Efficiency of Recycled Biogas Digestates as Phosphorus Fertilizers for Maize
Abstract
:1. Introduction
2. Materials and Methods
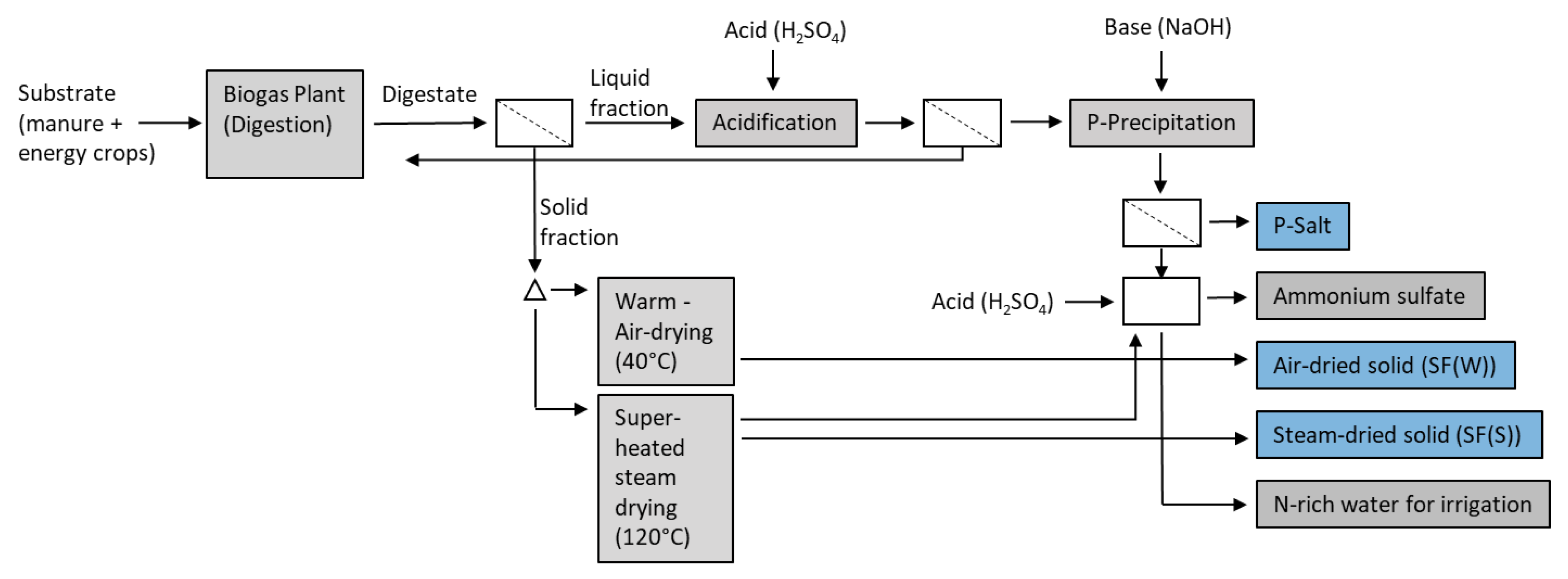
2.1. Recycled P-Fertilizers
2.2. Soil Characteristics
2.3. Soil Sampling and Analysis of P, K, Mg, Corg, Nt and pH
2.4. Fertilizer Dosing
2.5. Experimental Details
2.6. Maize Harvest and P, Mg and Ca Analysis
2.7. Evaluation of Synergistic Effects
2.8. Statistical Analysis
3. Results
3.1. Effect of P-Fertilizers on Biomass Yield and Plant Nutrient Concentration
3.2. Effect of P-Fertilizers on Plant Nutrient Content
3.3. Effect of P-Fertilizers on Plant Available P (CAL-P) in Soil
4. Discussion
4.1. Effects of P-Fertilizers on Biomass Yield, Plant Nutrient Uptake, and CAL-P in Soil
4.2. Fertilizer Effects of Different Fertilizer Combinations and Soil Application Techniques
4.3. Effects of Different Drying Procedures of the Solids (Air Dried vs. Steam Dried) on Fertilization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011, 91, 173–184. [Google Scholar] [CrossRef]
- Scholz, R.W.; Roy, A.H.; Hellums, D.T.; Ulrich, A.E. Sustainable Phosphorus Management—A Global Transdisciplinary Challenge; Springer: Berlin, Germany, 2014; pp. 1–128. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2021.
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants—Facing phosphorus scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef]
- Eichler-Löbermann, B.; Köhne, S.; Köppen, D. Effect of organic, inorganic, and combined organic and inorganic P fertilization on plant P uptake and soil P pools. J. Plant Nutr. Soil Sci. 2007, 170, 623–628. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. J. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Najera, I.; Baixauli, C.; Caravaca, F.; Roldan, A.; Cegarra, J.; Bernal, M.P. Agrucultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Bachmann, S.; Uptmoor, R.; Eichler-Löbermann, B. Phosphorus distribution and availability in untreated and mechanically separated biogas digestates. Sci. Agric. 2016, 73, 9–17. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- European Commission Communities (COM). Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions; European Commission Communities: Brussels, Belgium, 2007. [Google Scholar]
- Renewable Energy Directive (2009/28/EC). Official Journal of the European Union: Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. L140/16. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:140:0016:0062:en:PDF (accessed on 16 May 2021).
- EBA. European Biogas Association: Statistical Report; EBA: Brussels, Belgium, 2018. [Google Scholar]
- Döhler, H. Faustzahlen für die Landwirtschaft, 14th ed.; KTBL: Darmstadt, Germany, 2009. [Google Scholar]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Johnston, A.E.; Richards, I.R. Effectiveness of different precipitated phosphates as phosphorus sources for plants. Phosphorus Res. Bull. 2004, 15, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Römer, W. Vergleichende Untersuchungen zur Pflanzenverfügbarkeit von Phosphat aus verschiedenen P-Recycling-Produkten im Keimpflanzenversuch. J. Plant Nutr. Soil Sci. 2006, 169, 826–832. [Google Scholar] [CrossRef]
- Bach, I.-M.; Idler, L.; Lewandowski, I.; Müller, T. Einfluss Verschiedener Gärrest-Aufbereitungsprodukte auf die P-Aufnahme bei Mais in Einem Löss- und Einem Tonigen Lehmboden im Vergleich zu Einer Mineraldüngung. 2015. Available online: https://eprints.dbges.de/1157/ (accessed on 16 May 2021).
- Bilbao, J.; Frank, D.; Hirth, T. Combined Recovery of Phosphorus, Potassium and Nitrogen from Aqueous Residual Material; International Classification, Cooperative Patent Classification C01B25/45; Fraunhofer GES zur Förderung der Angewandten Forschung EV: Munich, Germany, 2015. [Google Scholar]
- Muhammad, S.; Müller, T.; Joergensen, R.G. Compost and P amendments for stimulating microorganisms and maize growth in a saline soil from Pakistan in comparison with a nonsaline soil from Germany. J. Plant Nutr. Soil Sci. 2007, 170, 745–752. [Google Scholar] [CrossRef]
- German Biogas Association. Biogas Market Data in Germany 2019/2020. Available online: https://www.biogas.org/edcom/webfvb.nsf/id/EN-German-biogas-market-data/$file/20-07-23_Biogasindustryfigures-2019-2020_english.pdf (accessed on 20 May 2021).
- Salamat, R.; Scaar, H.; Weigler, F.; Berg, W.; Mellmann, J. Drying of biogas digestate: A review with a focus on available drying techniques, drying kinetics, and gaseous emission behavior. Dry. Technol. 2020. [Google Scholar] [CrossRef]
- Awiszus, S.; Meissner, K.; Reyer, S.; Müller, J. Ammonia and Methane Emissions during Drying of dewatered biogas Digestate in a Two-Belt Conveyor Dryer. Bioresour. Technol. 2018, 247, 419–425. [Google Scholar] [CrossRef]
- Lemming, C.; Scheutz, C.; Bruun, S.; Jensen, L.S.; Magid, J. Effects of Thermal Drying on Phosphorus Availability from Iron-Precipitated Sewage Sludge. J. Plant Nutr. Soil Sci. 2017, 180, 720–728. [Google Scholar] [CrossRef]
- VDLUFA. Methodenbuch Bd. II: Die Untersuchung von Düngemitteln. 1995. Available online: https://www.vdlufa.de/Methodenbuch/index.php?option=com_content&view=article&id=6&Itemid=109&lang=de (accessed on 11 May 2021).
- Hedley, M.J.; Steward, J.W.B.; Chauhuan, B.S. Changes in organic and inorganic soil phosphorus fractions included by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 75–86. [Google Scholar]
- Wollmann, I.; Gauro, A.; Müller, T.; Möller, K. Phosphorus bioavailability of sewage sludge-based recycled fertilizers. J. Plant Nutr. Soil Sci. 2018, 181, 158–166. [Google Scholar] [CrossRef]
- Schüller, H. Die CAL-Methode, eine neue Methode zur Bestimmung des pflanzenverfügbaren Phosphates in Böden. Z. Pflanzenernähr. Düngg. Bodenk. 1969, 123, 48–63. [Google Scholar] [CrossRef]
- VDLUFA. Methode A 5.1.1, pH-Wert, in Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch). Bd. I Die Untersuchung von Böden. 1991. Available online: https://www.vdlufa.de/Methodenbuch/index.php?option=com_content&view=article&id=7&Itemid=108&lang=de&lang=de (accessed on 14 May 2021).
- VDLUFA Methode 2.1.1, Nasschemischer Aufschluss unter Druck, in Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. VII Umweltanalytik 4. Aufl. VDLUFA Verlag: Darmstadt, Germany, 2011. Available online: https://www.vdlufa.de/Methodenbuch/index.php?option=com_content&view=article&id=2&Itemid=113&lang=de (accessed on 10 May 2021).
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.4. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 16 May 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Piepho, H.-P. An Algorithm for a Letter-Based Representation of All-Pairwise Comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- BMEL, Bundesministerium für Ernährung und Landwirtschaft. Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der Guten Fachlichen Praxis beim Düngen (Düngeverordnung—DüV). Bundesgesetzblatt 2017, 1, 1305. Available online: https://www.gesetze-im-internet.de/d_v_2017/D%C3%BCV.pdf (accessed on 2 May 2021).
- Kern, J.; Heinzmann, B.; Markus, A.C.; Kaufmann, N.; Soethe, N.; Engels, C. Recycling and Assessment of Struvite Phosphorus from Sewage Sludge. Agric. Eng. Int. CIGR J. 2008, 10. Available online: https://www.semanticscholar.org/paper/Recycling-and-Assessment-of-Struvite-Phosphorus-Kern-Heinzmann/767c98cd80f35a7bd6b254552e38ec9bd7013152 (accessed on 2 May 2021).
- Ehmann, A.; Bach, I.-M.; Bilbao, J.; Lewandowski, I.; Müller, T. Phosphate recycled from semi-liquid manure and digestate are suitable alternative fertilizers for ornamentals. Sci. Hortic. 2018, 243, 440–450. [Google Scholar] [CrossRef]
- Ehmann, A.; Bach, I.-M.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can Phosphate Salts Recovered from Manure Replace Conventional Phosphate Fertilizer? Agriculture 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Vogel, T.; Nelles, M.; Eichler-Löbermann, B. Phosphorus application with recycled products from municipal waste water to different crop species. Ecol. Eng. 2015, 83, 466–475. [Google Scholar] [CrossRef]
- Lekfeldt, J.D.S.; Rex, M.; Mercl, F.; Kulhánek, M.; Tlustoš, P.; Magid, J.; de Neergaard, A. Effect of bioeffectors and recycled P-fertiliser products on the growth of spring Wheat. Chem. Biol. Technol. Agric. 2016, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Phosphorus Use Efficiency of Bio-Based Fertilizers: Bioavailability and Fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Römer, W.; Steingrobe, B. Fertilizer Effect of Phosphorus Recycling Products. Sustainability 2018, 10, 1166. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, H.; Tsuneda, S. (Eds.) Phosphorus Recovery and Recycling; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Shinde, R.; Sarkar, P.K.; Thombare, N. Soil Conditioners. Agric. Food 2019, 1, 2581–8317. [Google Scholar]
- Grüneberg, B.; Kern, J. P retention capacity of iron-ore and blast furnace slag in subsurface flow constructed wetlands. Water Sci. Technol. 2001, 44, 69–75. [Google Scholar] [CrossRef]
- Hylander, L.D.; Simán, G. Plant availability of P sorbed to potential wastewater treatment materials. Biol. Fert. Soils 2001, 34, 42–48. [Google Scholar] [CrossRef]
- Potarzycki, J. Effect of magnesium or zinc supplementation at the background of nitrogen rate on nitrogen management by maize canopy cultivated in monoculture. Plant Soil Environ. 2011, 57, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Grzebisz, W.; Potarzycki, J.; Lukowiak, R.; Przygocka-Cyna, K. The magnesium and calcium mineral status of maize at physiological maturity as a tool for an evaluation of yield forming conditions. J. Elem. 2016, 21, 881–897. [Google Scholar] [CrossRef]
- Weber, B.; Stadlbauer, E.A.; Schlich, E.; Eichenauer, S.; Kern, J.; Steffens, D. Phosphorus bioavailability of biochars produced by thermo-chemical conversion. J. Plant Nutr. Soil Sci. 2014, 177, 84–90. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of Nutrient-Enriched Biochar as a Soil Amendment during Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Johnson, M.G.; Spokas, K.A. Concentration and release of phosphorus and potassium from lignocellulosic- and manure-based biochars for fertilizer reuse. Front. Sustain. Food Syst. 2018, 2, 54. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Li, R.; Yin, J.; Wang, W.; Li, Y.; Zhang, Z. Transformation of phosphorus during drying and roasting of sewage Sludge. J. Waste Manag. 2014, 34, 1211–1216. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Speciation Dynamics of Phosphorus during (Hydro) Thermal Treatments of Sewage Sludge. Environ. Sci. Technol. 2015, 49, 14466–14474. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenes, J.P.; Carrere, H. Effects of thermal treatments on five different waste activated sludge samples solubilisation, physical properties and anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Qian, T.-T.; Jiang, H. Migration of Phosphorus in Sewage Sludge during Different thermal Treatment Processes. ACS Sustain. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Alfy, A.; Kiran, B.V.; Jeevitha, G.C.; Hebbar, H.U. Recent Developments in Superheated Steam Processing of Foods—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2191–2208. [Google Scholar] [CrossRef]






| Property/Variable | P-Salt (Precipitated from Liquid Digestate Fraction) | Steam-Dried Solids (Separated from Solid Digestate Fraction, Dried at 120 °C) | Air-Dried Solids (Separated from Solid Digestate Fraction, Dried at 40 °C) | Mineral TSP (Triple-Superphosphate, P Fertilizer as Reference) |
|---|---|---|---|---|
| Abbreviation | P-Salt | SF (S) | SF (W) | TSP |
| DM [% FM] | 69.7 | 91.6 | 95.4 | |
| Pt [% DM] | 11.3 | 2.3 | 2.1 | 19.0 |
| Water soluble P [% DM] | 0.13 | 0.35 | 0.35 | |
| Sequentially fractionated with … in (mg P (g DM)−1) | ||||
| NaHCO3 (easily available P) | 29.9 | 6.7 | 7.5 | |
| NaOH | 6.3 | 0.98 | 1.3 | |
| H2SO4 (sparingly available P) | 53.3 | 0.96 | 1.74 | |
| pH [CaCl2] | 8.3 | 8.5 | 7.1 | |
| Ca [% DM] | 8.2 | 1.8 | 1.7 | 15.0 |
| Mg [% DM] | 5.5 | 0.5 | 0.5 | |
| K [% DM] | 1.1 | 1.6 | 1.8 | |
| Na [% DM] | 0.43 | 0.07 | 0.08 | |
| Fe [% DM] | 0.55 | 0.13 | 0.14 | |
| Ammonium N (NH4-N) [% DM] | 2.1 | 1.4 | 1.5 | |
| Crop | Maize (Zea mays L. var. Carolinio), 3 seeds per pot; after germination reduction to 1 seedling per pot |
| Soil | Silty loam: Texture uL; pH [CaCl2] 7.3; nutrient status for P, K, Mg (all CAL) in mg/kg soil: 7; 71; 210; Corg%: 0.3; Nt%: 0.04 Clay loam: Texture tL; pH [CaCl2] 7.4; nutrient status for P, K, Mg (all CAL) in (mg (kg soil)−1): 26; 150; 580; Corg%: 3.6; Nt%: 0.24 |
| Additional mineral fertilization per pot (excluding P) | Before sowing: 200 (mg N (kg soil)−1) as NH4NO3, 200 (mg K (kg soil)−1) as K2SO4, 100 (mg Mg (kg soil)−1) as MgSO4·7H2O and 10 (mg (kg soil)−1) Fe–Sequestren (6%) 4 weeks after sowing: 200 (mg N (kg soil)−1) as NH4NO3 |
| Experimental Duration | Total: 50 days |
| Conditions | ambient greenhouse conditions (University of Hohenheim, Germany, June 2016), ca. 16 h light, 8 h dark, ca. 20 °C; initial watering to 70% water-holding capacity (WHC) with deionized (DI) water, additional watering when required (weight control every 2–3 days) |
| P-fertilizer treatments | |
| Recycled P-fertilizers | all mg below refers to P equivalents per 1 kg dry soil |
| P-Salt SF (W) SF (S) | 150 mg 150 mg 150 mg |
| SF (W) + P-Salt (1:1) | Dry mixed (dry): 75 mg SF (W) mixed into the soil, directly followed by 75 mg P-Salt mixed into the soil Suspended mixed (susp.): 75 mg SF (W) + 75 mg P-Salt + 50 mL DI water, pre-suspended in a separate vessel before mixing into soil |
| SF (W) + P-Salt (1:2) | Dry mixed (dry): 50 mg SF (W) mixed into the soil, directly followed by 100 mg P-Salt mixed into the soil Suspended mixed (susp.): 50 mg SF (W) + 100 mg P-Salt + 50 mL DI water, pre-suspended in a separate vessel before mixing into soil |
| SF(S) + P-Salt (1:1) | Dry mixed (dry): 75 mg SF (S) mixed into the soil, directly followed by 75 mg P-Salt mixed into the soil Suspended mixed (susp.): 75 mg SF (S) + 75 mg P-Salt + 50 mL DI water, pre-suspended in a separate vessel before mixing into soil |
| SF(S) + P-Salt (1:2) | Dry mixed (dry): 50 mg SF (S) mixed into the soil, directly followed by 100 mg P-Salt mixed into the soil Suspended mixed (susp.): 50 mg SF (S) + 100 mg P-Salt + 50 mL DI water, pre-suspended in a separate vessel before mixing into soil |
| Control treatments Triple superphosphate (TSP) Negative control | Positive reference; 150 mg DI water |
| P-Sources | Application Technique | CAL-P 2 Days after Fertilizer Incubation | CAL-P after Maize Harvest | CAL-P 2 Days after Fertilizer Incubation | CAL-P after Maize Harvest |
|---|---|---|---|---|---|
| Silty Loam [mg kg−1] | Silty Loam [mg kg−1] | Clay Loam [mg kg−1] | Clay Loam [mg kg−1] | ||
| Control | 24 G | 15 F | 73 g | 54 e | |
| TSP | 61 F | 142 AB | 121 cde | 97 cd | |
| P-Salt | 205 A | 173 A | 151 abc | 139 a | |
| SF(W) | 97 E | 52 E | 78 fg | 91 d | |
| SF(S) | 68 F | 59 E | 94 efg | 101 bcd | |
| SF(W)+P-Salt (1:1) | suspended | 113 CDE | 80 D | 123 BCDE | 115 abcd |
| dry | 130 BCD | 123 BC | 103 def | 109 ABCD | |
| SF(W)+P-Salt (1:2) | suspended | 101 DE | 125 BC | 117 CDE | 110 ABCD |
| dry | 147 BC | 138 ABC | 141 ABC | 133 AB | |
| SF(S)+P-Salt (1:1) | suspended | 104 DE | 104 CD | 161 ab | 105 ABCD |
| dry | 145 BC | 129 ABC | 146 ABC | 122 ABCD | |
| SF(S)+P-Salt (1:2) | suspended | 151 B | 119 BC | 167 A | 127 ABC |
| dry | 148 BC | 139 ABC | 135 ABCD | 110 abcd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach, I.-M.; Essich, L.; Müller, T. Efficiency of Recycled Biogas Digestates as Phosphorus Fertilizers for Maize. Agriculture 2021, 11, 553. https://doi.org/10.3390/agriculture11060553
Bach I-M, Essich L, Müller T. Efficiency of Recycled Biogas Digestates as Phosphorus Fertilizers for Maize. Agriculture. 2021; 11(6):553. https://doi.org/10.3390/agriculture11060553
Chicago/Turabian StyleBach, Inga-Mareike, Lisa Essich, and Torsten Müller. 2021. "Efficiency of Recycled Biogas Digestates as Phosphorus Fertilizers for Maize" Agriculture 11, no. 6: 553. https://doi.org/10.3390/agriculture11060553
APA StyleBach, I.-M., Essich, L., & Müller, T. (2021). Efficiency of Recycled Biogas Digestates as Phosphorus Fertilizers for Maize. Agriculture, 11(6), 553. https://doi.org/10.3390/agriculture11060553






