Antioxidant Activity of Elderberry Fruits during Maturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Extraction
2.4. Determination of Antioxidant Activity
2.5. Determination of Total Phenolic Content and Total Ascorbic Acid Content
2.6. Statistical Analysis
3. Results and Discussion
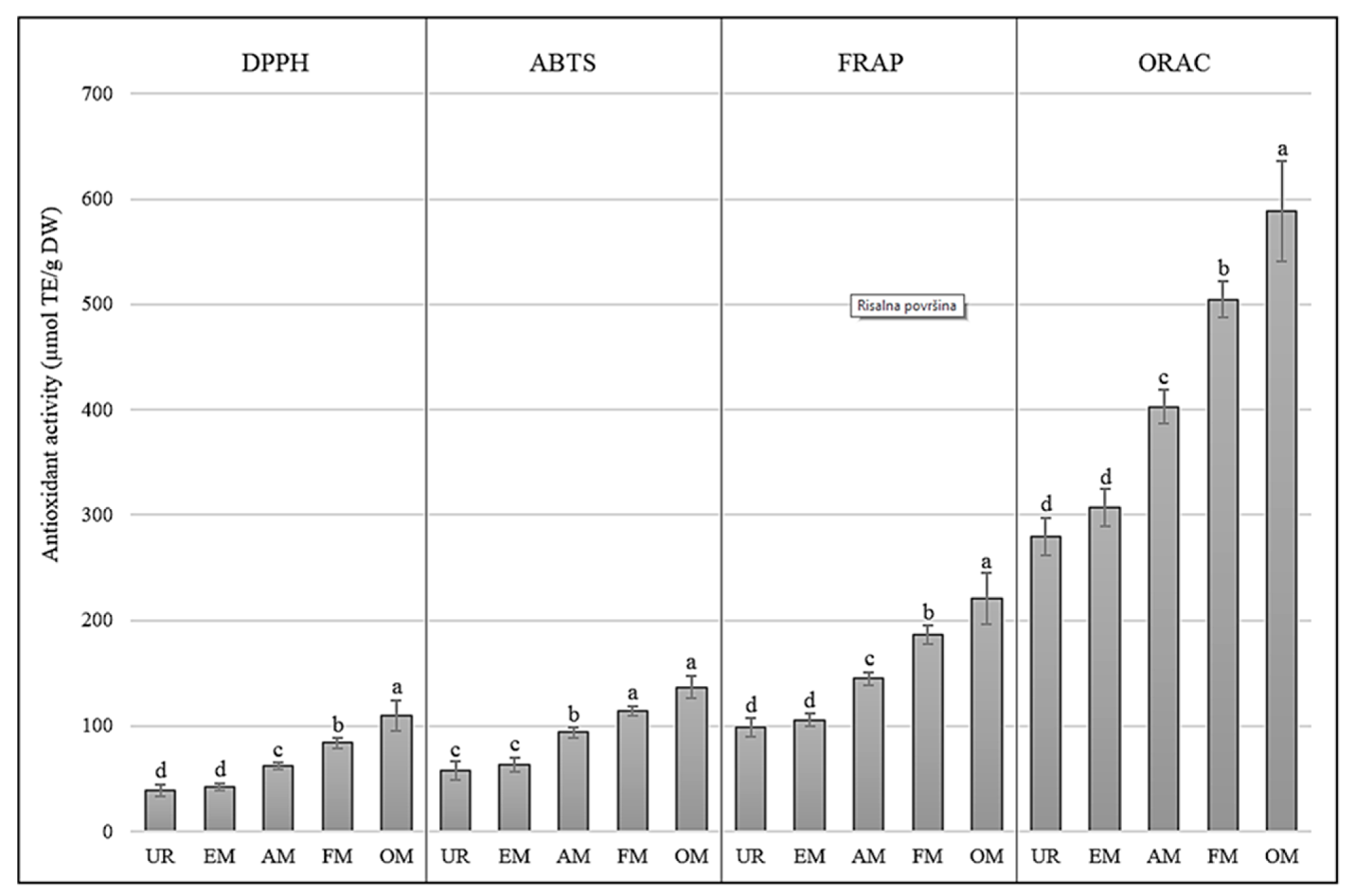
3.1. Antioxidant Activity during Elderberry Maturation Process
3.2. Correlations of AOA Based on Different Assays with Total Phenolic (TP) Content and Total Ascorbic Acid (TAA) Content
3.3. Clustering of Elderberry Interspecific Hybrids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2011, 1, 106. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.N. Natural and biological antioxidants in foods and biological systems. Their mechanism of action, applications and implications. Lipid Technol. 1995, 7, 77–80. [Google Scholar]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm.-Rundsch. 2007, 103, 369–377. [Google Scholar]
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornamental Plant Res. 2009, 17, 115–120. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Semik-Szczurak, D.; Wajda, L. Diversity and bioavailability of fruit polyphenols. J. Food Nutr. Res. 2017, 56, 167–178. [Google Scholar]
- Granato, D.; Karnopp, A.R.; van Ruth, S.M. Characterization and comparison of phenolic composition, antioxidant capacity and instrumental taste profile of juices from different botanical origins. J. Sci. Food Agric. 2014, 95, 1997–2006. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur. Food Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Nowak, D.; Goslinski, M.; Szwengiel, A. Multidimensional comparative analysis of phenolic compounds in organic juices with high antioxidant capacity. J. Sci. Food Agric. 2017, 97, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch. Lebensm.-Rundsch. 2007, 103, 58–64. [Google Scholar]
- Slatnar, A.; Jakopic, J.; Stampar, F.; Veberic, R.; Jamnik, P. The effect of bioactive compounds on in vitro and in vivo antioxidant activity of different berry juices. PLoS ONE 2012, 7, e47880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Bolli, R. Revision of the Genus Sambucus; J. Cramer: Berlin, Germany, 1994. [Google Scholar]
- Mathieu, F.; Charlebois, D.; Charles, M.T.; Chevrier, N. Biochemical changes in American elder (Sambucus canadensis) fruits during development. In Proceedings of the Acta Horticulturae, I International Symposium on Elderberry, Columbia, MO, USA, 9–14 June 2013; Thomas, A.L., Ed.; International Society for Horticultural Science: Korbeek-Lo, Belgium, 2015; pp. 61–72. [Google Scholar]
- Koss-Mikolajczyk, I.; Lewandowska, A.; Pilipczuk, T.; Kusznierewicz, B.; Bartoszek, A. Composition of bioactive secondary metabolites and mutagenicity of Sambucus nigra L. Fruit at different stages of ripeness. Acta Aliment. 2016, 45, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Kaack, K.; Frette, X.C.; Christensen, L.P.; Landbo, A.K.; Meyer, A.S. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- Salvador, A.C.; Rudnitskaya, A.; Silvestre, A.J.D.; Rocha, S.M. Metabolomic-based strategy for fingerprinting of Sambucus nigra L. berry volatile terpenoids and norisoprenoids: Influence of ripening and cultivar. J. Agric. Food Chem. 2016, 64, 5428–5438. [Google Scholar] [CrossRef]
- Szaloki-Dorko, L.; Steger-Mate, M.; Abranko, L. Evaluation of colouring ability of main European elderberry (Sambucus nigra L.) varieties as potential resources of natural food colourants. Int. J. Food Sci. Technol. 2015, 50, 1317–1323. [Google Scholar] [CrossRef]
- Salvador, A.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crops Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Akbulut, M.; Ercisli, S.; Tosun, M. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacogn. Mag. 2009, 5, 320–323. [Google Scholar]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmianski, J.; Skupien, K. Chemical composition, phenolics, and firmness of small black fruits. J. Appl. Bot. Food Qual. 2009, 83, 64–69. [Google Scholar]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Anton, A.M.; Pintea, A.M.; Rugina, D.O.; Sconta, Z.M.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary studies on the chemical characterization and antioxidant capacity of polyphenols from Sambucus sp. Dig. J. Nanomater. Biostructures 2013, 8, 973–980. [Google Scholar]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambuclus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgen, M.; Scheerens, J.C.; Reese, R.N.; Miller, R.A. Total phenolic, anthocyanin contents and antioxidant capacity of selected elderberry (Sambucus canadensis L.) accessions. Pharmacogn. Mag. 2010, 6, 198–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins-Veazie, P.; Thomas, A.L.; Byers, P.L.; Finn, C.E. Fruit composition of elderberry (Sambucus spp.) genotypes grown in oregon and missouri, USA. In Proceedings of the Acta Horticulturae, I International Symposium on Elderberry, Columbia, MO, USA, 9–14 June 2015; Thomas, A.L., Ed.; International Society for Horticultural Science: Korbeek-Lo, Belgium, 2015; pp. 219–224. [Google Scholar]
- Mudge, E.; Applequist, W.L.; Finley, J.; Lister, P.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J. Food Compos. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Dudonne, S.; Dube, P.; Anhe, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Harris, C.; Dewailly, E.; Desjardins, Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. J. Food Compos. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Rimpapa, Z.; Toromanovic, J.; Tahirovic, I.; Šapčanin, A.; Sofic, E. Total content of phenols and anthocyanins in edible fruits from Bosnia. Bosnian J. Basic Med. Sci. 2007, 7, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, C.; Rea, P.; Lynch, M.E.; Blumenthal, M. Herbal supplement sales rise in all channels in 2009. HerbalGram 2010, 86, 62–65. [Google Scholar]
- Milena, V.; Tatjana, M.; Gökhan, Z.; Ivana, B.; Aleksandra, C.; Mohammad, M.F.; Marija, R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crops Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.-L.; Li, J.-Y.; Liang, Y.-J.; Yang, R.-Q.; Liu, J.-Y.; Ma, Z.; Wu, L. Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotechnol. Biotechnol. Equip. 2018, 32, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Duymus, H.G.; Goger, F.; Baser, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Imenšek, N.; Ivančič, A.; Kraner-Šumenjak, T.; Islamčević-Razboršek, M.; Kristl, J. The effect of maturation on chemical composition and harvest of fruits of diverse elderberry interspecific hybrids. Eur. J. Hortic.Sci. 2021, in press. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4.5. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 30 April 2021).
- Mahmood, T.; Anwar, F.; Bhatti, I.A.; Iqbal, T. Effect of maturity on proximate composition, phenolics and antioxidant attributes of cherry fruit. Pak. J. Bot 2013, 45, 909–914. [Google Scholar]
- Shin, G.R.; Lee, S.; Lee, S.; Do, S.-G.; Shin, E.; Lee, C.H. Maturity stage-specific metabolite profiling of Cudrania tricuspidata and its correlation with antioxidant activity. Ind. Crops Prod. 2015, 70, 322–331. [Google Scholar] [CrossRef]
- Lee, M.Y.; Seo, H.S.; Singh, D.; Lee, S.J.; Lee, C.H. Unraveling dynamic metabolomes underlying different maturation stages of berries harvested from Panax ginseng. J. Ginseng Res. 2020, 44, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Veberič, R.; Stampar, F.; Koron, D. Quality parameters of black and red currants during ripening. In Proceedings of the Acta Horticulture, III Balkan Symposium on Fruit Growing, Belgrade, Serbia, 16–18 September 2015; Milatović, D., Ed.; International Society for Horticultural Science: Korbeek-Lo, Belgium, 2016; pp. 651–656. [Google Scholar]
- Acosta-Montoya, Ó.; Vaillant, F.; Cozzano, S.; Mertz, C.; Pérez, A.M.; Castro, M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010, 119, 1497–1501. [Google Scholar] [CrossRef]
- Josiane, K.R.; Glenise, B.V.; Rui Carlos, Z. Influence of the degree of maturation on the bioactive compounds in blackberry (Rubus spp.) cv. Tupy. Food Nutr. Sci. 2012, 3, 1453–1460. [Google Scholar]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Csorba, V.; Magdolna, T.; Laszlo, A.M.; Kardos, L.; Kovacs, S. Cultivar and year effects on the chemical composition of elderberry (Sambucus nigra L.) fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 770–782. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. J. Food Compos. Anal. 2004, 17, 407–422. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Andrade, P.B.; Valentão, P.; Romano, A. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Jiménez, N.; Carrillo-Hormaza, L.; Pujol, A.; Álzate, F.; Osorio, E.; Lara-Guzman, O. Antioxidant capacity and phenolic content of commonly used anti-inflammatory medicinal plants in Colombia. Ind. Crops Prod. 2015, 70, 272–279. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar] [PubMed]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Kim, D.-O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.; Talcott, S.T.; Goodner, K.; Baldwin, E.A. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]

| DPPH | ABTS | FRAP | ORAC | TP | |
|---|---|---|---|---|---|
| ABTS | 0.633 ** | ||||
| FRAP | 0.529 * | 0.698 ** | |||
| ORAC | 0.524 * | 0.851 ** | 0.576 ** | ||
| TP | 0.070 | 0.259 | 0.734 ** | 0.254 | |
| TAA | 0.409 | 0.594 ** | 0.352 | 0.286 | 0.183 |
| DPPH | ABTS | FRAP | ORAC | TP | |
|---|---|---|---|---|---|
| ABTS | 0.764 ** | ||||
| FRAP | 0.832 ** | 0.885 ** | |||
| ORAC | 0.621 ** | 0.720 ** | 0.868 ** | ||
| TP | 0.544 * | 0.641 ** | 0.635 ** | 0.626 ** | |
| TAA | 0.385 | 0.214 | 0.348 | 0.377 | 0.023 |
| Group | DPPH | FRAP | ORAC | ABTS | TP | TAA |
|---|---|---|---|---|---|---|
| 1 | 96 ± 14 | 208 ± 25 | 527 ± 60 | 130 ± 14 | 1130 ± 81 | 158 ± 59 |
| 2 | 100 ± 16 | 214 ± 33 | 589 ± 89 | 124 ±13 | 2801 ± 246 | 170 ±45 |
| 3 | 66 ± 21 | 172 ± 26 | 450 ± 24 | 106 ± 25 | 1762 ± 76 | 205 ±75 |
| 4 | 73 ± 19 | 156 ± 36 | 463 ± 59 | 98 ± 17 | 1361 ± 170 | 183 ± 125 |
| UR | DPPH | ABTS | FRAP | ORAC | TP | TAA | |
|---|---|---|---|---|---|---|---|
| FM | |||||||
| DPPH | 0.384 | ||||||
| ABTS | 0.300 | ||||||
| FRAP | 0.232 | ||||||
| ORAC | −0.155 | ||||||
| TP | 0.265 | ||||||
| TAA | 0.827 ** | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imenšek, N.; Kristl, J.; Kraner Šumenjak, T.; Ivančič, A. Antioxidant Activity of Elderberry Fruits during Maturation. Agriculture 2021, 11, 555. https://doi.org/10.3390/agriculture11060555
Imenšek N, Kristl J, Kraner Šumenjak T, Ivančič A. Antioxidant Activity of Elderberry Fruits during Maturation. Agriculture. 2021; 11(6):555. https://doi.org/10.3390/agriculture11060555
Chicago/Turabian StyleImenšek, Nataša, Janja Kristl, Tadeja Kraner Šumenjak, and Anton Ivančič. 2021. "Antioxidant Activity of Elderberry Fruits during Maturation" Agriculture 11, no. 6: 555. https://doi.org/10.3390/agriculture11060555





