Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Growth Conditions
2.2. Soil Analysis
2.3. Determination of Growth Biomarkers
2.4. Determination of Leaf Characteristics
2.5. Determination of Photosynthetic Pigments
2.6. Determination of Nitrate Reductase Activity, Protein and Carbohydrate Content
2.7. Assay of Antioxidant Enzyme Activity
2.8. Quantification and Histochemical Localization of Superoxide Anion (O2−) and Determination of SOD Activity
2.9. Determination of the Root Cell Viability through Confocal Laser Scanning Microscopy
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of the Soil
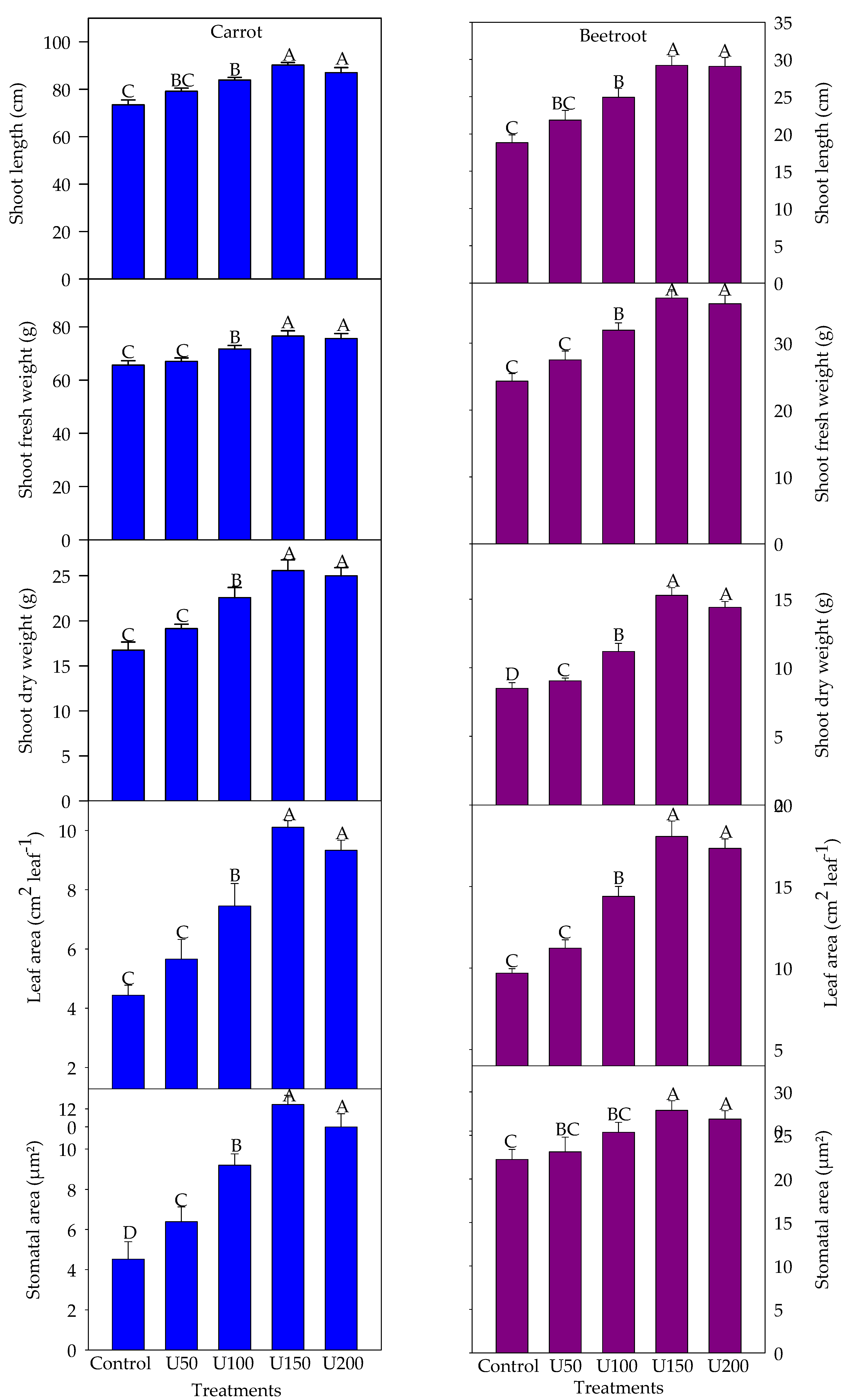
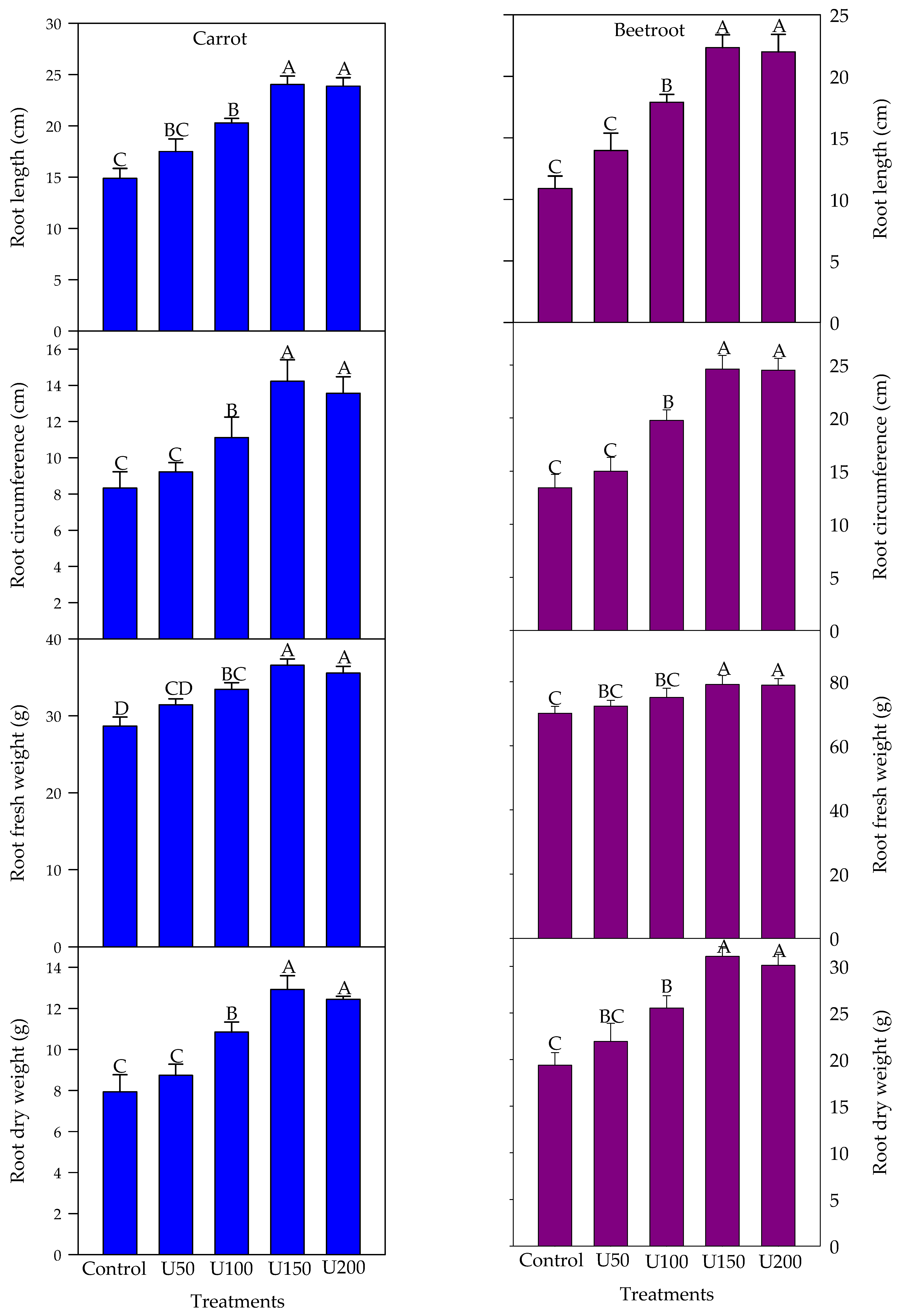
3.2. Effect of N on the Growth and Yield Attributes of Carrot and Beetroot
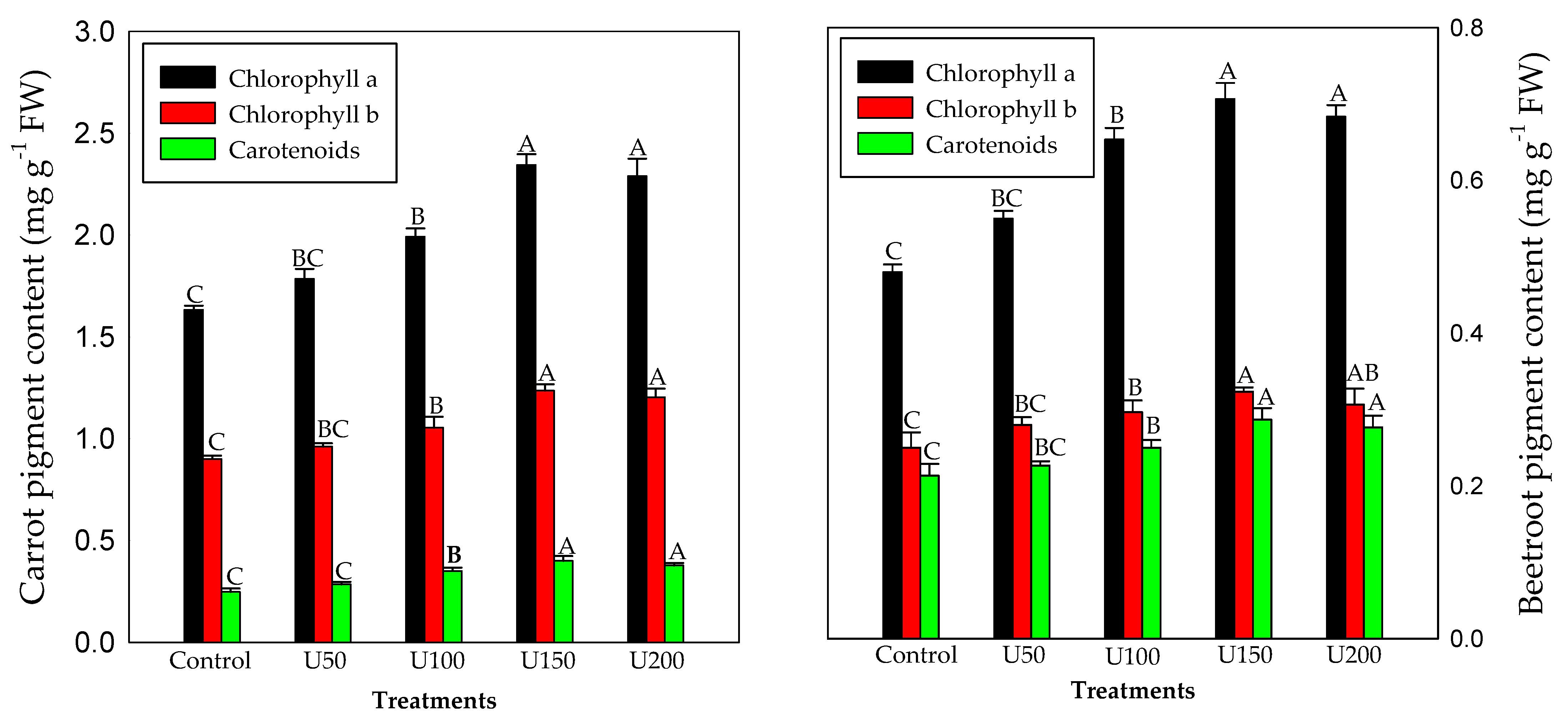
3.3. Effect of N on the Photosynthetic Pigment Content of Carrot and Beetroot
3.4. Effect of N on the NR Activity, Carbohydrate and Protein, and Nitrogen Contents of the Carrot and Beetroot
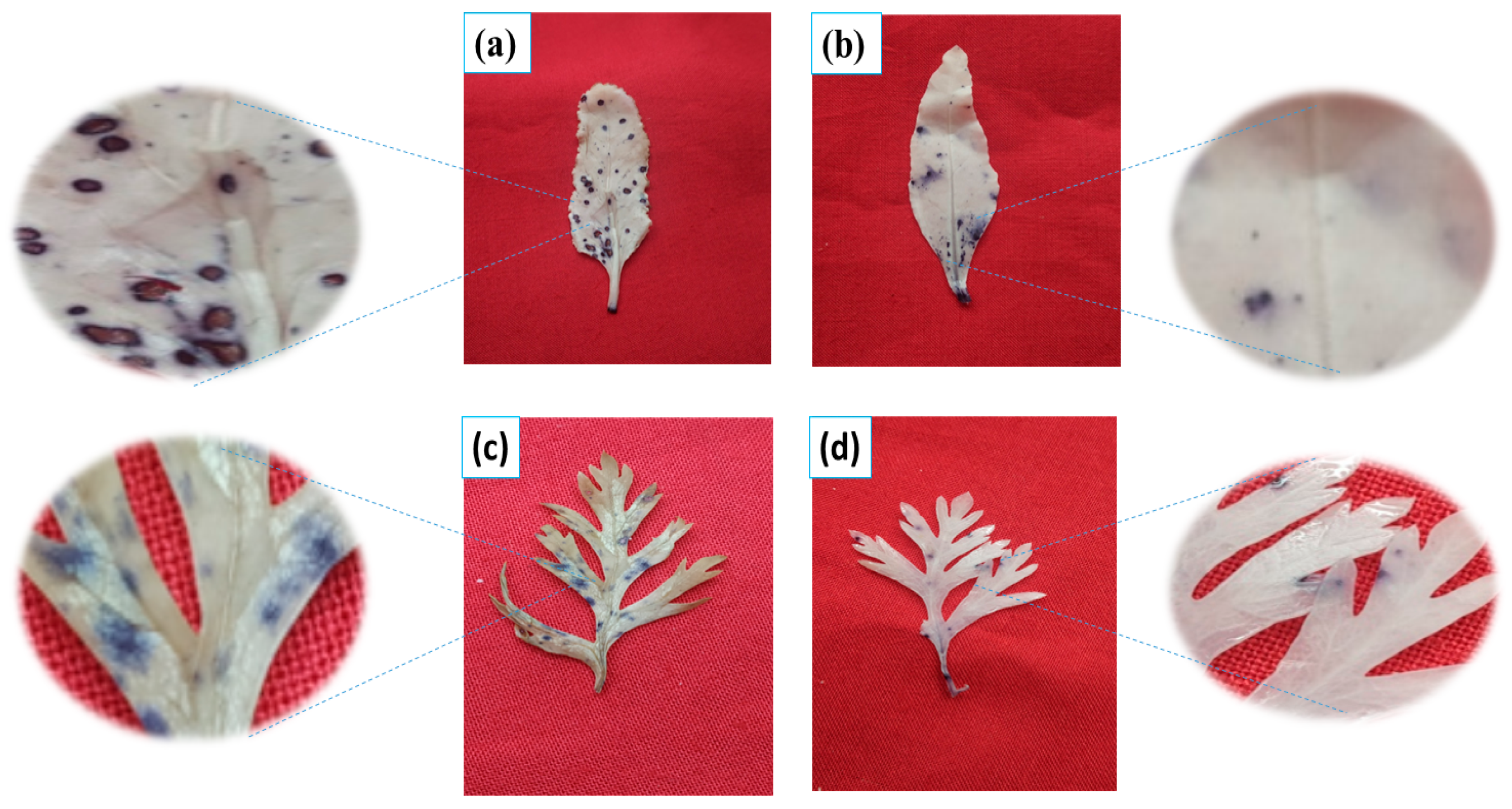
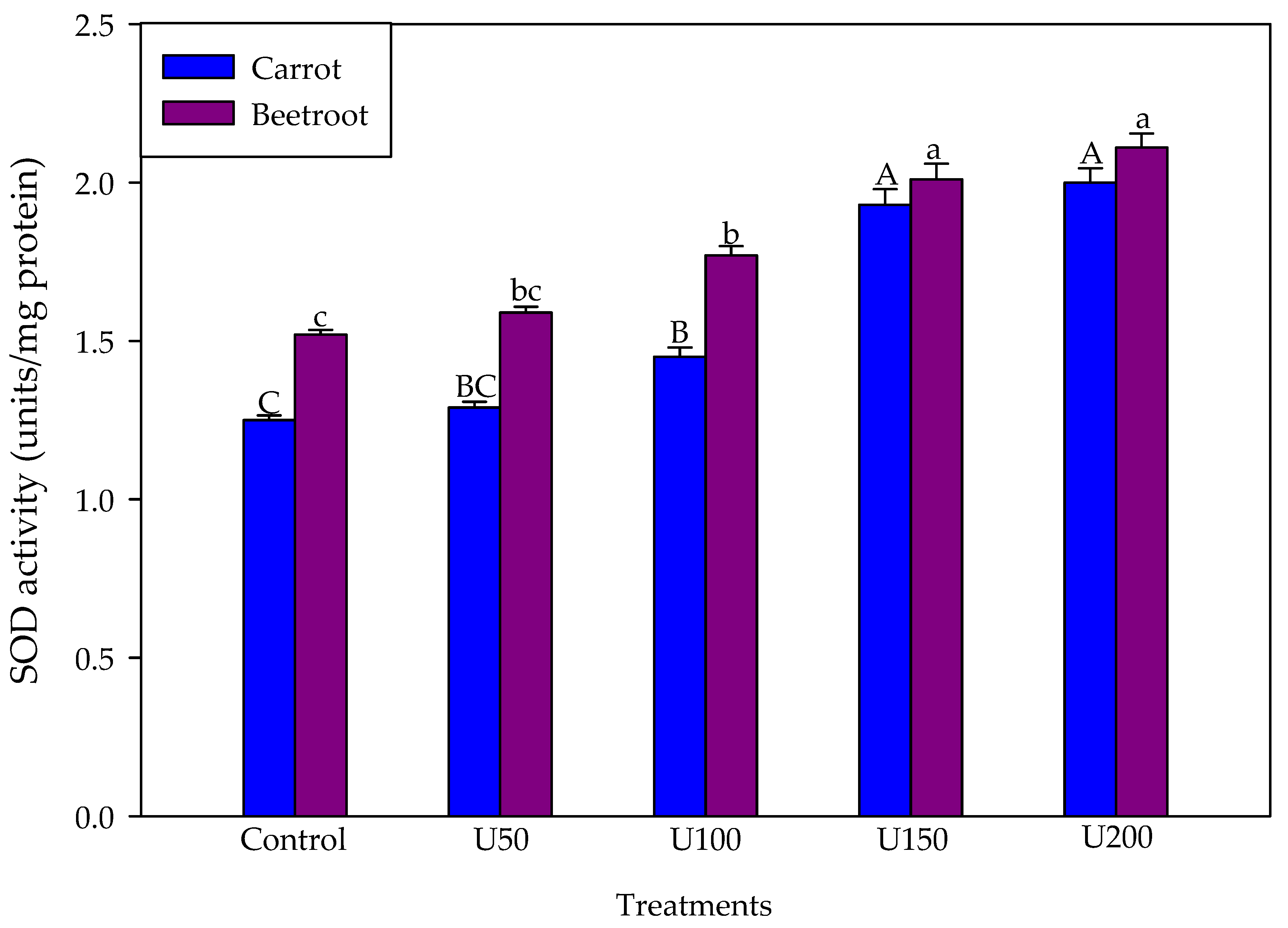
3.5. Effect of N on Superoxide Anions and SOD Activity of the Carrot and Beetroot
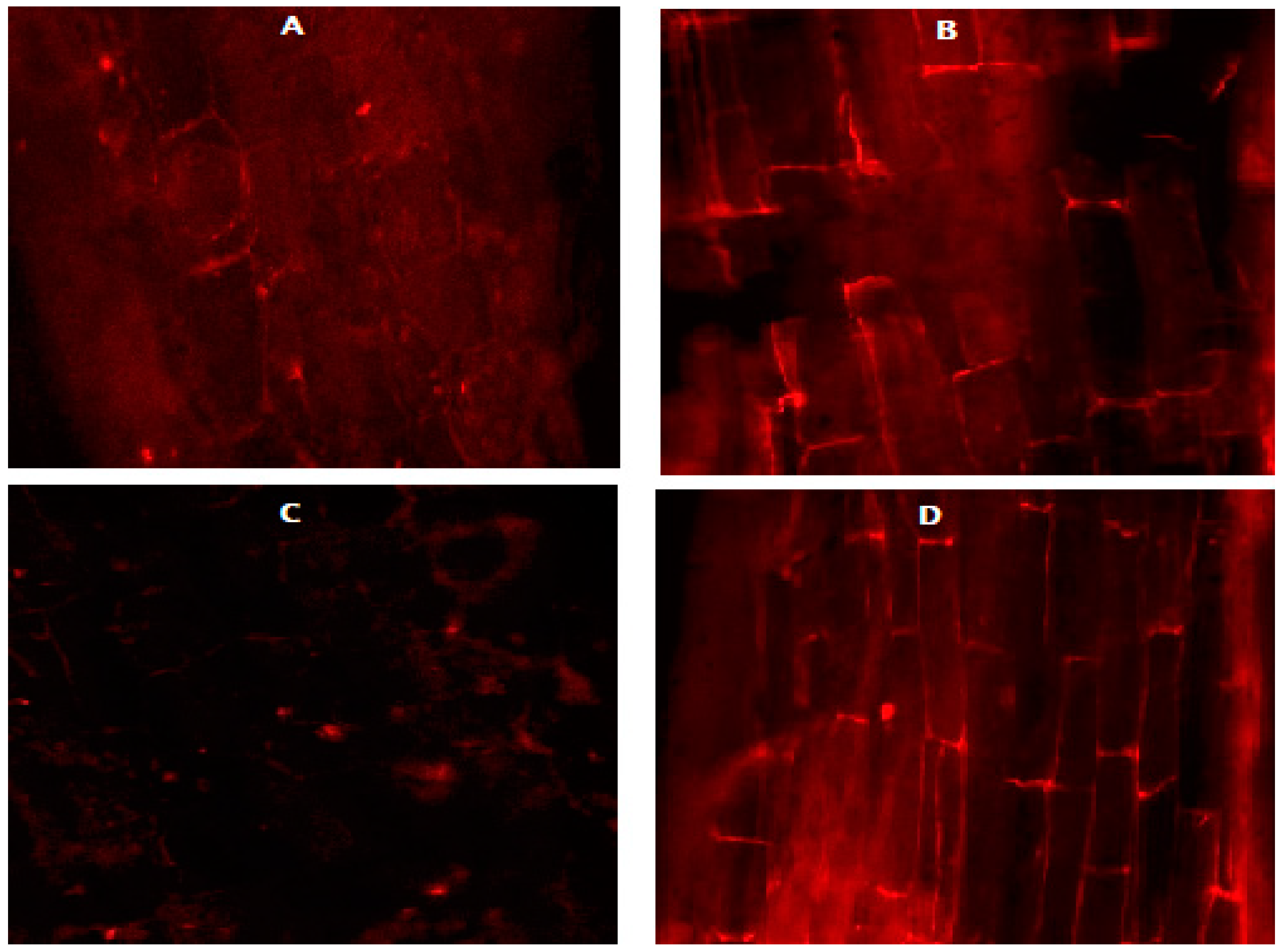
3.6. Effect of N on the Root Cell Viability of the Carrot and Beetroot
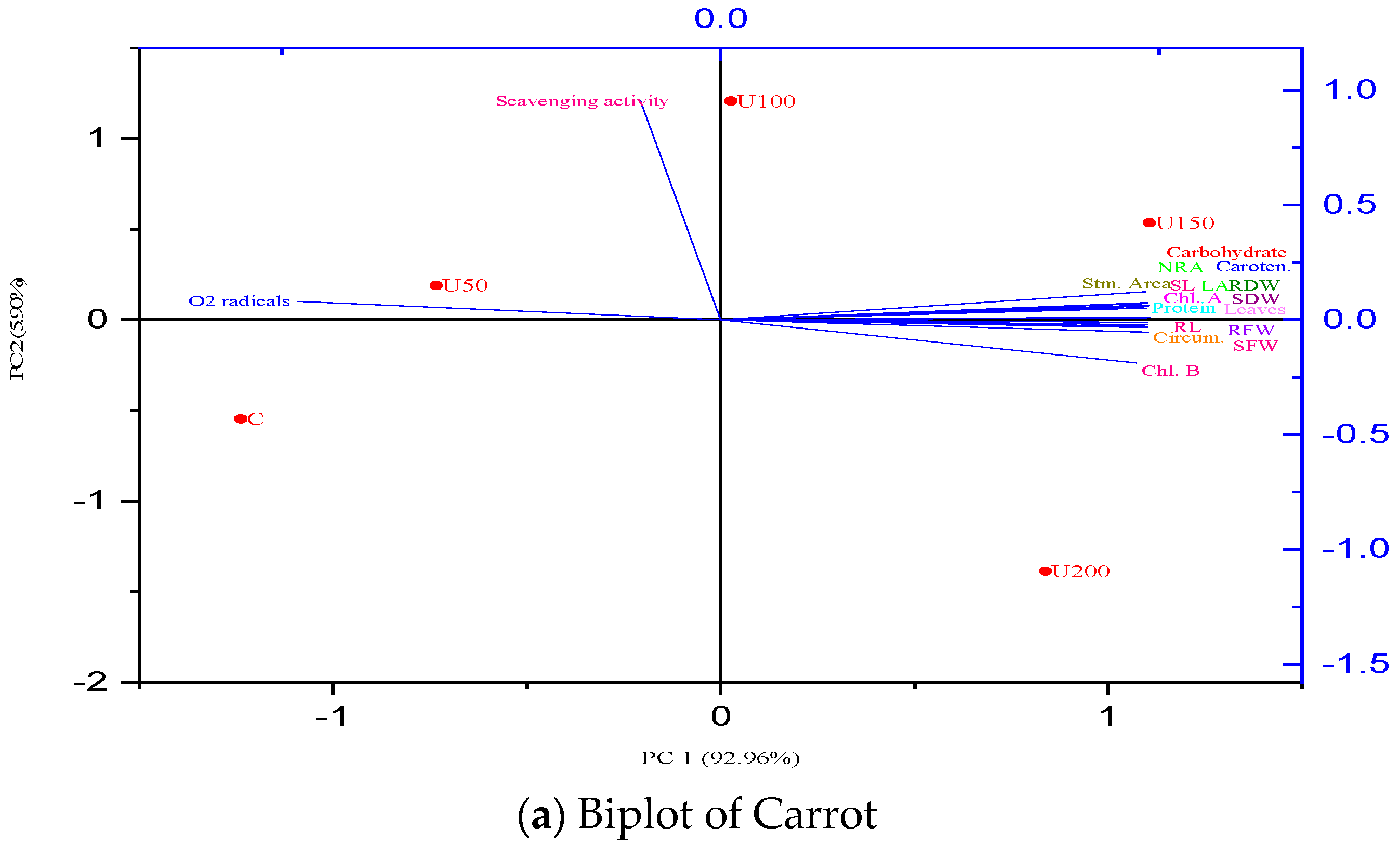
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhao, P.; Chi, Y.; Wang, D.; Wang, P.; Liu, N.; Cai, D.; Wu, N.; Zhong, N. Controlling the hydrolysis and loss of nitrogen fertilizer (urea) by using a nanocomposite favors plant growth. ChemSusChem 2017, 10, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Higher yield with less nitrogen fertilizer. Nature Plants 2020, 6, 1078–1079. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Shi, Y.; Yu, Z. Optimized split nitrogen fertilizer increase photosynthesis, grain yield, nitrogen use efficiency and water use efficiency under water-saving irrigation. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Jiao, X.; Lyu, Y.; Wu, X.; Li, H.; Cheng, L.; Zhang, C.; Yuan, L.; Jiang, R.; Jiang, B.; Rengel, Z. Grain production versus resource and environmental costs: Towards increasing sustainability of nutrient use in China. J. Exp. Bot. 2016, 67, 4935–4949. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Aghavani, M.; Zaeimzadeh, A.; Savadkoohi, S.; Samiei, L. Salinity tolerance of Kentucky bluegrass as affected by nitrogen fertilization. J. Biol. Sci. 2017, 368, 20130164. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.; Wu, D.; Xia, T.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. A RNA-Seq analysis of the response of photosynthetic system to low nitrogen supply in maize leaf. Int. J. Mol. Sci. 2017, 18, 2624. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity, and nitrogen metabolism in ramie under nitrogen fertilizer. Agron. J. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Z.; Fang, B.; Chen, J.; Zhang, X.; Luo, Z.; Huang, L.; Zou, H.; Yang, Y. Involvement of nitrogen in storage root growth and related gene expression in sweet potato (Ipomoea batatas). Plant Biol. 2020, 22, 376–385. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 1621. [Google Scholar]
- Hill, J.; Goodkind, A.; Tessum, C.; Thakrar, S.; Tilman, D.; Polasky, S.; Smith, T.; Hunt, N.; Mullins, K.; Clark, M.; et al. Air-quality-related health damages of maize. Nat. Sust. 2019, 2, 397–403. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2020. [Google Scholar] [CrossRef]
- Department of Agriculture, Cooperation & Farmers Welfare, Ministry of Agriculture and Farmers Welfare, Govt. of India, Krishi Bhawan, New Delhi-110001. Annual Report (2016–2017). Available online: www.agricoop.nic.in (accessed on 17 December 2020).
- Singh, B.K.; Koley, T.K.; Maurya, A.; Singh, P.M.; Singh, B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. & Martens). Physiol. Mol. Biol. Plants 2018, 24, 99–907. [Google Scholar]
- Venkataramanan, R.; Gunasekaran, S.; Sreekumar, C.; Anilkumar, R.; Iyue, M. Nutritional value and suitability of carrot whole top as green fodder. Ind. J. Veter. Anim. Sci. Res. 2015, 44, 49–52. [Google Scholar]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, R.G.; Sawate, A.R.; Kshirsagar, R.B.; Patil, B.M.; Mane, R.P. Studies on evaluation of physical and chemical composition of beetroot (Beta vulgaris L.). Int. J. Chem. Stud. 2018, 6, 2977–2979. [Google Scholar]
- Panghal, A.; Virkar, K.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Development of probiotic beetroot drink. Curr. Res. Nutr. Food Sci. 2017, 24, 5. [Google Scholar] [CrossRef]
- Shakeel, A.; Khan, A.; Hakeem, K.R. Growth, biochemical, and antioxidant response of beetroot (Beta vulgaris L.) grown in fly ash-amended soil. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Priha, O.; Smolander, A. Nitrogen transformations in soil under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Soil Biol. Biochem. 1999, 31, 965–977. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall: New Delhi, India, 1958. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Mount Waverley, Australia, 1992. [Google Scholar]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 336. [Google Scholar]
- Dickman, S.R.; Bray, R.H. Colorimetric determination of phosphate. Ind. Eng. Chem. Anal. 1940, 12, 665–668. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T.; Whistler, R.L. Methods in Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang., H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, I.; Kirat, K.; Pati, P.K. Detection of reactive oxygen species in Oryza sativa L. (rice). Bio-Protocol 2016, 6, 2061. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Throne, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lilpd peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Kamada-Nobusada, T.; Makita, N.; Kojima, M.; Sakakibara, H. Nitrogen-dependent regulation of de novo cytokinin biosynthesis in rice: The role of glutamine metabolism as an additional signal. Plant Cell Physiol. 2013, 54, 18811893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef]
- Basi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.; Andreotti, M.C.M.; Arf, S.B.M.; Eustaquio de Sa, M.O. Application times, sources and doses of nitrogen on wheat cultivars under no till in the Cerrado region. Cienc. Rural St. Maria 2011, 41, 1375–1382. [Google Scholar] [CrossRef]
- Hong, L.; Li, M.; Luo, J.; Cao, X.; Qu, L.; Gai, Y.; Jiang, X.; Liu, T.; Janz, H.; Bai, D.; et al. N fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow- and fast growing Populus species. J. Exp. Bot. 2012, 63, 6173–6185. [Google Scholar]
- Li, Y.; Liu, K.; Zhu, J.; Jiang, Y.; Huang, Y.; Zhou, Z.; Chen, C.; Yu, F. Manganese accumulation and plant physiology behavior of Camellia oleifera in response to different levels of nitrogen fertilization. Ecotoxicol. Environ. Saf. 2019, 184, 109603. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 2019, 141, 466–476. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Texture | pH | EC (μ mhos cm−1) | Porosity (%) | Water Holding Capacity (%) | N (%) | p (%) | K (%) |
|---|---|---|---|---|---|---|---|---|
| Results | Sandy loam | 7.62 | 278.44 | 35.87 | 40.67 | 1.53 | 1.93 | 12.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeel, A.; Khan, A.A.; Alharby, H.F.; Bamagoos, A.A.; Alabdallah, N.M.; Hakeem, K.R. Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer. Agriculture 2021, 11, 704. https://doi.org/10.3390/agriculture11080704
Shakeel A, Khan AA, Alharby HF, Bamagoos AA, Alabdallah NM, Hakeem KR. Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer. Agriculture. 2021; 11(8):704. https://doi.org/10.3390/agriculture11080704
Chicago/Turabian StyleShakeel, Adnan, Abrar Ahmad Khan, Hesham F. Alharby, Atif A. Bamagoos, Nadiyah M. Alabdallah, and Khalid Rehman Hakeem. 2021. "Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer" Agriculture 11, no. 8: 704. https://doi.org/10.3390/agriculture11080704
APA StyleShakeel, A., Khan, A. A., Alharby, H. F., Bamagoos, A. A., Alabdallah, N. M., & Hakeem, K. R. (2021). Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer. Agriculture, 11(8), 704. https://doi.org/10.3390/agriculture11080704







