The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Preparation of Soil Samples
2.2. Soil pH Manipulation
2.3. Greenhouse Bioassay
2.4. Evaluation of Pea Susceptibility
2.5. Statistical Analysis
3. Results
3.1. Visual Injuries of Pea Plants at 14, 28 and 35 Days after Treatment
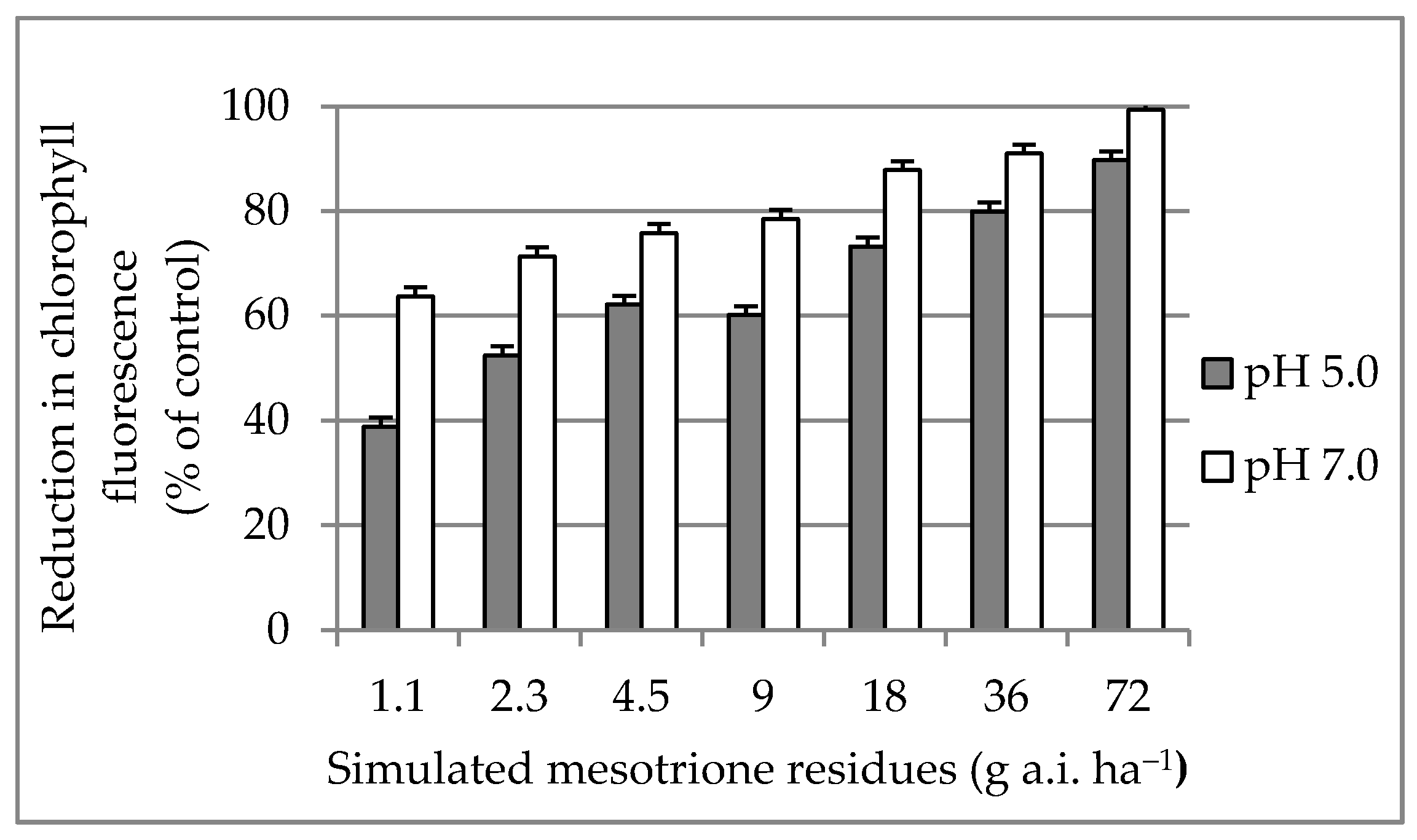
3.2. Chlorophyll Fluorescence Reduction at 35 Days after Treatment
3.3. Aboveground Dry Biomass Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anonymous. Callisto 480 SC Herbicide Label. Available online: File:///C:/Users/Korisnik/Downloads/Callisto%20480%20SC%20(9).pdf (accessed on 7 May 2021).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Ondrašek, G.; Romić, D.; Bakić Begić, H.; Bubalo Kovačić, M.; Husnjak, S.; Mesić, M.; Šestak, I.; Salajpal, K.; Barić, K.; Bažok, R.; et al. Establishment of Priority Areas for Groundwater Monitoring within the Intensive Agricultural Area (SAGRA 2); University of Zagreb Faculty of Agriculture: Zagreb, Croatia, 2019; pp. 197–220. [Google Scholar]
- Robinson, D.E. Atrazine accentuates carryover injury from mesotrione in vegetable crops. Weed Technol. 2008, 22, 641–645. [Google Scholar] [CrossRef]
- Riddle, R.N.; O’Sullivan, J.; Swanton, C.J.; Van Acker, R.C. Crop Response to Carryover of Mesotrione Residues in the Field. Weed Technol. 2013, 27, 92–100. [Google Scholar] [CrossRef]
- Wichert, R.A.; Townson, J.K.; Bartlett, D.W.; Foxon, G.A. Technical review of mesotrione, a new maize herbicide. In Proceedings of the Brighton Crop Protection Conference, Weeds, BCPC, Farnham, Surrey, Brighton, UK, 15–18 November 1999; pp. 105–110. [Google Scholar]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest. Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Soltani, N.; Sikkema, P.H.; Robinson, D.E. Response of four market classes of dry bean to mesotrione soil residues. Crop. Prot. 2007, 26, 1655–1659. [Google Scholar] [CrossRef]
- Torma, M.; Radvany, B.; Hodi, L. Effect of mesotrione residues on following crops. J. Plant Dis. Prot. 2004, 19, 801–805. [Google Scholar]
- Dyson, J.S.; Beulke, S.; Brown, C.D.; Lane, M.C.G. Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. J. Environ. Qual. 2002, 31, 613–618. [Google Scholar] [CrossRef]
- Chaabane, H.; Vulliet, E.; Calvayrac, C.; Coste, C.M.; Cooper, J.F. Behaviour of sulcotrione and mesotrione in two soils. Pest. Manag. Sci. 2008, 64, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Burnside, O.C.; Fenster, C.R.; Wicks, G.A.; Drew, J.V. Effect of soil and climate on herbicide dissipation. Weed Sci. 1969, 17, 241–245. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Wu, R.; Xu, H.; Xue, F.; Lu, C. Degradation of Mesotrione Affected by Environmental Conditions. Bull. Environ. Contam. Toxicol. 2017, 98, 212–217. [Google Scholar] [CrossRef]
- Pintar, A.; Stipicevic, S.; Lakic, J.; Baric, K. Phytotoxicity of mesotrione residues on sugar beet (Beta vulgaris L.) in agricultural soils differing in adsorption affinity. Sugar Tech. 2020, 22, 137–142. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World reference base for soil resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports Nr. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Cheng, Y.; Cai, Z.; Chang, S.X.; Wang, J.; Zhang, J. Effects of soil pH and salt on N2O production in adjacent forest and grassland soils in central Alberta, Canada. J. Soils Sediments 2013, 13, 863–868. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. PP 1/135 (4) Phytotoxicity assessment. Bull. OEPP/EPPO Bull. 2014, 44, 265–273. [Google Scholar] [CrossRef]
- Estime, N.; Teychené, S.; Autret, J.M.; Biscans, B. Influence of pH, temperature and impurities on the solubility of an Active Pharmaceutical Ingredient (API). Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Boesten, J.J. Bioavailability of organic chemicals in soil related to their concentration in the liquid phase: A review. Sci. Total Environ. 1993, 134, 397–407. [Google Scholar] [CrossRef]
- Fuscaldo, F.; Bedmar, F.; Monterubbianesi, G. Persistence of atrazine, metribuzin and simazine herbicides in two soils. Pesqui. Agropecuária Bras. 1999, 34, 2037–2044. [Google Scholar] [CrossRef]
- Weber, J.B.; Perry, P.W.; Ibaraki, K. Effect of pH on the phytotoxicity of prometryne applied to synthetic soil media. Weed Sci. 1968, 16, 134–136. [Google Scholar] [CrossRef]
- Ni, Y.; Lai, J.; Wan, J.; Chenb, L. Photosynthetic responses and accumulation of mesotrione in two freshwater algae. Environ. Sci. Process. Impacts 2014, 16, 2288. [Google Scholar] [CrossRef]
- Sousa, C.P.; Pinto, J.J.O.; Martinazzo, E.G.; Perboni, A.T.; Farias, M.E.; Bacarin, M.A. Chlorophyll a fluorescence in rice plants exposed of herbicides of group imidazolinone. Planta Daninha 2014, 32, 141–150. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Hao, H.; An, S.; Lu, C.; Wu, R.; Su, W. Effects of bensulfuron-methyl residue on photosynthesis and chlorophyll fluorescence in leaves of cucumber seedlings. PLoS ONE 2019, 14, e0215486. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Huang, Y.Y.; Wang, L.; Huang, L.F.; Yu, Y.L.; Zhou, Y.H.; Yu, J.Q. Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic. Biochem. Phys. 2006, 86, 42–48. [Google Scholar] [CrossRef]
- Silva, L.G.B.; da Silva Araújo, L.; Gonçalves, D.J.; Valente, M.S.; da Silva, A.R.; Nascimento, W.M.; da Cunha, P.C.R. Selectivity of diphenyl-ether herbicides with postemergence applications in chickpea. J. Plant Prot. Res. 2019, 59, 350–354. [Google Scholar]


| Soil | pH (H2O) | pH (KCl) | Humus (%) | CEC (cmol kg−1) | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|
| (%) | |||||||
| Gleysol | 7.7 | 7.02 | 4.2 | 33.8 | 1.1 | 59.6 | 39.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintar, A.; Svečnjak, Z.; Lakić, J.; Magdić, I.; Brzoja, D.; Barić, K. The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation. Agriculture 2021, 11, 688. https://doi.org/10.3390/agriculture11080688
Pintar A, Svečnjak Z, Lakić J, Magdić I, Brzoja D, Barić K. The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation. Agriculture. 2021; 11(8):688. https://doi.org/10.3390/agriculture11080688
Chicago/Turabian StylePintar, Ana, Zlatko Svečnjak, Josip Lakić, Ivan Magdić, Dragojka Brzoja, and Klara Barić. 2021. "The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation" Agriculture 11, no. 8: 688. https://doi.org/10.3390/agriculture11080688
APA StylePintar, A., Svečnjak, Z., Lakić, J., Magdić, I., Brzoja, D., & Barić, K. (2021). The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation. Agriculture, 11(8), 688. https://doi.org/10.3390/agriculture11080688





