNO2 treatment of sludge: An alternative way of sludge usage as fertilizer. J. Environ. Manag. 2020, 258, 110016. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Dimon, B.; Mama, D.; Yu, C.-P. Heavy metal removal from sludge with organic chelators: Comparative study of N, N-bis(carboxymethyl) glutamic acid and citric acid. J. Environ. Manag. 2016, 166, 341–347. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, G.; Zhang, P.; Zhang, C.; Ren, M.; Zhang, J.; Chen, M. Feasibility of bioleaching combined with Fenton-like reaction to remove heavy metals from sewage sludge. Bioresour. Technol. 2013, 142, 530–534. [Google Scholar] [CrossRef]
- Veeken, A.H.M.; Hamelers, H.V.M. Removal of heavy metals from sewage sludge by extraction with organic acids. Water Sci. Technol. 1999, 40, 129–136. [Google Scholar] [CrossRef]
- Jenkins, R.L.; Scheybeler, B.J. Metals removal and recovery from municipal sludge. J. Water Pollut. Control Fed. 1981, 5, 25–31. [Google Scholar]
- Stylianou, M.A.; Kollia, D.; Haralambous, K.-J.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Effect of acid treatment on the removal of heavy metals from sewage sludge. Desalination 2007, 215, 73–81. [Google Scholar] [CrossRef]
- Gaber, S.E.; Rizk, M.S.; Yehia, M.M. Extraction of certain heavy metals from sewage sludge using different types of acids. Biokemistri 2011, 23, 41–48. [Google Scholar]
- Marchioretto, M.M.; Bruning, H.; Loan, N.T.P.; Rulkens, W.H. Heavy metals extraction from anaerobically digested sludge. Water Sci. Technol. 2002, 46, 1–8. [Google Scholar] [CrossRef]
- Veeken, A. Remediation technologies for conversion of heavy metal polluted organic wastes into compost. In Resource Recovery and Reuse in Organic Solid Waste Management; Lens, P., Hamelers, B., Hoitink, H., Bidlingmaier, W., Eds.; IWA Publishing: London, UK, 2004; pp. 503–521. [Google Scholar]
- Wu, C.-H.; Kuo, C.-Y.; Lo, S.-L. Removal of Metals from Industrial Sludge by Extraction with Different Acids. J. Environ. Sci. Health Part A 2004, 39, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M.; Pode, R.; Manea, F. Comparative heavy metal chemical extraction from anaerobically digested biosolids. Hydrometallurgy 2011, 108, 115–121. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Liu, T.; Xin, X.; Hu, H. Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 2017, 189, 599–608. [Google Scholar] [CrossRef]
- Kazi, F.K.M.; Cooper, P.A. Rapid-extraction oxidation process to recover and reuse copper chromium and arsenic from industrial wood preservative sludge. Waste Manag. 2002, 22, 293–301. [Google Scholar] [CrossRef]
- Mingot, J.I.; Obrador, A.; Alvarez, J.M.; Rico, M.I. Acid Extraction and Sequential Fractionation of Heavy Metals in Water Treatment Sludges. Environ. Technol. 1995, 16, 869–876. [Google Scholar] [CrossRef]
- Naoum, C.; Fatta, D.; Haralambous, K.J.; Loizidou, M. Removal of heavy metals from sewage sludge by acid treatment. J. Environ. Sci. Health Part A 2001, 36, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Soares, D.; Paiva, A.P.; Labrincha, J.A.; Castro, F. Leaching behaviour of a galvanic sludge in sulphuric acid and ammoniacal media. J. Hazard. Mater. 2005, 121, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, S.; Tomida, T. Principle and Process of Heavy Metal Removal from Sewage Sludge. Environ. Sci. Technol. 2000, 34, 1572–1575. [Google Scholar] [CrossRef]
- Deng, J.; Feng, X.; Qiu, X. Extraction of heavy metal from sewage sludge using ultrasound-assisted nitric acid. Chem. Eng. J. 2009, 152, 177–182. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Waste Catalyst Utilization: Extraction of Valuable Metals from Spent Hydroprocessing Catalysts by Ultrasonic-Assisted Leaching with Acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Petrier, C.; Jiang, Y.; Lamy, M.-F. Ultrasound and Environment: Sonochemical Destruction of Chloroaromatic Derivatives. Environ. Sci. Technol. 1998, 32, 1316–1318. [Google Scholar] [CrossRef]
- Chu, C.P.; Chang, B.-V.; Liao, G.S.; Jean, D.S.; Lee, D.J. Observations on changes in ultrasonically treated waste-activated sludge. Water Res. 2001, 35, 1038–1046. [Google Scholar] [CrossRef]
- Feng, X.; Lei, H.; Deng, J.; Yu, Q.; Li, H. Physical and chemical characteristics of waste activated sludge treated ultrasonically. Chem. Eng. Process. Process Intensif. 2009, 48, 187–194. [Google Scholar] [CrossRef]
- Wang, F.; Lu, S.; Ji, M. Components of released liquid from ultrasonic waste activated sludge disintegration. Ultrason. Sonochem. 2006, 13, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Song, W.; Li, J.; Zhang, X. Bioleaching of heavy metals from wastewater sludge with the aim of land application. Chemosphere 2020, 249, 126134. [Google Scholar] [CrossRef]



| Cu | Zn | |
|---|---|---|
| Recovery (%) (n = 2) | 106 | 112 |
| SD | 5.06 | 8.03 |
| Method detection limit (mg/kg dry solids) | 0.027 | 0.036 |
| Reagent | Cu | Zn | ||
|---|---|---|---|---|
| RE (%) | SD | RE (%) | SD | |
| HNO3 | 80.5 | 1.98 | 83.6 | 2.15 |
| H2SO4 | 91.4 | 0.24 | 90.1 | 1.04 |
| Citric acid | 12.9 | 5.82 | 64.9 | 1.46 |
| Oxalic acid | 50.9 | 3.01 | 56.6 | 3.49 |
| Metal Removal Efficiency (%) | |||||
|---|---|---|---|---|---|
| Concentration | Solid to Liquid Ratio | Reaction Time | Cu | Zn | Referecnce |
| 10% | 1:5 | 30 min | 54 | 70 | Stylianou et al., 2007 |
| 20% | 1:5 | 60 min | 86 | 72 | Stylianou et al., 2007 |
| 100 g/L | 1:5 | 25 h | 88.6 | 99.2 | Silva et al., 2002 |
| 0.5 M | 1 h | 20 | 78 | Yoshizaki and Tomida, 2000 | |
| 0.5 g/250 mL | 12 h | 57.8 | 82.3 | Mingot et al., 1995 | |
| 0.5 M | 24 h | 1 | 72 | Jenkins and Scheybeler, 1981 | |
| 10% | 1:50 | 5 h | 91.4 | 90.1 | This study |
| Cu | Zn | |||
|---|---|---|---|---|
| Reaction Time (Hour) | 1% H2SO4 | 10% H2SO4 | 1% H2SO4 | 10% H2SO4 |
| 1 | 68.3 ± 1.01 | 85.6 ± 2.90 | 83.2 ± 1.40 | 92.0 ± 0.21 |
| 2 | 75.8 ± 0.58 | 93.1 ± 0.29 | 83.4 ± 1.93 | 91.4 ± 0.16 |
| 4 | 69.8 ± 0.11 | 88.8 ± 1.36 | 79.2 ± 4.35 | 92.0 ± 0.13 |
| RE(%) | SD | ||
|---|---|---|---|
| HNO3 | – | – | |
| H2SO4 | 51.1 | 9.6 | |
| Citric acid | 16.8 | 5.5 | |
| Oxalic acid | 14.9 | 10.0 | |
| H2SO4 | 0.5 | – | – |
| concentrations | 1 | – | – |
| (%) | 2 | – | – |
| 5 | 26.7 | 0.48 | |
| 7 | 41.0 | 1.84 | |
| 10 | 54.9 | 3.83 | |
| 15 | 71.7 | 7.47 | |
| reaction time | 1 | 53.3 | 0.68 |
| (hour) | 2 | 51.5 | 0.03 |
| 3.5 | 52.1 | 1.73 | |
| 8 | 56.4 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, M.-J.; Lee, S.-M. Chemical Removal of Cu and Zn from Swine Feces before Soil Application. Agriculture 2021, 11, 377. https://doi.org/10.3390/agriculture11050377
Shim M-J, Lee S-M. Chemical Removal of Cu and Zn from Swine Feces before Soil Application. Agriculture. 2021; 11(5):377. https://doi.org/10.3390/agriculture11050377
Chicago/Turabian StyleShim, Moo-Joon, and Seung-Mok Lee. 2021. "Chemical Removal of Cu and Zn from Swine Feces before Soil Application" Agriculture 11, no. 5: 377. https://doi.org/10.3390/agriculture11050377






