Impacts of Cd on Temporal Dynamics of Nutrient Distribution Pattern of Bletilla striata, a Traditional Chinese Medicine Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Growth Parameter Measurement
2.3. Data Calculation and Analysis
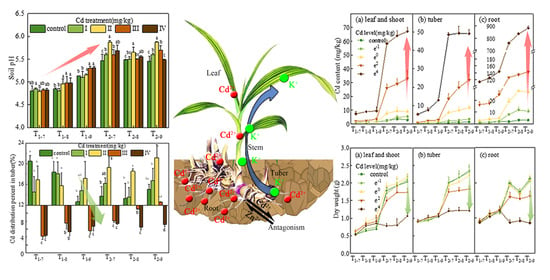
3. Result
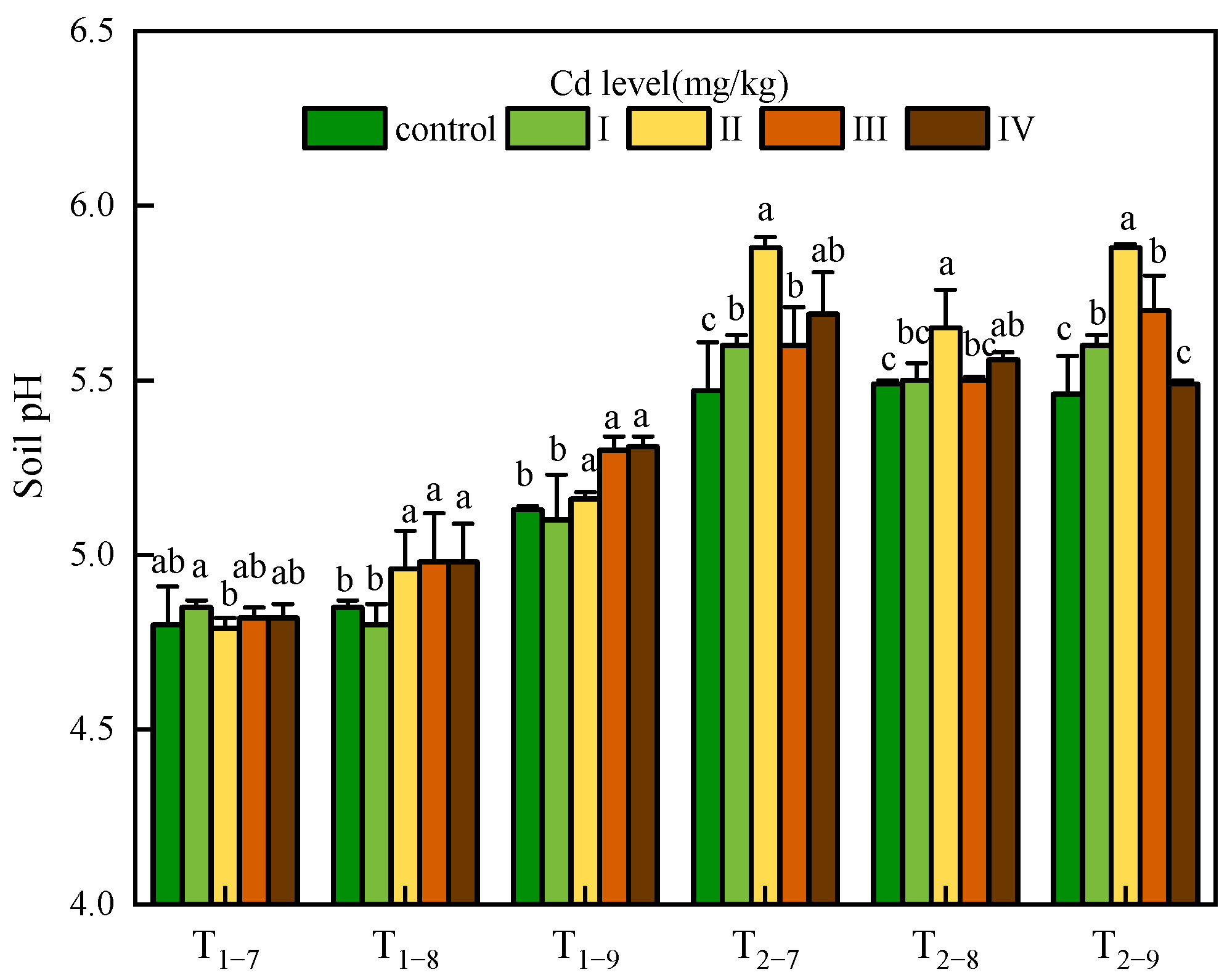
3.1. Soil pH and Available Cd Content
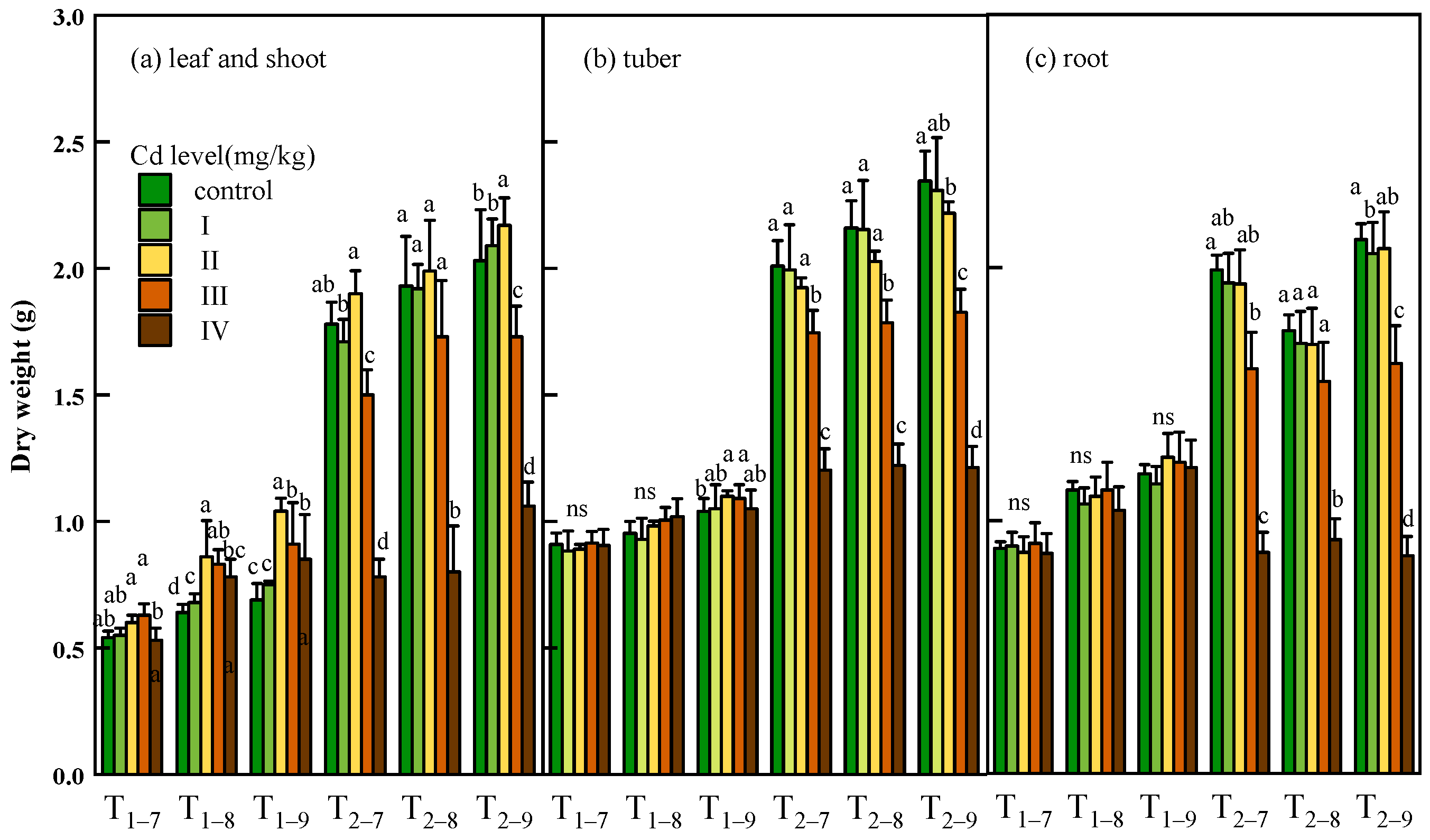
3.2. Effect of Cd on Plant Growth
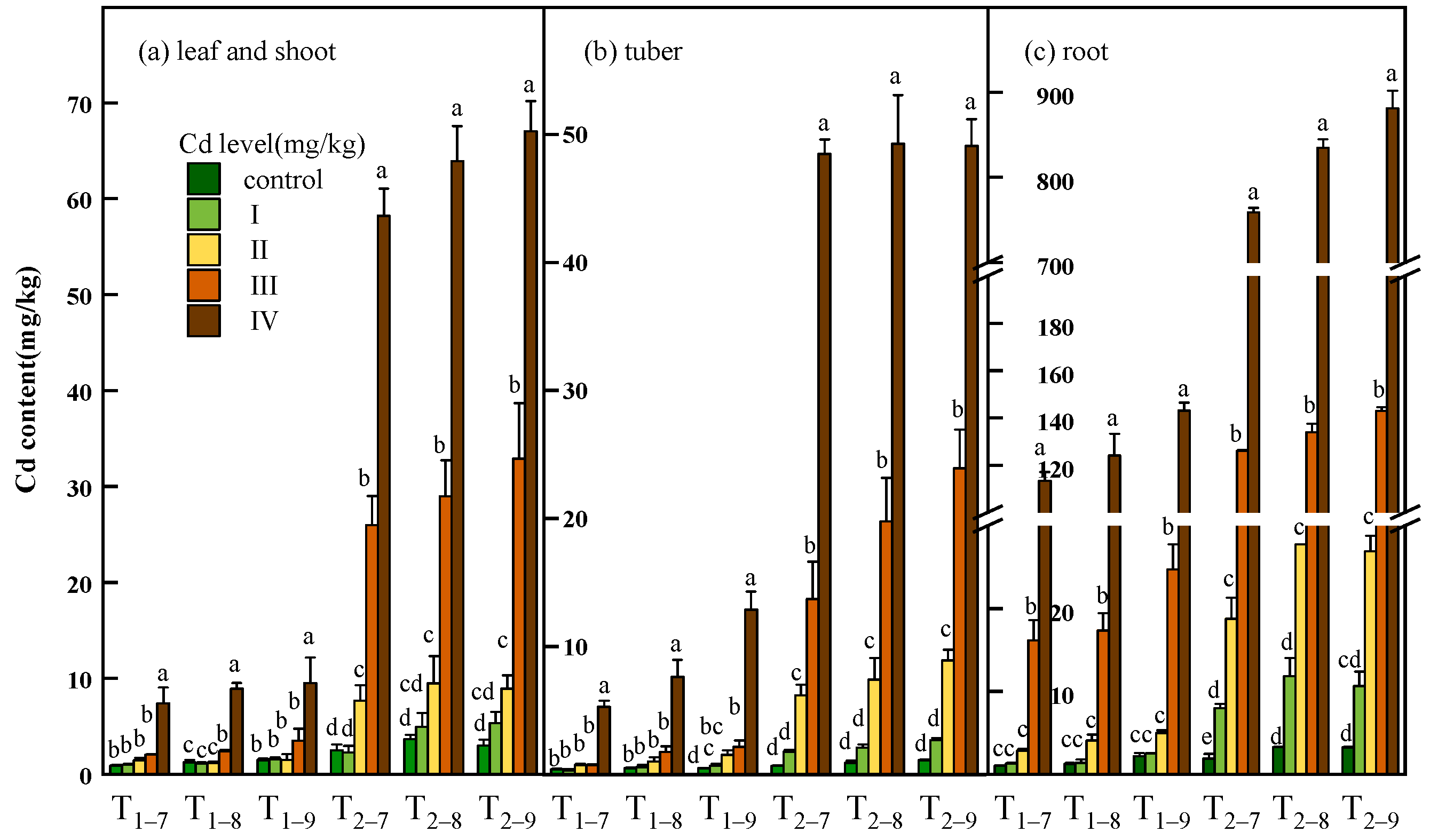
3.3. Cd Content, Accumulation and Distribution
3.4. Effect of Cd Exposure on Nutrient Distribution Pattern
3.5. Accumulation of K, Mg, Ca and Zn in Tubers of Plants
3.6. Relationship between Cd Accumulation and Nutrient Allocation Pattern
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, X.; Wang, X.; Fang, J.; Zhao, Z.; Huang, L.; Guo, H.; Zheng, X. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017, 195, 20–38. [Google Scholar] [CrossRef]
- Wang, C.; Tian, M. Seventeen novel microsatellite markers for a valuable medicinal plant Bletilla striata and cross-amplification in B. ochracea. Conserv. Genet. Resour. 2015, 7, 715–716. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.Z. Research survey and countermeasure on resources utilization in stem tuber of Bletilla striata. Chin. Tradit. Herb. Drugs 2006, 37, 1751–1755. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Shah, K.; Dubey, R.S. Cadmium elevates level of protein, amino acids and alters activity of proteolytic enzymes in germinating rice seeds. Acta Physiol. Plant. 1998, 20, 189–196. [Google Scholar] [CrossRef]
- Laporte, M.-A.; Sterckeman, T.; Dauguet, S.; Denaix, L.; Nguyen, C. Variability in cadmium and zinc shoot concentration in 14 cultivars of sunflower (Helianthus annuus L.) as related to metal uptake and partitioning. Environ. Exp. Bot. 2015, 109, 45–53. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Ma, Y.; Wang, L.; Wei, D.; Hua, L. Genotypic variations in the accumulation of Cd exhibited by different vegetables. J. Environ. Sci. 2010, 22, 1246–1252. [Google Scholar] [CrossRef]
- Tanwir, K.; Akram, M.S.; Masood, S.; Chaudhary, H.J.; Lindberg, S.; Javed, M.T. Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L.). Environ. Sci. Pollut. Res. 2015, 22, 9193–9203. [Google Scholar] [CrossRef]
- Chen, X.D.; Shan, C.J. Cerium nitrate improves salt tolerance of wheat seedlings by regulating the antioxidant capacity of chloroplasts. Biol. Plant. 2019, 63, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Tessier, J.T.; McNaughton, S.J.; Raynal, D.J. Influence of nutrient availability and tree wildling density on nutrient uptake by Oxalis acetosella and Acer saccharum. Environ. Exp. Bot. 2001, 45, 11–20. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Pellerin, S. Shoot and root growth of hydroponic maize (Zea mays L.) as influenced by K deficiency. Plant Soil 2008, 304, 157–168. [Google Scholar] [CrossRef]
- Ouzounidou, G. Copper-induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environ. Exp. Bot. 1994, 34, 165–172. [Google Scholar] [CrossRef]
- Greger, M.; Lindberg, S. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). I. Cd2+ uptake and sugar accumulation. Physiol. Plant. 1986, 66, 69–74. [Google Scholar] [CrossRef]
- Shukla, U.C.; Murthy, R.C.; Kakkar, P. Combined effect of ultraviolet-B radiation and cadmium contamination on nutrient uptake and photosynthetic pigments in Brassica campestris L. seedlings. Environ. Toxicol. 2008, 23, 712–719. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Sun, X.; Ren, W. Effects of cadmium on uptake and translocation of nutrient elements in different welsh onion (Allium fistulosum L.) cultivars. Food Chem. 2016, 194, 101–110. [Google Scholar] [CrossRef]
- Liu, J.G.; Liang, J.S.; Li, K.Q.; Zhang, Z.J.; Yu, B.Y.; Lu, X.L.; Yang, J.C.; Zhu, Q.S. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Santos, C.; Soares, A.M.V.M.; Mann, R.M. Assessment of biomarkers of cadmium stress in lettuce. Ecotoxicol. Environ. Saf. 2009, 72, 811–818. [Google Scholar] [CrossRef]
- Zorrig, W.; Rouached, A.D.; Shahzad, Z.; Abdelly, C.; Davidian, J.C.; Berthomieu, P. Identification of three relationships linking cadmium accumulation to cadmium tolerance and zinc and citrate accumulation in lettuce. J. Plant Physiol. 2010, 167, 1239–1247. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yin, H.; Liu, X.; Sun, H.; Mi, Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 2010, 326, 321–330. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Kurtyka, R.; Malkowski, E.; Kita, A.; Karcz, W. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Pol. J. Environ. Stud. 2008, 17, 51–56. Available online: https://www.researchgate.net/publication/259852721 (accessed on 12 February 2021).
- Drazic, G.; Mihailovic, N. Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci. 2005, 168, 511–517. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.; Rivera-Olivares, L.; Tejeda-Sartorius, O. Cadmium-induced changes in leaf nutrient concentrations in sugarcane. J. Food Agric. Environ. 2014, 12, 879–885. Available online: https://www.mendeley.com/catalogue/c83e97c3-0fa5-32ad-966e-79a2e3dad58c/#cited%20by-title (accessed on 8 February 2021).
- Yang, D.; Guo, Z.; Green, I.D.; Xie, D. Effect of cadmium accumulation on mineral nutrient levels in vegetable crops: Potential implications for human health. Environ. Sci. Pollut. Res. 2016, 23, 19744–19753. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.F.; Antes, F.G.; Maldaner, J.; Pereira, L.B.; Tabaldi, L.A.; Rauber, R.; Rossato, L.V.; Bisognin, D.A.; Dressler, V.L.; de Moraes Flores, É.M.; et al. Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol. Biochem. 2009, 47, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Nocito, F.F.; Espen, L.; Crema, B.; Cocucci, M.; Sacchi, G.A. Cadmium induces acidosis in maize root cells. New Phytol. 2008, 179, 700–711. [Google Scholar] [CrossRef]
- Qin, R.; Hirano, Y.; Brunner, I. Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physiol. 2007, 27, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Liu, Z.; Zeng, Q.; Zhu, M.; Wang, A.; Chen, B. Nutrient allocation might affect the cadmium accumulation of Bermuda grass (Cynodon dactylon). Chemosphere 2020, 252, 126512. [Google Scholar] [CrossRef]
- Grant, C.A.; Buckley, W.T.; Bailey, L.D.; Selles, F. Cadmium accumulation in crops. Can. J. Plant Sci. 1998, 78, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Wang, X.; Luo, Y.; Du, W.; Guo, H.; Yin, D. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 2007, 69, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; He, Y.F.; Luo, Y.M.; Yu, Y.L.; Lin, Q.; Wong, M.H. Physiological mechanism of plant roots exposed to cadmium. Chemosphere 2003, 50, 789–793. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef]
- Ghasemi, R.; Share, H.; Sharifi, R.; Boyd, R.S.; Rajakaruna, N. Inducing Ni sensitivity in the Ni hyperaccumulator plant Alyssum inflatum Nyárády (Brassicaceae) by transforming with CAX1, a vacuolar membrane calcium transporter. Ecol. Res. 2018, 33, 737–747. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Shi, J. Plant Resources and Utilization of Bletilla striata. Master’s Thesis, University of Hainan, Haikou, China, 2010; pp. 2–36. [Google Scholar]
- Huang, Y.; Zhu, Z.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Lower cadmium accumulation and higher antioxidative capacity in edible parts of Brassica campestris L. seedlings applied with glutathione under cadmium toxicity. Environ. Sci. Pollut. Res. 2019, 26, 13235–13245. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Wang, Q.; Luo, S.; Chen, B.; Khan, K.Y.; Yang, X.; Feng, Y. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 2016, 154, 358–366. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Wang, L.; Li, Y.C.; Tong, Z.; Huo, W.; Chi, K.; Fan, H. Cadmium absorption and translocation of amaranth (Amaranthus mangostanus L.) affected by iron deficiency. Environ. Pollut. 2020, 256, 113410. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shang, S.; Feng, X.; Zhang, G.; Wu, F. Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ. Toxicol. Chem. 2015, 34, 92–99. [Google Scholar] [CrossRef]
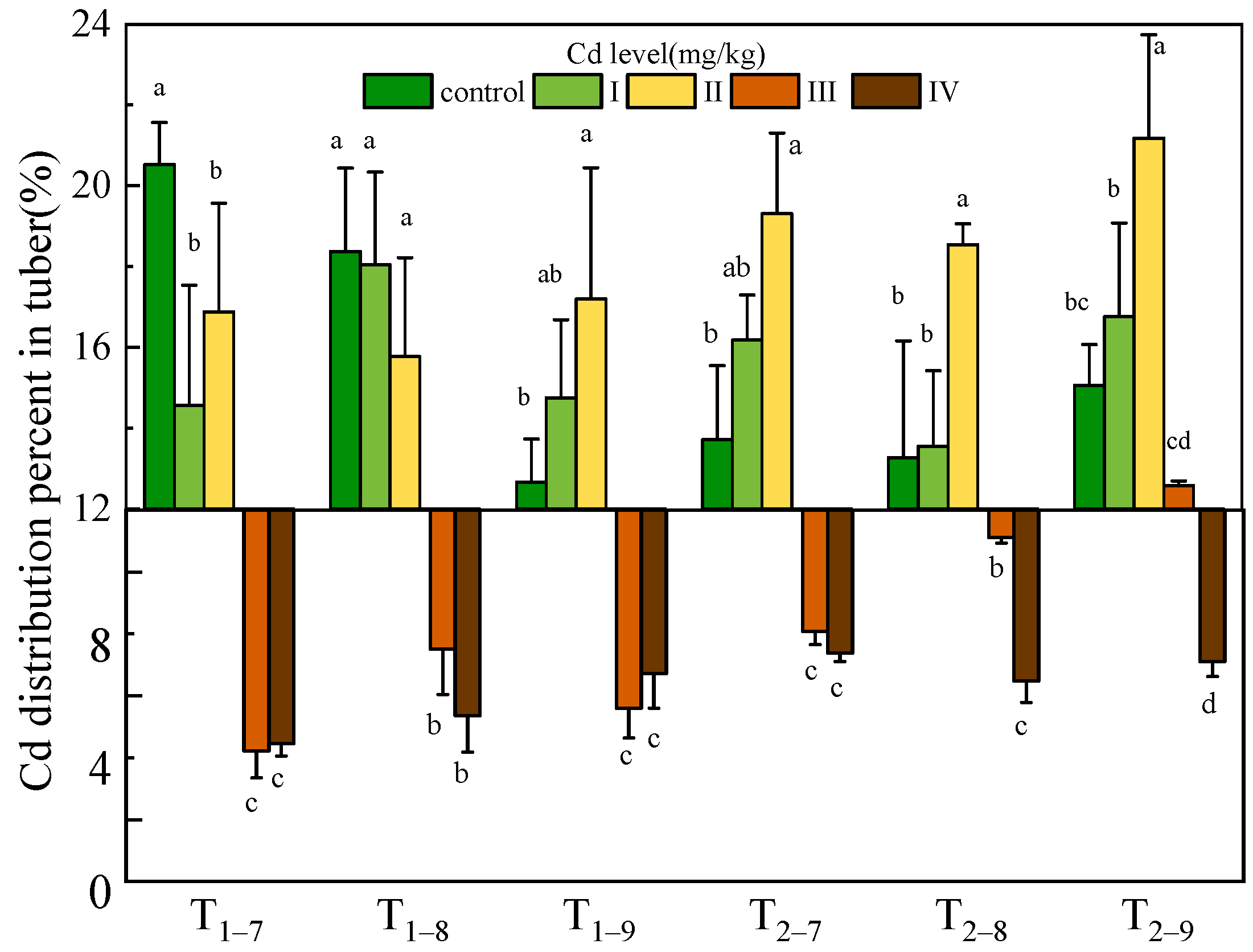
| Cd Treatment | T1–7 | T1–8 | T1–9 | T2–7 | T2–8 | T2–9 | |
|---|---|---|---|---|---|---|---|
| control | 0.49 ± 0.02 c | 0.48 ± 0.02 d | 0.61 ± 0.01 c | 1.12 ± 0.01 c | 1.15 ± 0.04 d | 0.86 ± 0.03 d | |
| available Cd (mg/kg) | I | 0.88 ± 0.03 c | 0.57 ± 0.03 d | 1.21 ± 0.01 c | 1.23 ± 0.02 c | 1.77 ± 0.43 c | 1.44 ± 0.05 d |
| II | 1.05 ± 0.09 c | 1.38 ± 0.07 c | 1.86 ± 0.00 c | 1.97 ± 0.08 c | 1.89 ± 0.32 c | 1.71 ± 0.07 c | |
| III | 4.59 ± 0.02 b | 7.33 ± 0.04 b | 11.02 ± 0.53 b | 11.92 ± 2.42 b | 14.83 ± 2.43 b | 10.31 ± 0.35 b | |
| IV | 49.44 ± 0.85 a | 54.24 ± 1.53 a | 54.26 ± 3.53 a | 61.42 ± 5.63 a | 68.96 ± 6.42 a | 59.16 ± 4.53 a |
| Pearson | K1 | M1 | C1 | Z1 | TFK1 | TFM1 | TFC1 | TFZ1 | K2 | M2 | C2 | Z2 | TFK2 | TFM2 | TFC2 | TFZ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AccumuT1 | 0.404 ** | −0.024 | 0.009 | 0.136 | 0.572 ** | 0.016 | −0.125 | 0.435 ** | −0.199 | −0.439 ** | −0.583 ** | −0.111 | 0.447 ** | −0.112 | −0.317 * | 0.109 |
| AccumuR1 | 0.303 * | −0.062 | −0.058 | 0.067 | 0.566 ** | 0.015 | −0.114 | 0.405 ** | −0.293 | −0.543 ** | −0.686 ** | −0.198 | 0.635 ** | 0.015 | −0.371 * | 0.186 |
| TFCd1 | 0.132 | 0.046 | 0.022 | 0.024 | −0.199 | 0.041 | −0.007 | −0.437 ** | 0.265 | 0.485 ** | 0.616 ** | 0.211 | −0.589 ** | −0.144 | 0.261 | −0.569 ** |
| AccumuT2 | 0.095 | 0.063 | 0.02 | 0.106 | 0.437 ** | 0.113 | 0.008 | 0.545 ** | −0.153 | −0.517 ** | −0.705 ** | −0.142 | 0.689 ** | 0.049 | −0.365 * | 0.298 * |
| AccumuR2 | 0.145 | −0.099 | −0.082 | −0.047 | 0.410 ** | −0.044 | −0.122 | 0.409 ** | −0.357 * | −0.590 ** | −0.772 ** | −0.27 | 0.760 ** | 0.125 | −0.419 ** | 0.323 * |
| TFCd2 | 0.045 | 0.212 | 0.066 | 0.14 | −0.284 | 0.178 | 0.057 | −0.296 * | 0.286 | 0.373 * | 0.738 ** | 0.158 | −0.665 ** | −0.249 | 0.571 ** | −0.499 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, H.; Qin, J.; Wang, X.; Chen, W. Impacts of Cd on Temporal Dynamics of Nutrient Distribution Pattern of Bletilla striata, a Traditional Chinese Medicine Plant. Agriculture 2021, 11, 594. https://doi.org/10.3390/agriculture11070594
Yang J, Sun H, Qin J, Wang X, Chen W. Impacts of Cd on Temporal Dynamics of Nutrient Distribution Pattern of Bletilla striata, a Traditional Chinese Medicine Plant. Agriculture. 2021; 11(7):594. https://doi.org/10.3390/agriculture11070594
Chicago/Turabian StyleYang, Jiyuan, Hui Sun, Jihong Qin, Xiaoqin Wang, and Wenqing Chen. 2021. "Impacts of Cd on Temporal Dynamics of Nutrient Distribution Pattern of Bletilla striata, a Traditional Chinese Medicine Plant" Agriculture 11, no. 7: 594. https://doi.org/10.3390/agriculture11070594
APA StyleYang, J., Sun, H., Qin, J., Wang, X., & Chen, W. (2021). Impacts of Cd on Temporal Dynamics of Nutrient Distribution Pattern of Bletilla striata, a Traditional Chinese Medicine Plant. Agriculture, 11(7), 594. https://doi.org/10.3390/agriculture11070594