Effects of Water Stress and Auxin Application on Growth and Yield of Two Sugarcane Cultivars under Greenhouse Conditions
Abstract
:1. Introduction
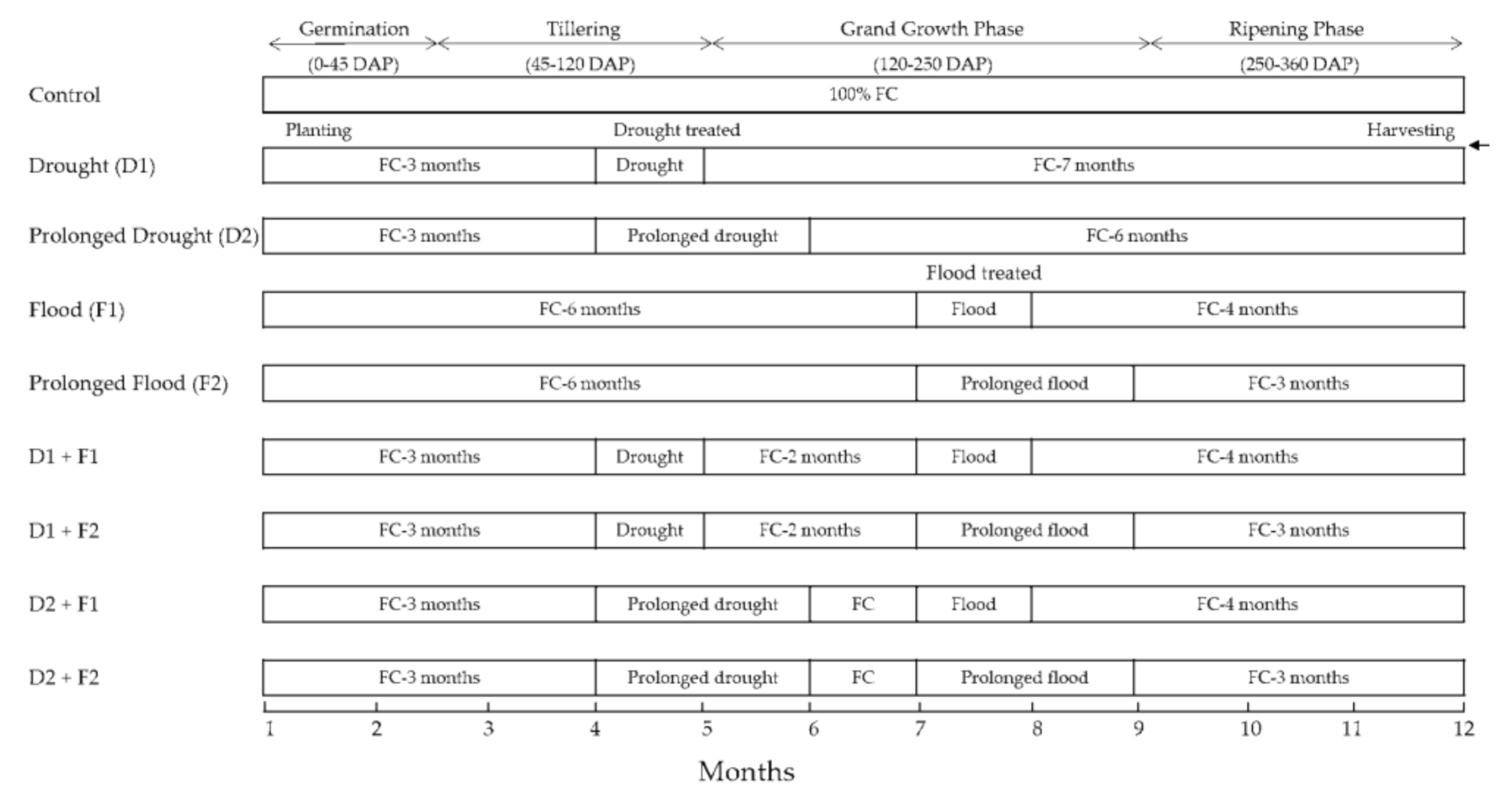
2. Materials and Methods
2.1. Cultural Detailed
2.2. Measurement
2.2.1. Soil Property
2.2.2. Physiological Characters
2.2.3. Root Growth
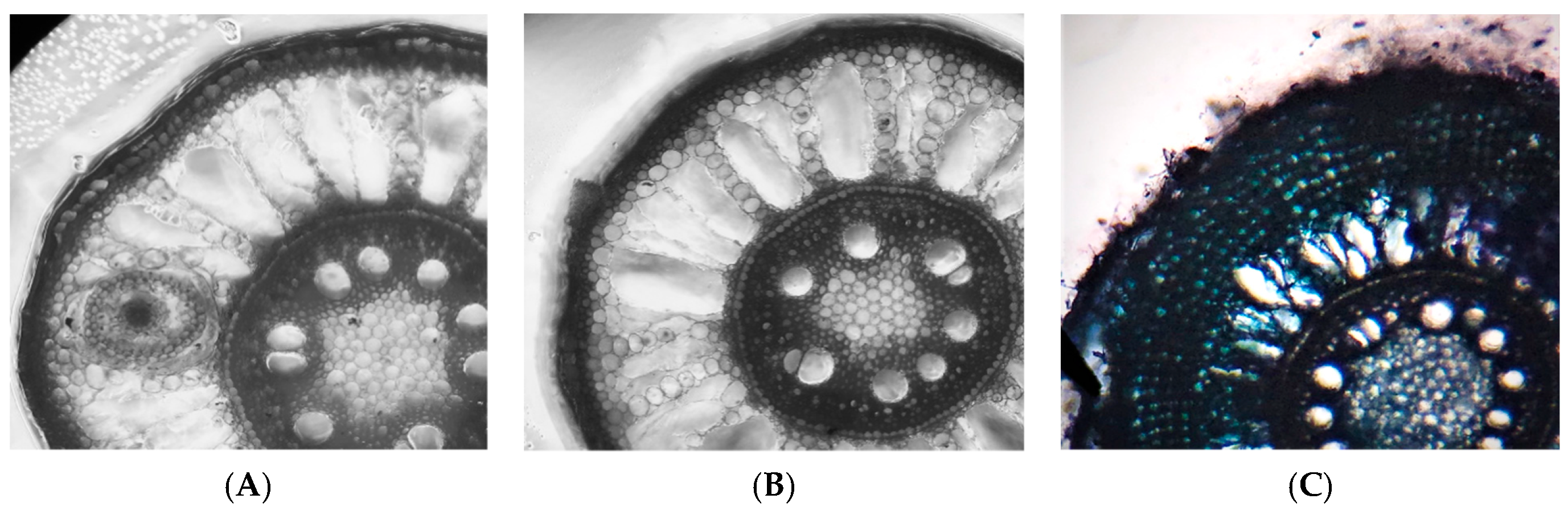
2.2.4. Aerenchyma Formation
2.2.5. Shoot Growth and Sugar Quality
2.3. Statistical Analysis
3. Results
3.1. Physiological Characters Performance
3.1.1. Stomatal Conductance
3.1.2. SPAD Chlorophyll Meter Reading (SCMR)
3.1.3. Chlorophyll a Fluorescence
3.2. Root Growth and Development
3.3. Shoot Growth and Yield
3.4. Sugar Quality
3.5. Adventitious Root Development
3.6. Aerenchyma Formation
3.7. Growth Parameters and Stayed Green Leaf Performance
3.8. Sugar Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, E.A. Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ. 2002, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tollennar, M.; Aguilera, A. Radiation use efficiency of an old and a new maize hybrid. Agron. J. 1992, 84, 536–541. [Google Scholar] [CrossRef]
- Qing, Z.M.; Jing, L.G.; Kai, C.R. Photosynthesis characteristics in eleven cultivars of sugarcane and their responses to water stress during the elongation stage. Proc. Int. Society Sugar Cane Technol. 2011, 24, 642–643. [Google Scholar]
- Ramesh, P.; Mahadevaswamy, M.; Ramesh, P.; Mahadevaswamy, M. Effect of formative Phase drought on different classes of shoots, shoot mortality cane attributes yield and quality of four sugarcane cultivars. J. Agron. Crop. Sci. 2000, 185, 249–258. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Davies, W.J.; Wilkinson, S.; Loveys, B. Stomatal control by chemical signaling and the exploitation of this mechanism to increase water use efficiency in agriculture. N. Phytol. 2002, 153, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of water stress on leaf area development, photosynthesis, and productivity in Chardonnay and Airén grapevines. Am. J. Enol. Viticult. 2002, 53, 138–143. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006; ISBN 0-87893-856-8. [Google Scholar]
- Jangpromma, N.; Thammasirirak, S.; Jaisil, P.; Songsri, P. Effects of drought and recovery from drought stress on above ground and root growth, and water use efficiency in sugarcane (Saccharum officinarum L.). Aust. J. Crop. Sci. 2012, 6, 1298–1304. [Google Scholar]
- Da Graça, J.P.; Rodrigues, F.A.; Farias, J.R.B.; De Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar]
- Silva, M.D.A.; Jifon, J.L.; Da Silva, J.A.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, R.; Ou, H.; Gui, Y.; Wei, J.; Zhou, H.; Tan, H.; Li, Y. Comprehensive transcriptome analysis reveals genes in response to water deficit in the leaves of Saccharum narenga (Nees ex Steud.) hack. BMC Plant Biol. 2018, 18, 250. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 1–25. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Brestic, M.; Landi, M.; Skalicky, M. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, A.; Pandey, N.; Singh, S. Assessment of waterlogging induced physio-biochemical changes in sugarcane varieties and its association with waterlogging tolerance. J. Environ. Biol. 2019, 40, 384–392. [Google Scholar] [CrossRef]
- Bradford, K.J. Effect of soil flooding on leaf gas exchange of tomato plants. Plant Physiol. 1983, 73, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Pezeshki, S.R.; Anderson, P.H. Responses of three bottomland species with different flood tolerance capabilities to various flooding regimes. Wetl. Ecol. Manag. 1996, 4, 245–256. [Google Scholar] [CrossRef]
- Tamang, B.G.; Li, S.; Rajasundaram, D.; Lamichhane, S.; Fukao, T. Overlapping and stress-specific transcriptomic and hormonal responses to flooding and drought in soybean. Plant J. 2021. [Google Scholar] [CrossRef]
- Sugarcane Breeding Institute. Effect of Waterlogging in Sugarcane and Its Management; Abi Print: Coimbatore, India, 2010; p. 18. [Google Scholar]
- Ezint, V.; De la Pena, R.; Ahanchede, A. Flooding Tolerance of Tomato Genotypes During Vegetative and Reproductive Stages. Braz. J. Plant. Physiol. 2010, 9, 1665–1678. [Google Scholar]
- Gomathi, R.; Gururaja Rao, P.N.; Chandran, K. Adaptive Responses of Sugarcane to Flooding Stress: An Over View. Sugar Tech. 2015, 17, 325–338. [Google Scholar] [CrossRef]
- Gilbert, R.A.; Rainbolt, C.R.; Morris, D.R.; Bennett, A.C. Morphological Responses of Sugarcane to Long-Term Flooding. Agron. J. 2007, 99, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Worasant, U. Effect of waterlogged conditions and growth, yield and quality of sugarcane. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Plant Science, Graduate School, Khon Kaen University, Khon Kaen, Thailand, 2010. [Google Scholar]
- Gomathi, R.; Chandran, K. Effect of flooding on growth and yield of sugarcane clones. Sugarcane Breeding Institute (SBI-ICAR). Q. News Lett. 2009, 29, 1–2. [Google Scholar]
- Gomathi, R.; Chandran, K.; Gururaja Rao, P.N.; Rakkiyappan, P. Effect of waterlogging in sugarcane and its management. Published by The Director, Sugarcane Breeding Institute (SBI-ICAR). Extension Pub: Coimbatore, India; p. 185.
- Avivi, S.; Soeparjono, S.; Slameto; Ramadhan, R.A. Physiological Characters of Sugarcane after Flooding Stress. Agric. Agric. Sci. Procedia 2016, 9, 31–39. [Google Scholar] [CrossRef]
- Begum, M.K.; Alam, M.R.; Islam, M.S. Adaptive mechanisms of sugarcane genotypes under flood stress condition. World J. Agric. Sci. 2013, 1, 56–64. [Google Scholar]
- Kozlowski, T.; Pallardy, S.G. Effects of flooding on water, carbohydrate and mineral relations. In Flooding and Plant Growth; Kozlowski, T.T., Ed.; Academic Press Inc.: Orlando, FL, USA, 1984; pp. 165–193. [Google Scholar]
- Jackson, M.B.; Drew, M.C.; Giffard, S.C. Effects of applying ethylene to the root system of Zea mays on growth and nutrient concentration in relation to flooding. Physiol. Plant 1981, 52, 23–28. [Google Scholar] [CrossRef]
- Tsukuhara, H.; Kozlowski, T.T. Importance of adventitious roots to growth of flooded Platanus occidental seedlings. Plant Soil 1985, 88, 123–132. [Google Scholar] [CrossRef]
- Drew, M.C. Soil aeration and plant root metabolism. Soil Sci. 1992, 154, 259–268. [Google Scholar] [CrossRef]
- Jaipong, T.; Tominaga, J.; Watanabe, K.; Nakabaru, M.; Takaragawa, H.; Suwa, R.; Ueno, M.; Kawamitsu, Y. Effects of duration and combination of drought and flood conditions on leaf photosynthesis, growth and sugar content in sugarcane. Plant Prod. Sci. 2016, 19, 427–437. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Heijink, C.J.; van Hout, K.J.G.M.; Voesenek, L.A.C.J.; Barendse, G.W.M.; Blom, C.W.P.M. Regulatory role of auxin in adventitious root formation in two species of Rumex, differing in their sensitivity to flooding. Physiol. Plant 1995, 93, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.O.; Bradford, K.J. Ethylene synthesis and growth of tomato hypocotyls: Induction by auxin and fusicoccin and inhibition by vanadate. J. Plant Growth Regul. 1990, 9, 43. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Cohen, J.D.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L.A.C.J. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Lakshmanan, P.; He, W.; Tan, H.; Liu, L.; Liu, H.; Liu, J.; Huang, D.; Chen, Z. Transcriptome profiling provided molecular insights into auxin-induced adventitious root formation in sugarcane (Saccharum spp.). Interspecific hybrids) microshoot. Plants 2020, 9, 931. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Vachova, P.; Hajihashemi, S.; Martinkova, J.; Hejnak, V. Effect of Gibberellic Acid on Growing-Point Development of Non-Vernalized Wheat Plants under Long-Day Conditions. Plants 2020, 9, 1735. [Google Scholar] [CrossRef]
- Hidaka, T.; Karim, M.A. Flooding tolerance of sugarcane in relation to growth, physiology and root structure. S. Pac. Stud. 2007, 28, 9–22. [Google Scholar]
- Mano, Y.; Omori, F.; Takamizo, T.; Kindinger, B.; Bird, R.M.; Laoisigi, C.H. Variation of root aerenchyma formation in flooded and non-flooded maize and Teosinte seedings. Plant Soil 2006, 281, 269–279. [Google Scholar] [CrossRef]
- Pardales, J.R., Jr.; Kono, Y.; Yamauchi, A. Response of the different root system components of sorghum to incidence of waterlogging. Environ. Exp. Bot. 1991, 31, 107–115. [Google Scholar] [CrossRef]
- Samanhudi. Pengujian Cepat Ketahanan Tanaman Sorgum Manis Terhadap Cekaman Kekeringan. Agrosains 2010, 12, 9–13. [Google Scholar]
- Inman-Bamber, N.; Smith, D. Water relations in sugarcane and response to droughts. Field Crops Res. 2005, 92, 185–202. [Google Scholar] [CrossRef]
- Endres, L.; Silva, J.V.; Ferreira, V.M.; Barbosa, G.V.S. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. J. 2010, 11, 31–37. [Google Scholar] [CrossRef]
- Du, Y.; Kawamitsu, Y.; Nose, A.; Hiyane, S.; Murayama, S.; Wasano, K. Effects of water stress on carbon exchange rate and activities of photosynthetic enzymes in leaves of sugarcane (Saccharum sp.). Aust. J. Plant Physiol. 1996, 23, 719–726. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Gonkhamdee, S.; Jongrungklang, N. Understanding drought responses of sugarcane cultivars controlled under low water potential conditions. Chil. J. Agric Res. 2020, 80, 370–380. [Google Scholar] [CrossRef]
- Marquard, R.D.; Tipton, J.L. Relationship between extractable chlorophyll and an In Situ method to estimate leaf greenness. Hort Sci. 1987, 22, 1327. [Google Scholar]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Rong-hua, L.; Pei-guo, G.; Baum, M.; Grando, S.; Ceccarell, S. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric Sci. China 2006, 5, 751–757. [Google Scholar]
- Ecco, M.; Santiago, E.F.; Lima, P.R. Chlorophyll a fluorescence in two varieties of sugarcane subjected to aluminum and water stress. Afr. J. Agric Res 2013, 8, 4941–4948. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Ray, C.J.; Noble, R. Effects of flooding on young sugarcane plants. Aust. Sugarcane 1998, 2, 28–30. [Google Scholar]
- Robertson, M.J.; Inman-Bamber, N.G.; Muchow, R.C.; Wood, A.W. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crop. Res. 1999, 64, 211–227. [Google Scholar] [CrossRef]
- Gentile, A.; Dias, L.I.; Mattos, R.S.; Ferreira, T.H.; Menossi, M. MicroRNAs and drought responses in sugarcane. Front. Plant Sci. 2015, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, P.W.; Hitchcock, A.E. Substituted Phenoxy and Benzoic acid Growth Substances and the Relation of Structure to Physiological Activity. Contributions of the Boyce Thompson Institute: Ithaca, NY, USA, 1942; p. 12. [Google Scholar]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-Induced Adventitious Root Formation in Nodal Cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.W.; Hall, W.C. Effect of 2,4-dichlorophenoxyacetic acid on the production of ethylene by cotton and grain sorghum. Physiol. Plant 1962, 15, 420–427. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Niuzi, N.K. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2017, 7, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; White, J.C.; Zhao, J.; Zhao, Q.; Xing, B. Uptake of engineered nanoparticles by food crops: Characterization, mechanisms, and implications. Annu. Rev. Food Sci. Technol. 2018, 9, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Yamauchi, A.; Suralta, R.R. Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environ. Exp. Bot. 2008, 64, 75–82. [Google Scholar]
- Tavares, E.Q.P.; Grandis, A.; Lembke, C.G.; Souza, G.M.; Purgatto, E.; De Souza, A.P.; Buckeridge, M.S. Roles of auxin and ethylene in aerenchyma formation in sugarcane roots. Plant Signal. Behav. 2018, 13, e1422464. [Google Scholar] [CrossRef]
- Colmer, T.D.; Greenway, H. Oxygen transport, respiration and anaerobic carbohydrate catabolism in roots in flooded soil. In Plant Respiration: From Cell to Ecosystem; Lambers, H., Rivas-Carbo, M., Eds.; Springer: Heidelberg, Germany; pp. 137–158.

| Cultivar | Water Regime | Stomatal Conductance (μmol/m2/s) | |||
|---|---|---|---|---|---|
| 1 DAT | 2 DAT | 4 DAT | 8 DAT | ||
| KK3 | Control | 274.5ab | 342.2 | 299.73 | 285.8ab |
| Drought (D1) | 114.7c | 234.3 | 237.94 | 195.2abc | |
| Prolonged Drought (D2) | 222.4bc | 474.9 | 228.75 | 286.4a | |
| Flood (F1) | 288.5a | 210.4 | 282 | 255.6ab | |
| Prolonged Flood (F2) | 167.2c | 182.9 | 177.43 | 176.4bc | |
| D1 + F1 | 169.4c | 155.8 | 119.2 | 219.7abc | |
| D1 + F2 | 241.6ab | 267.2 | 285.36 | 253.6ab | |
| D2 + F1 | 246.0ab | 279.9 | 248.72 | 147.1c | |
| D2 + F2 | 173.0c | 266.9 | 336.72 | 170.1bc | |
| F-test | * | ns | ns | * | |
| CV (%) | 18.9 | 36.7 | 30.1 | 21.62 | |
| K93-219 | Control | 269.1 | 309.8ab | 229.7 | 350.4a |
| Drought (D1) | 152.7 | 195.3abc | 219.1 | 200.0c | |
| Prolonged Drought (D2) | 200.4 | 295.5ab | 230.0 | 166.9c | |
| Flood (F1) | 182.5 | 288.6ab | 345.6 | 250.0bc | |
| Prolonged Flood (F2) | 136.7 | 162.3c | 152.4 | 172.7c | |
| D1 + F1 | 149.8 | 186.9bc | 210.4 | 179.9c | |
| D1 + F2 | 207.9 | 341.2a | 216.0 | 189.4c | |
| D2 + F1 | 263.7 | 314.07ab | 287.4 | 299.5ab | |
| D2 + F2 | 220.0 | 195.3abc | 246.0 | 151.6c | |
| F-test | Ns | * | ns | * | |
| CV (%) | 32 | 15.1 | 24.82 | 20.7 | |
| Cultivar | Water Regime | SCMR | ||||
|---|---|---|---|---|---|---|
| 3 MOA | 5 MOA | 7 MOA | 9 MOA | Harvest | ||
| KK3 | Control | 43.2 | 61.5 | 74.1 | 67.8 | 32.6 |
| Drought (D1) | 45.5 | 52.6 | 66.3 | 59.5 | 22.3 | |
| Prolonged Drought (D2) | 47.4 | 78.4 | 80.2 | 79.3 | 24.0 | |
| Flood (F1) | 44.0 | 71.7 | 59.0 | 65.3 | 26.5 | |
| Prolonged Flood (F2) | 48.2 | 52.9 | 69.4 | 61.2 | 31.9 | |
| D1 + F1 | 43.0 | 32.4 | 44.0 | 38.2 | 32.7 | |
| D1 + F2 | 47.5 | 92.9 | 81.1 | 87.0 | 23.3 | |
| D2 + F1 | 47.9 | 58.4 | 65.9 | 62.2 | 32.0 | |
| D2 + F2 | 43.5 | 71.9 | 82.5 | 77.2 | 28.1 | |
| F-test | Ns | ns | ns | Ns | ns | |
| CV (%) | 12.9 | 27.5 | 30.3 | 26.3 | 21.3 | |
| K93-219 | Control | 55.1 | 92.1 | 87.0 | 89.5 | 60.6 |
| Drought (D1) | 42.8 | 74.0 | 91.8 | 82.9 | 32.1 | |
| Prolonged Drought (D2) | 45.5 | 65.0 | 76.3 | 70.6 | 68.5 | |
| Flood (F1) | 51.4 | 85.3 | 88.3 | 86.7 | 35.4 | |
| Prolonged Flood (F2) | 49.4 | 94.1 | 85.3 | 89.7 | 32.9 | |
| D1 + F1 | 47.3 | 88.3 | 92.9 | 90.6 | 31.5 | |
| D1 + F2 | 50.1 | 68.8 | 85.8 | 77.2 | 33.9 | |
| D2 + F1 | 48.6 | 83.8 | 76.3 | 80.5 | 34.8 | |
| D2 + F2 | 44.5 | 90.6 | 88.5 | 89.5 | 49.5 | |
| F-test | Ns | ns | ns | Ns | ns | |
| CV (%) | 10.8 | 12.7 | 11.3 | 9 | 5.29 | |
| Cultivar | Water Regime | Chlorophyll a Fluorescence Ratio (Fv/Fm) | ||||
|---|---|---|---|---|---|---|
| 3 MOA | 5 MOA | 7 MOA | 9 MOA | Harvest | ||
| KK3 | Control | 689.6 | 792.7 | 722.5 | 819 | 780.1 |
| Drought (D1) | 632.6 | 794.6 | 738.0 | 813.3 | 779.0 | |
| Prolonged Drought (D2) | 682.6 | 802.3 | 691.0 | 799.6 | 789.3 | |
| Flood (F1) | 709.1 | 798.3 | 746.6 | 789.9 | 779.4 | |
| Prolonged Flood (F2) | 701.0 | 772.3 | 743.6 | 745.6 | 782.0 | |
| D1 + F1 | 695.3 | 788.0 | 725.8 | 798.4 | 798.2 | |
| D1 + F2 | 673.6 | 782.6 | 756.6 | 804.3 | 752.6 | |
| D2 + F1 | 681.3 | 778.3 | 727.6 | 776.6 | 769.6 | |
| D2 + F2 | 670.6 | 768.6 | 744.0 | 808.3 | 775.3 | |
| F-test | ns | Ns | ns | ns | ns | |
| CV (%) | 7.7 | 3.22 | 4.66 | 3.31 | 3.23 | |
| K93-219 | Control | 750.3a | 803.3 | 742.7 | 798.1 | 808.0 |
| Drought (D1) | 743.4ab | 737.6 | 824.5 | 836.3 | 818.6 | |
| Prolonged Drought (D2) | 584.0d | 780.7 | 734.9 | 723.4 | 748.8 | |
| Flood (F1) | 737.3abc | 796.3 | 752.0 | 764.3 | 763.3 | |
| Prolonged Flood (F2) | 684.6bc | 786.3 | 739.0 | 788.0 | 770.0 | |
| D1 + F1 | 671.8c | 774.3 | 737.2 | 783.1 | 778.5 | |
| D1 + F2 | 698.6abc | 773.5 | 745.8 | 824.1 | 769.4 | |
| D2 + F1 | 700.3abc | 761.6 | 753.0 | 809.7 | 793.3 | |
| D2 + F2 | 741.6abc | 801.9 | 811.4 | 801.7 | 786.3 | |
| F-test | * | Ns | ns | ns | ns | |
| CV (%) | 4.01 | 4.46 | 5.24 | 3.16 | 2.8 | |
| Cultivar | Water Regime | Adventitious Root (No. Plant−1) | Adventitious Root Weight (g) | Original Root Weight (g) | |||
|---|---|---|---|---|---|---|---|
| 3 DAT | 7 DAT | Fresh Weight | Dry Weight | Fresh Weight | Dry Weight | ||
| KK3 | Control | 0 | 0 | 0 | 0 | 78.7a | 19.4a |
| Drought (D1) | 0 | 0 | 0 | 0 | 40.3c | 12.5c | |
| Prolonged Drought (D2) | 0 | 0 | 0 | 0 | 34.2cd | 16.1b | |
| Flood (F1) | 20 | 60 | 28.0 | 7.4 | 64.2bc | 18.7ab | |
| Prolonged Flood (F2) | 15 | 56 | 36.0 | 6.9 | 42.6c | 13.9c | |
| D1 + F1 | 17 | 54 | 32.2 | 7.4 | 26.9d | 10.5d | |
| D1 + F2 | 19 | 46 | 31.8 | 8.3 | 54.6bc | 12.1c | |
| D2 + F1 | 18 | 48 | 30.5 | 8.7 | 57.9bc | 12.9c | |
| D2 + F2 | 21 | 48 | 34.2 | 8.9 | 66.3bc | 13.3c | |
| F-test | ns | ns | ns | Ns | ** | * | |
| CV (%) | 8.4 | 12.9 | 5.9 | 15 | 18.5 | 15.9 | |
| K93-219 | Control | 0 | 0 | 0 | 0 | 63.6a | 15.8a |
| Drought (D1) | 0 | 0 | 0 | 0 | 36.2bc | 12.6cd | |
| Prolonged Drought (D2) | 0 | 0 | 0 | 0 | 30.9c | 10.6d | |
| Flood (F1) | 23 | 60 | 47.3 | 8.8 | 41.3bc | 13.4c | |
| Prolonged Flood (F2) | 19 | 63 | 51.1 | 10.2 | 67.6a | 15.9a | |
| D1 + F1 | 21 | 57 | 49.2 | 10.9 | 25.7d | 10.8d | |
| D1 + F2 | 22 | 51 | 45.7 | 9.4 | 34.3c | 10.5d | |
| D2 + F1 | 17 | 48 | 48.9 | 9.5 | 49.5b | 14.4bc | |
| D2 + F2 | 21 | 54 | 52.2 | 9.1 | 53.6ab | 14.8b | |
| F-test | ns | ns | ns | Ns | * | * | |
| CV (%) | 8.4 | 16.5 | 5.9 | 14.2 | 15.8 | 17.49 | |
| Cultivar | Water Regime | Tiller (No. Plant−1) | Stem Number(No. Plant−1) | Plant Height (cm) | Stem Fresh Weight | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 MOA | 5 MOA | 9 MOA | Harvest | 3 MOA | 5 MOA | 9 MOA | Harvest | (kg Plant−1) | ||
| KK3 | Control | 4.1 | 4.7 | 5.2 | 2.2 | 144.3 | 147.3 | 195.0 | 310.0 | 2.1a |
| Drought (D1) | 4.6 | 8.1 | 11.0 | 3.3 | 149.3 | 135.1 | 199.6 | 278.4 | 1.4bc | |
| Prolonged Drought (D2) | 3.6 | 7.0 | 10.0 | 3.6 | 152.0 | 138.6 | 220.3 | 256.6 | 1.7ab | |
| Flood (F1) | 3.1 | 2.7 | 4.3 | 2.8 | 126.0 | 117.3 | 216.7 | 263.7 | 1.7ab | |
| Prolonged Flood (F2) | 3.3 | 4.0 | 4.6 | 3.6 | 137.0 | 153.0 | 202.6 | 280.0 | 1.9ab | |
| D1 + F1 | 4.3 | 4.6 | 4.8 | 4.1 | 145.7 | 142.5 | 214.7 | 238.4 | 1.3bc | |
| D1 + F2 | 4.3 | 6.3 | 8.3 | 2.3 | 127.0 | 135.6 | 192.6 | 310.0 | 0.9c | |
| D2 + F1 | 5.0 | 5.5 | 6.0 | 3.3 | 162.3 | 160.6 | 184.6 | 300.0 | 1.2bc | |
| D2 + F2 | 5.3 | 6.8 | 8.3 | 2.3 | 146.3 | 140.0 | 211.3 | 296.6 | 1.6ab | |
| F-test | ns | ns | ns | ns | ns | ns | Ns | ns | * | |
| CV (%) | 39.1 | 33 | 41.26 | 30.5 | 9.0 | 9.0 | 15.1 | 11.3 | 10.9 | |
| K93-219 | Control | 4.5 | 6.2 | 7.8c | 2.7 | 156.3 | 150.9 | 213.8 | 300.5a | 2.0a |
| Drought (D1) | 6.0 | 7.2 | 8.3bc | 2.6 | 133.4 | 108.5 | 193.6 | 199.4b | 1.1b | |
| Prolonged Drought (D2) | 4.8 | 10.0 | 11.2ab | 2.6 | 158.2 | 157.0 | 207.6 | 169.4b | 1.1b | |
| Flood (F1) | 5.0 | 5.5 | 6.0c | 2.3 | 137.0 | 148.7 | 205.0 | 310.0a | 1.8ab | |
| Prolonged Flood (F2) | 5.3 | 7.3 | 9.3bc | 2.6 | 142.0 | 152.7 | 206.6 | 290.0a | 1.7ab | |
| D1 + F1 | 4.5 | 6.2 | 7.8c | 2.7 | 114.3 | 119.4 | 224.3 | 320.5a | 0.8b | |
| D1 + F2 | 5.3 | 7.8 | 10.2bc | 2.0 | 142.3 | 133.6 | 224.9 | 279.2a | 0.9b | |
| D2 + F1 | 4.3 | 6.8 | 9.3bc | 2.3 | 141.3 | 128.7 | 200.0 | 306.0a | 1.1b | |
| D2 + F2 | 6.3 | 11.0 | 14a | 3.2 | 134.3 | 162.8 | 208.1 | 291.5a | 1.1b | |
| F-test | ns | ns | * | ns | ns | ns | Ns | ** | * | |
| CV (%) | 32.1 | 19.39 | 22.35 | 22.48 | 14.9 | 11.6 | 4.2 | 6.4 | 25.4 | |
| Cultivar | Water Regime | Brix (%) | Polarity (%) | Fiber (%) | CCS (%) | Purity (%) |
|---|---|---|---|---|---|---|
| KK3 | Control | 20.7 | 9.7 | 15.1 | 6.0 | 47.0 |
| Drought (D1) | 21.9 | 13.5 | 16.6 | 7.3 | 62.6 | |
| Prolonged Drought (D2) | 25.4 | 16.1 | 15.5 | 8.8 | 64.1 | |
| Flood (F1) | 24.9 | 15.1 | 19.2 | 7.3 | 60.0 | |
| Prolonged Flood (F2) | 20.5 | 9.8 | 15.4 | 3.5 | 47.5 | |
| D1 + F1 | 20.5 | 8.5 | 14.6 | 3.3 | 41.4 | |
| D1 + F2 | 18.4 | 8.3 | 16.1 | 5.5 | 41.0 | |
| D2 + F1 | 17.2 | 7.3 | 13.8 | 4.9 | 40.1 | |
| D2 + F2 | 17.2 | 6.8 | 15.2 | 1.4 | 39.3 | |
| F-test | ns | ns | ns | ns | ns | |
| CV (%) | 24.4 | 24.7 | 6.2 | 34.9 | 31.2 | |
| K93-219 | Control | 25.2 | 16.5 | 19.2 | 5.7 | 69.2ab |
| Drought (D1) | 23.2 | 10.1 | 14.6 | 3.2 | 42.0b | |
| Prolonged Drought (D2) | 15.4 | 5.6 | 15.3 | 4.8 | 36.5b | |
| Flood (F1) | 17.9 | 6.8 | 16.8 | 2.9 | 37.3b | |
| Prolonged Flood (F2) | 19.4 | 12.0 | 16.6 | 6.3 | 62.5ab | |
| D1 + F1 | 21.6 | 19.4 | 15.9 | 10.9 | 93.1a | |
| D1 + F2 | 19.6 | 9.8 | 20.0 | 5.3 | 49.4b | |
| D2 + F1 | 16.9 | 5.9 | 18.0 | 6.8 | 33.2bc | |
| D2 + F2 | 16.6 | 4.0 | 13.0 | 8.0 | 25.3c | |
| F-test | ns | ns | ns | ns | * | |
| CV (%) | 22.6 | 35.6 | 24.0 | 22.2 | 19.4 |
| Treatment | AR Fresh Weight (g Plant−1) | AR Dry Weight (g Plant−1) | Number of Adventitious Roots | Aerenchyma Score | |
|---|---|---|---|---|---|
| 3 DAT | 7 DAT | ||||
| Auxin Rates (A) | |||||
| 0 mg L−1 | 15.1c | 4.8 | 38 | 86b | 2 |
| 10 mg L−1 | 28.5a | 6.4 | 61 | 102a | 3 |
| 20 mg L−1 | 23.9b | 5.9 | 50 | 88b | 3 |
| 30 mg L−1 | 16.7c | 6.3 | 48 | 81c | 3 |
| F-test | * | ns | ns | ** | ns |
| Growth stages (G) | |||||
| 4 MOA | 24.4 | 6.2 | 44 | 89 | 3 |
| 5 MOA | 19.2 | 5.3 | 39 | 87 | 3 |
| 7 MOA | 20.3 | 6.2 | 55 | 90 | 3 |
| F-test | ns | ns | ns | ns | ns |
| Cultivars (C) | |||||
| KK3 | 22.7b | 6.1 | 39b | 80b | 3 |
| K93-219 | 32.4a | 7.28 | 52a | 97a | 3 |
| F-test | ** | ns | * | * | ns |
| Interaction | |||||
| AxG | ns | ns | ns | ns | ns |
| AxC | * | ns | ns | * | ns |
| GxC | * | ns | ns | ns | ns |
| AxGxC | ns | ns | ns | ns | ns |
| Auxin Rates (mg L−1) (A) | Cultivars (C) | AR Fresh Weight (gm Plant−1) | AR Number |
|---|---|---|---|
| 0 | KK3 | 15.4d | 78de |
| K93-219 | 20.0bc | 89bcd | |
| 10 | KK3 | 22.6ab | 92bc |
| K93-219 | 27.2a | 113a | |
| 20 | KK3 | 18.0bcd | 80cde |
| K93-219 | 20.3b | 94b | |
| 30 | KK3 | 15.1d | 72e |
| K93-219 | 18.4bcd | 87bcd | |
| F-test | * | * | |
| Growth Stages (G) | Cultivars (C) | AR Fresh Weight (gm Plant−1) |
|---|---|---|
| 4 MOA | KK3 | 20.3c |
| K93-219 | 28.5a | |
| 5 MOA | KK3 | 20.1c |
| K93-219 | 22.7bc | |
| 7 MOA | KK3 | 20.8c |
| K93-219 | 25.6b | |
| F-test | * | |
| Treatment | Plant Height (cm) | Stayed Green Leaf (No. Plant−1) | Leaf Width (cm) | Stem Number (No. Plant−1) | Stem Fresh Weight (kg Plant−1) |
|---|---|---|---|---|---|
| Auxin Rates (A) | |||||
| 0 mg L−1 | 132 | 26 | 4.7 | 3 | 5.8 |
| 10 mg L−1 | 124 | 25 | 5.8 | 3 | 6.4 |
| 20 mg L−1 | 123 | 26 | 6.7 | 3 | 6 |
| 30 mg L−1 | 116 | 34 | 6.9 | 4 | 6 |
| F-test | Ns | Ns | ns | ns | ns |
| Growth stages (G) | |||||
| 4 MOA | 120 | 31 | 4.9c | 3 | 4.1b |
| 5 MOA | 124 | 25 | 7.1a | 3 | 5.9a |
| 7 MOA | 129 | 26 | 6.1b | 3 | 5.7a |
| F-test | Ns | Ns | * | ns | * |
| Cultivars (C) | |||||
| KK3 | 115b | 29 | 5.6 | 3 | 6.4 |
| K93-219 | 133a | 27 | 6.4 | 3 | 7.5 |
| F-test | * | Ns | ns | ns | ns |
| Interaction | |||||
| AxG | Ns | Ns | ns | ns | ns |
| AxC | Ns | Ns | ns | ns | ns |
| GxC | Ns | Ns | ns | ns | ns |
| AxGxC | Ns | Ns | ns | ns | ns |
| Treatment | Brix (%) | Polarity (%) | Purity (%) | Fiber (%) | CCS (%) |
|---|---|---|---|---|---|
| Auxin Rates (A) | |||||
| 0 mg L−1 | 19.8 | 69.2 | 83.9 | 14.1 | 12.0 |
| 10 mg L−1 | 19.2 | 64.4 | 82.2 | 14.7 | 10.8 |
| 20 mg L−1 | 18.8 | 63.4 | 81.2 | 16.4 | 10.6 |
| 30 mg L−1 | 19.2 | 66.2 | 80.4 | 26.0 | 11.1 |
| F-test | ns | Ns | Ns | ns | ns |
| Growth stages (G) | |||||
| 4 MOA | 19.1 | 64.9 | 81.4 | 16.5 | 11.2 |
| 5 MOA | 19.0 | 64.1 | 81.0 | 16.1 | 10.7 |
| 7 MOA | 19.7 | 68.5 | 83.3 | 13.8 | 11.8 |
| F-test | ns | Ns | Ns | ns | ns |
| Cultivars (C) | |||||
| KK3 | 19.2 | 65.5 | 82.1 | 15.0 | 11.1 |
| K93-219 | 19.4 | 66.1 | 81.7 | 20.6 | 11.4 |
| F-test | ns | Ns | Ns | ns | ns |
| Interaction | |||||
| AxG | ns | Ns | Ns | ns | ns |
| AxC | ns | Ns | Ns | ns | ns |
| GxC | ns | Ns | Ns | ns | ns |
| AxGxC | ns | Ns | Ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamrungrai, J.; Tubana, B.; Tre-loges, V.; Promkhambut, A.; Polthanee, A. Effects of Water Stress and Auxin Application on Growth and Yield of Two Sugarcane Cultivars under Greenhouse Conditions. Agriculture 2021, 11, 613. https://doi.org/10.3390/agriculture11070613
Bamrungrai J, Tubana B, Tre-loges V, Promkhambut A, Polthanee A. Effects of Water Stress and Auxin Application on Growth and Yield of Two Sugarcane Cultivars under Greenhouse Conditions. Agriculture. 2021; 11(7):613. https://doi.org/10.3390/agriculture11070613
Chicago/Turabian StyleBamrungrai, Jiraporn, Brenda Tubana, Vidhaya Tre-loges, Arunee Promkhambut, and Anan Polthanee. 2021. "Effects of Water Stress and Auxin Application on Growth and Yield of Two Sugarcane Cultivars under Greenhouse Conditions" Agriculture 11, no. 7: 613. https://doi.org/10.3390/agriculture11070613





