Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Growing Conditions
2.2. Water Use and Yield at Physiological Maturity
2.3. Root Harvest and Root Density at Physiological Maturity
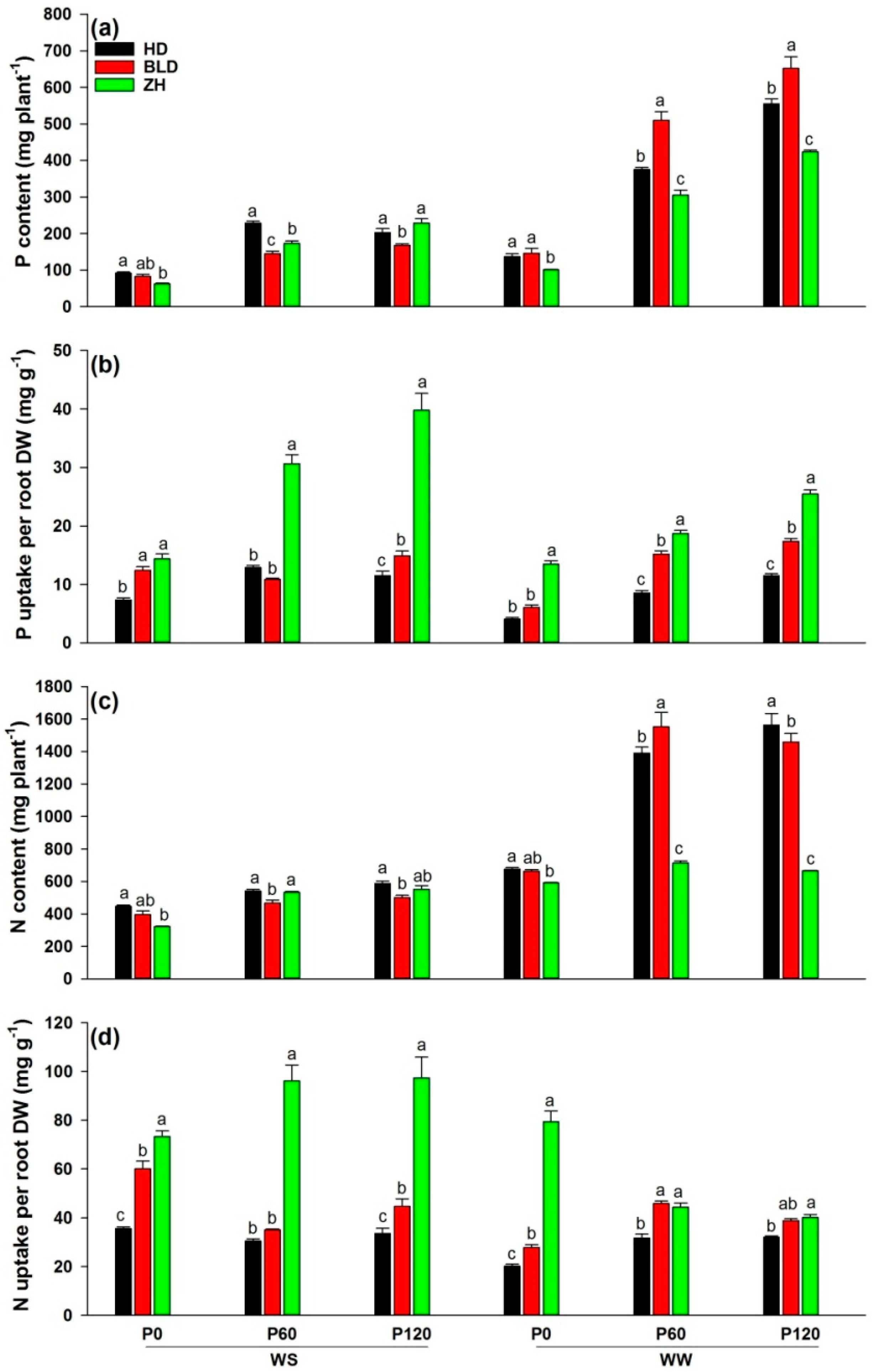
2.4. P and N Concentrations
2.5. Statistics
3. Results
3.1. Grain Yield and Water-Use Efficiency under Water- and P-Limited Conditions
3.2. Shoot to Root Ratio, Water- and Nutrient-Uptake Efficiencies and Root Density under Water and P Deficits
3.3. Correlation and Principal Component Analysis
4. Discussion
4.1. Root Efficiency vs. Root Size on Yield Performance
4.2. Root Traits and Root Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.S.P.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Xi, Y.; Li, F.M. Old and new cultivars of soya bean (Glycine max L.) subjected to soil drying differ in abscisic acid accumulation, water relations characteristics and yield. J. Agron. Crop. Sci. 2016, 202, 372–383. [Google Scholar] [CrossRef]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.W.; et al. Conserved water use improves the yield performance of soybean (Glycine max (L. Merr.)) under drought. Agric. Water Manag. 2017, 179, 236–245. [Google Scholar] [CrossRef]
- Yang, M.H.; Jahufer, M.; He, J.; Dong, R.; Hofmann, R.; Siddique, K.H.M.; Li, F.M. Effect of traditional soybean breeding on water use strategy in arid and semi-arid areas. Eur. J. Agron. 2020, 120, 126128. [Google Scholar] [CrossRef]
- Xu, Q.P.; Luo, C.Y.; Liao, H.; Yan, X.L.; Nian, H. Study on the response of soybean varieties to P deficiency. Soybean Sci. 2003, 22, 108–114. [Google Scholar]
- He, J.; Du, Y.L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.M.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, N.C.; Molyneux, N.; Yang, S.; Xiong, Y.C.; Siddique, K.H.M. Climate change in south-west Australia and north-west China: Challenges and opportunities for crop production. Crop. Pasture Sci. 2011, 62, 445–456. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Turner, N.C.; Chen, Z.; Liu, H.Y.; Wang, X.L.; Siddique, K.H.M.; Li, F.M. Phosphorus application increases root growth, improves daily water use during the reproductive stage, and increases grain yield in soybean subjected to water shortage. Env. Exp. Bot. 2019, 166, 103816. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Johansen, C.; Slinkard, A.E.; Rao, R.C.N.; Saxena, N.P.; Chauhan, Y.S. Strategies for improving drought resistance in grain legumes. Cri.t Rev. Plant Sci. 1995, 14, 469–523. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Jin, Y.; Turner, N.C.; Li, F.M. Irrigation during flowering improves subsoil water uptake and grain yield in rainfed soybean. Agronomy 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Brown, K.M. Topsoil foraging–An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Liu, P.; Lynch, J.P. Greater lateral root branching density in maize (Zea mays L.) improves phosphorus acquisition from low phosphorus soil. J. Exp. Bot. 2018, 69, 4961–4970. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Gao, Y.; Lynch, J. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol. 2018, 177, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, H.; Postma, J.; Lynch, J.P. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann. Bot. 2018, 122, 485–499. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Mori, A.; Fukuda, T.; Vejchasarn, P.; Nestler, J.; Pariasca-Tanaka, J.; Wissuwa, M. The role of root size versus root efficiency in phosphorus acquisition in rice. J. Exp. Bot. 2016, 67, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Rubio, G. Root morphological traits related to phosphorus-uptake efficiency of soybean, sunflower, and maize. J. Plant. Nutr. Soil Sci. 2015, 178, 807–815. [Google Scholar] [CrossRef]
- Fehr, W.; Caviness, C.; Burmood, D.; Pennington, J. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop. Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Messina, C.D.; Beatty, A.; Samples, M. Assessment across the united states of the benefits of altered soybean drought traits. Agron. J. 2010, 102, 475–482. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Jenkinson, D.M.; Vadez, V.A. conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J. Exp. Bot. 2011, 62, 4239–4252. [Google Scholar] [CrossRef] [Green Version]
- Vadez, V.; Kholova, J.; Zaman-Allah, M.; Belko, N. Water: The most important ‘molecular’ component of water stress tolerance research. Funct. Plant Biol. 2013, 40, 1310–1322. [Google Scholar] [CrossRef] [Green Version]
- Waddell, H.A.; Simpson, R.J.; Henderson, B.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and implications for grassland management. Grass Forage Sci. 2015, 71, 245–258. [Google Scholar] [CrossRef]
- Haling, R.E.; Yang, Z.; Shadwell, N.; Culvenor, R.A.; Stefanski, A.; Ryan, M.H.; Sandral, G.A.; Kidd, D.R.; Lambers, H.; Simpson, R. Growth and root dry matter allocation by pasture legumes and a grass with contrasting external critical phosphorus requirements. Plant Soil 2016, 407, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Funayama-Noguchi, S.; Noguchi, K.O.; Terashima, I. Comparison of the response to phosphorus deficiency in two lupin species, Lupinus albus and L. angustifolius, with contrasting root morphology. Plant Cell Environ. 2015, 38, 399–410. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, A.F.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol 1998, 116, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus-and moisture-limited environments. Ann. Bot. 2010, 105, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Plant responses to limited moisture and phosphorus availability. Adv. Agron. 2014, 124, 143–200. [Google Scholar]
- Waddell, H.A.; Simpson, R.J.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 2016, 412, 7–19. [Google Scholar] [CrossRef]



| Genotype (G) | Water Treatment (W) | P Rate (p) | Grain Yield | WUEG | Shoot DW | Root DW | SRR |
|---|---|---|---|---|---|---|---|
| ZH | WS | P0 | 8.4 | 0.72 | 24.0 | 4.4 | 5.4 |
| P60 | 12.7 | 0.81 | 45.0 | 5.7 | 8.1 | ||
| P120 | 13.8 | 0.84 | 45.1 | 6.2 | 7.3 | ||
| BLD | WS | P0 | 9.6 | 0.52 | 36.6 | 6.8 | 5.4 |
| P60 | 6.3 | 0.27 | 56.0 | 13.3 | 4.3 | ||
| P120 | 10.2 | 0.39 | 59.5 | 11.5 | 5.2 | ||
| HD | WS | P0 | 10.0 | 0.42 | 54.1 | 12.5 | 4.3 |
| P60 | 10.8 | 0.37 | 76.5 | 18.0 | 4.4 | ||
| P120 | 12.5 | 0.42 | 81.9 | 18.2 | 4.8 | ||
| ZH | WW | P0 | 17.0 | 0.74 | 47.2 | 7.6 | 6.3 |
| P60 | 24.3 | 0.67 | 88.5 | 16.2 | 5.5 | ||
| P120 | 23.9 | 0.69 | 90.2 | 16.7 | 5.4 | ||
| BLD | WW | P0 | 15.0 | 0.29 | 98.2 | 24.2 | 4.1 |
| P60 | 35.1 | 0.43 | 170.4 | 34.4 | 5.1 | ||
| P120 | 32.3 | 0.40 | 172.3 | 37.8 | 4.7 | ||
| HD | WW | P0 | 14.2 | 0.23 | 111.8 | 34.0 | 3.4 |
| P60 | 32.9 | 0.37 | 179.9 | 44.6 | 4.1 | ||
| P120 | 37.0 | 0.41 | 192.7 | 48.6 | 4.0 | ||
| G | ** (1.7) | *** (0.04) | *** (7.8) | *** (2.9) | *** (1.1) | ||
| W | *** (1.4) | ** (0.03) | *** (6.4) | *** (2.4) | * (0.9) | ||
| P | *** (1.7) | n.s | *** (7.8) | *** (2.9) | n.s | ||
| G×W | *** (2.4) | n.s | *** (11.1) | *** (4.2) | n.s | ||
| G×P | n.s | n.s | n.s | n.s | n.s | ||
| W×P | *** (2.4) | ** (0.06) | *** (11.1) | * (4.2) | n.s | ||
| G×W×P | ** (4.2) | *** (0.10) | n.s | n.s | n.s |
| Source of variability | G | W | P | G×W | G×P | W×P | G×W×P |
|---|---|---|---|---|---|---|---|
| Water use | *** (2.4) | *** (1.9) | *** (2.4) | *** (3.4) | ** (4.2) | *** (3.4) | n.s |
| Water uptake per unit root DW | *** (0.3) | n.s | n.s | n.s | n.s | n.s | n.s |
| P content | *** (28.5) | *** (23.2) | *** (28.4) | *** (40.2) | n.s | *** (40.2) | ** (69.7) |
| P uptake per unit root DW | *** (2.2) | *** (1.8) | *** (2.2) | *** (3.1) | *** (3.8) | n.s | *** (5.3) |
| N content | *** (77.4) | *** (63.2) | *** (77.4) | *** (109) | ** (134) | *** (109) | *** (189) |
| N uptake per unit root DW | *** (7.4) | *** (6.0) | n.s | *** (10.5) | n.s | n.s | *** (18.1) |
| Adventitious root density | *** (1.2) | n.s | n.s | n.s | n.s | n.s | n.s |
| AD branching density | *** (0.30) | * (0.24) | *** (0.30) | n.s | n.s | n.s | *** (0.74) |
| Lateral root density | *** (0.42) | n.s | * (0.42) | *** (0.59) | n.s | * (0.59) | n.s |
| LR branching density | *** (0.38) | n.s | n.s | n.s | n.s | n.s | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Jin, Y.; Siddique, K.H.M.; Li, F.-M. Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels. Agriculture 2021, 11, 481. https://doi.org/10.3390/agriculture11060481
He J, Jin Y, Siddique KHM, Li F-M. Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels. Agriculture. 2021; 11(6):481. https://doi.org/10.3390/agriculture11060481
Chicago/Turabian StyleHe, Jin, Yi Jin, Kadambot H. M. Siddique, and Feng-Min Li. 2021. "Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels" Agriculture 11, no. 6: 481. https://doi.org/10.3390/agriculture11060481
APA StyleHe, J., Jin, Y., Siddique, K. H. M., & Li, F.-M. (2021). Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels. Agriculture, 11(6), 481. https://doi.org/10.3390/agriculture11060481








