Effect of Inorganic Zinc on Selected Immune Parameters in Chicken Blood and Jejunum after A. galli Infection
Abstract
:1. Introduction
2. Material and methods
2.1. Chickens
2.2. Infective Material and Inoculation
2.3. Homogenization of Jejunum and Isolation of Total RNA of Interleukins (IL-4, IL-17), IFN-γ, and TNF-α Gene
2.4. Relative Expression of Interleukins, IFN-γ, and TNF-α Gene in Quantitative Real-Time PCR (qRT-PCR)
2.5. White Blood Cell Count (WBC)
- absolute leukocyte count × relative % of a different type of WBC/100 counted cells.
2.6. Isolation of Lamina Propria Lymphocytes (LPL)
2.7. Staining of Lymphocytes by Direct Immunofluorescence
2.8. Flow Cytometry Analysis of Stained Cells (FC)
2.9. Statistical Analysis
3. Results
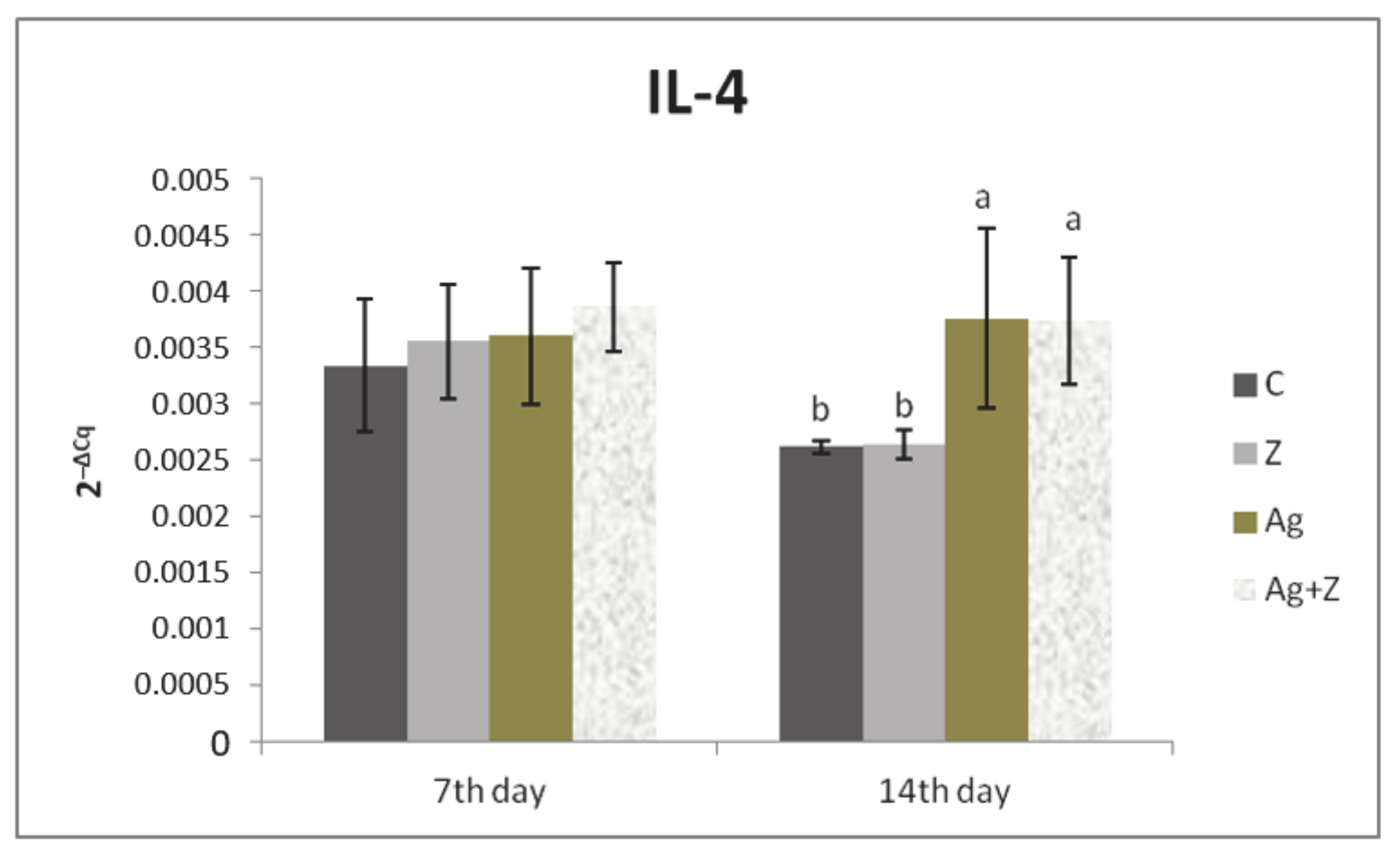
3.1. Relative Expression for Target Genes
3.2. White Blood Cell Count (WBC)
3.3. Immunophenotyping of Lymphocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferdushy, T.; Nejsum, P.; Roepstorff, A.; Thamsborg, S.M.; Kyvsgaard, N.C. Ascaridia galli in chickens: Intestinal localization and comparison of methods to isolate the larvae within the first week of infection. Parasitol. Res. 2012, 111, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.H.; Znada, N.Y.A. Morphology and life history of Ascaridia galli in the domestic fowl that are raised in Jeddah. Science 1992, 4, 87–99. [Google Scholar] [CrossRef]
- Marcos-Atxutegi, C.; Gandolfi, B.; Arangüena, T.; Sepúlveda, R.; Arévalo, M.; Simón, F. Antibody and inflammatory responses in laying hens with experimental primary infections of Ascaridia galli. Vet. Parasitol. 2009, 161, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, A.; Gauly, M.; Abel, H.; Daş, G.; Humburg, J.; Rohn, K.; Breves, G.; Rautenschlein, S. Immunopathogenesis of Ascaridia galli infection in layer chicken. Dev. Comp. Immunol. 2011, 35, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Darmawi, U.; Balqis, M.; Hambal, R.; Tiuria, F.; Priosoeryanto, B.P. Mucosal mast cells response in the jejunum of Ascaridia galli-infected laying hens. Media Peternak. 2013, 36, 113–119. [Google Scholar] [CrossRef]
- Norup, L.R.; Dalgaard, T.S.; Pleidrup, J.; Permin, A.; Schou, T.W.; Jungersen, G.; Fink, D.R.; Juul-Madsen, H.R. Comparison of parasite-specific immunoglobulin levels in two chicken lines during sustained infection with Ascaridia galli. Vet. Parasitol. 2013, 191, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Degen, W.G.J.; Daal, N.V.; Rothwell, L.; Kaiser, P.; Schijns, V.E.J.C. Th1/Th2 polarization by viral and helminth infection in birds. Vet. Microbiol. 2005, 105, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.E.; Wynn, T.A. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011, 7, e1002003. [Google Scholar] [CrossRef] [Green Version]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Logan, N.; Rückerl, D.; Humbles, A.A.; Allan, S.M.; Papayannopoulos, V.; Stockinger, B.; Maizels, R.M.; Allen, J.E. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 2014, 15, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Ajendra, J.; Chenery, A.L.; Parkinson, J.E.; Chan, B.H.K.; Pearson, S.; Colombo, S.A.P.; Boon, L.; Grencis, R.K.; Sutherland, T.E.; Allen, J.E. IL-17A both initiates, via IFNγ suppression, and limits the pulmonary type-2 immune response to nematode infection. Mucosal Immunol. 2020, 13, 958–968. [Google Scholar] [CrossRef]
- Dahl, C.; Permin, A.; Christensen, J.P.; Bisgaard, M.; Muhairwa, A.P.; Petersen, K.M.; Poulsen, J.S.; Jensen, A.L. The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Vet. Microbiol. 2002, 86, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Hunt, P.W.; Hine, B.C.; Ruhnke, I. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: New insights into an old problem. Poult. Sci. 2019, 98, 6517–6526. [Google Scholar] [CrossRef]
- Mudroňová, D.; Nemcová, R.; Lauková, A.; Koščová, J.; Strompfová, V.; Györyová, K.; Szunyogová, E.; Lazar, G. Effect of Lactobacillus fermentum alone, and in combination with zinc(ii) propionate on Salmonella Enterica serovar. Dusseldorf in Japanese quails. Biologia 2006, 6, 797–801. [Google Scholar] [CrossRef]
- Sloup, V.; Jankovská, I.; Nechybová, S.; Peřinková, P.; Langrová, I. Zinc in the animal organism: A review. Sci. Agric. Bohem. 2017, 48, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Permin, A.; Bojesen, M.; Nansen, P.; Bisgaard, M.; Frandsen, F.; Pearman, M. Ascaridia galli populations in chickens following single infections with different dose levels. Parasitol. Res. 1997, 83, 614–617. [Google Scholar] [CrossRef]
- Karaffová, V.; Bobíková, K.; Levkut, M.; Revajová, V.; Ševčíková, Z.; Levkut, M. The influence of Farmatan® and Flimabend® on the mucosal immunity of broiler chicken. Poult. Sci. 2019, 98, 1161–1166. [Google Scholar] [CrossRef]
- Truong, A.D.; Park, B.; Ban, J.; Hong, Y.H. The novel chicken interleukin 26 protein is overexpressed in T cells and induces proinflammatory cytokines. Vet. Res. 2016, 47, 65. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Berndt, A.; Wilhelm, A.; Jugert, C.; Pieper, J.; Sachse, K.; Methner, U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 2007, 75, 5993–6007. [Google Scholar] [CrossRef] [Green Version]
- Kolesarova, M.; Spisakova, V.; Matulova, M.; Crhanova, M.; Sisak, F.; Rychlik, I. Characterisation of basal expression of selected cytokines in the liver, spleen, and respiratory, reproductive and intestinal tract of hens. Vet. Med. 2011, 56, 325–332. [Google Scholar] [CrossRef] [Green Version]
- De Boever, S.; Vangestel, C.; De Backer, P.; Croubels, S.; Sys, S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008, 122, 312–317. [Google Scholar] [CrossRef]
- Fried, K.; Jantošovič, J. Blood sampling by cardiac punction. Vet. Cas. 1961, 10, 383–391. (In Slovak) [Google Scholar]
- Solano-Aguilar, G.I.; Vengroski, K.G.; Beshah, E.; Lunney, J.K. Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. J. Immunol. Methods 2000, 241, 185–199. [Google Scholar] [CrossRef]
- Bucková, B.; Revajová, V. Immunophenotyping of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the chicken intestine by flow cytometry. Folia Vet. 2014, 58, 75–77. [Google Scholar]
- Eigaard, N.M.; Schou, T.W.; Permin, A.; Christensen, J.P.; Ekstrøm, C.T.; Ambrosini, F.; Cianci, D.; Bisgaard, M. Infection and excretion of Salmonella enteritidis in two different chicken lines with concurrent Ascaridia galli infection. Avian Pathol. 2006, 35, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Permin, A.; Christensen, J.P.; Bisgaard, M. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet. Scand. 2006, 47, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleidrup, J.; Dalgaard, T.S.; Norup, L.R.; Permin, A.; Schou, T.W.; Skovgaard, K.; Vadekær, D.F.; Jungersen, G.; Sørensen, P.; Juul-Madsen, H.R. Ascaridia galli infection influences the development of both humoral and cell-mediated immunity after Newcastle disease vaccination in chickens. Vaccine 2014, 32, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Shea-Donohue, T.; Morris, S.C.; Gildea, L.; Strait, R.; Madden, K.B.; Schopf, L.; Urban, J.F. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004, 201, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Sutherland, T.E. Host protective roles of type 2 immunity: Parasite killing and tissue repair, flip sides of the same coin. Semin. Immunol. 2014, 26, 329–340. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Song, M.; Hu, Y.; Pan, B.; Cai, J.; Wang, M. Eimeria tenella: Interleukin 17 contributes to host immunopathology in the gut during experimental infection. Exp. Parasitol. 2013, 133, 121–130. [Google Scholar] [CrossRef]
- Cardenas, A.E.; Sánchez Tapia, M.T.; Muñoz Chacon, B.; Pastelin Palacios, R. Impact of zinc supplementation on the production of IL-17 in perinatal stages. In Proceedings of the 15th International Congress of Immunology (ICI), Milan, Italy, 22–27 August 2013. [Google Scholar]
- Gabrashanska, M.; Teodorova, S.E.; Galvez-Morros, M.M.; Tsocheva-Gaytandzhieva, N.; Mitov, M.; Ermidou-Pollet, S.; Pollet, S. Administration of Zn-Co-Mn basic salt to chickens with ascaridiosis. Parasitol. Res. 2004, 93, 235–241. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wang, J.F.; Zi, N.T.; Jing, M.Y.; Weng, X.Y. Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol. Trace Elem. Res. 2011, 143, 394–402. [Google Scholar] [CrossRef]
- Ruhnke, I.; Andronicos, N.M.; Swick, R.A.; Hine, B.; Sharma, N.; Kheravii, S.K.; Wu, S.B.; Hunt, P. Immune responses following experimental infection with Ascaridia galli and necrotic enteritis in broiler chickens. Avian Pathol. 2017, 46, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, R.K.; Mishra, S. Clinico-HaematoBiochemical studies on intestinal helminthiasis in poultry. Vet. Practitioner 2001, 2, 137–140. [Google Scholar]
- Kumar, R.; Sinha, S.R.P.; Verma, S.B.; Sinha, S. Haematological changes in the Japanese quails (Coturnix coturnix japonica) naturally infected with nematode Ascaridia galli. Ind. Vet. Med. J. 2003, 27, 297–299. [Google Scholar]
- Sudadi, A.M.; Hariyati, R.; Dewi, A.M.K. Influence of zinc supplementation in eosinophil nasal mucous count and quality of life in moderate to severe persistent allergic rhinitis patient in ENT clinic Dr. Kariadi hospital Semarang in November 2016 until January 2017. Bali Med. J. 2019, 8, 88–93. [Google Scholar] [CrossRef]
- Deka, K.; Borah, J. Haematological and biochemical changes in japanese quails Coturnix coturnix japonica and chickens due to Ascaridia galli infection. Int. J. Poult. Sci. 2008, 7, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Luna-Olivares, L.A.; Ferdushy, T.; Kyvsgaard, N.C.; Nejsum, P.; Thamsborg, S.M.; Roepstorff, A.; Iburg, T.M. Localization of Ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet. Parasitol. 2012, 185, 186–193. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef]
- Dahan, S.; Roda, G.; Pinn, D.; Roth-Walter, F.; Kamalu, O.; Martin, A.P.; Mayer, L. Epithelial: Lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology 2008, 134, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [Green Version]
- Miyai, T.; Hojyo, S.; Ikawa, T.; Kawamura, M.; Irié, T.; Ogura, H.; Hijikata, A.; Bin, B.H.; Yasuda, T.; Kitamura, H.; et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. USA. 2014, 111, 11780–11785. [Google Scholar] [CrossRef] [Green Version]
- Maiguma, M.; Suzuki, Y.; Suzuki, H.; Okazaki, K.; Aizawa, M.; Muto, M.; Tomino, Y. Dietary zinc is a key environmental modifier in the progression of IgA nephropathy. PLoS ONE 2014, 9, e90558. [Google Scholar] [CrossRef] [Green Version]






| Ingredients g/kg | BR1 |
|---|---|
| Wheat | 290 |
| Maize | 300 |
| Soybean meal | 320 |
| Rapeseed oil | 40 |
| Fish meal | 20 |
| Limestone | 12 |
| Dicalcium phosphate | 10 |
| Sodium chloride | 2 |
| DL-methionine | 1 |
| Vitamin-mineral mix | 5 |
| Composition by analysis (g/kg dry matter) | |
| dry matter | 899.9 |
| crude protein | 232.7 |
| fat | 64.5 |
| dietary fiber | 22.7 |
| ash | 53 |
| Ca | 90.4 |
| P total | 69.6 |
| Primer | Sequence 5′–3′ | Annealing/Temperature Time | References |
|---|---|---|---|
| IL-4 Fw | AGCACTGCCACAAGAACCTG | 60 °C /30 s | [19] |
| IL-4 Rev | CCTGCTGCCGTGGGACAT | ||
| IL-17 Fw | TATCAGCAAACGCTCACTGG | 59 °C /30 s | [20] |
| IL-17 Rev | AGTTCACGCACCTGGAATG | ||
| IFN-γ Fw | GCCGCACATCAAACACATATCT | 59 °C /30 s | [21] |
| IFN-γ Rev | TGAGACTGGCTCCTTTTCCTT | ||
| TNF-α Fw | AATTTGCAGGCTGTTTCTGC | 59 °C /30 s | [22] |
| TNF-α Rev | TATGAAGGTGGTGCAGATGG | ||
| GAPDH Fw | CCTGCATCTGCCCATTT | 59 °C /30 s | [23] |
| GAPDH Rev | GGCACGCCATCACTATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaffová, V.; Revajová, V.; Dvorožňáková, E.; Grešáková, Ľ.; Levkut, M.; Ševčíková, Z.; Herich, R.; Levkut, M. Effect of Inorganic Zinc on Selected Immune Parameters in Chicken Blood and Jejunum after A. galli Infection. Agriculture 2021, 11, 551. https://doi.org/10.3390/agriculture11060551
Karaffová V, Revajová V, Dvorožňáková E, Grešáková Ľ, Levkut M, Ševčíková Z, Herich R, Levkut M. Effect of Inorganic Zinc on Selected Immune Parameters in Chicken Blood and Jejunum after A. galli Infection. Agriculture. 2021; 11(6):551. https://doi.org/10.3390/agriculture11060551
Chicago/Turabian StyleKaraffová, Viera, Viera Revajová, Emília Dvorožňáková, Ľubomíra Grešáková, Martin Levkut, Zuzana Ševčíková, Róbert Herich, and Mikulas Levkut. 2021. "Effect of Inorganic Zinc on Selected Immune Parameters in Chicken Blood and Jejunum after A. galli Infection" Agriculture 11, no. 6: 551. https://doi.org/10.3390/agriculture11060551
APA StyleKaraffová, V., Revajová, V., Dvorožňáková, E., Grešáková, Ľ., Levkut, M., Ševčíková, Z., Herich, R., & Levkut, M. (2021). Effect of Inorganic Zinc on Selected Immune Parameters in Chicken Blood and Jejunum after A. galli Infection. Agriculture, 11(6), 551. https://doi.org/10.3390/agriculture11060551







