Accumulation of Potentially Toxic Metals in Egyptian Alluvial Soils, Berseem Clover (Trifolium alexandrinum L.), and Groundwater after Long-Term Wastewater Irrigation
Abstract
:1. Introduction
2. Materials and Methods
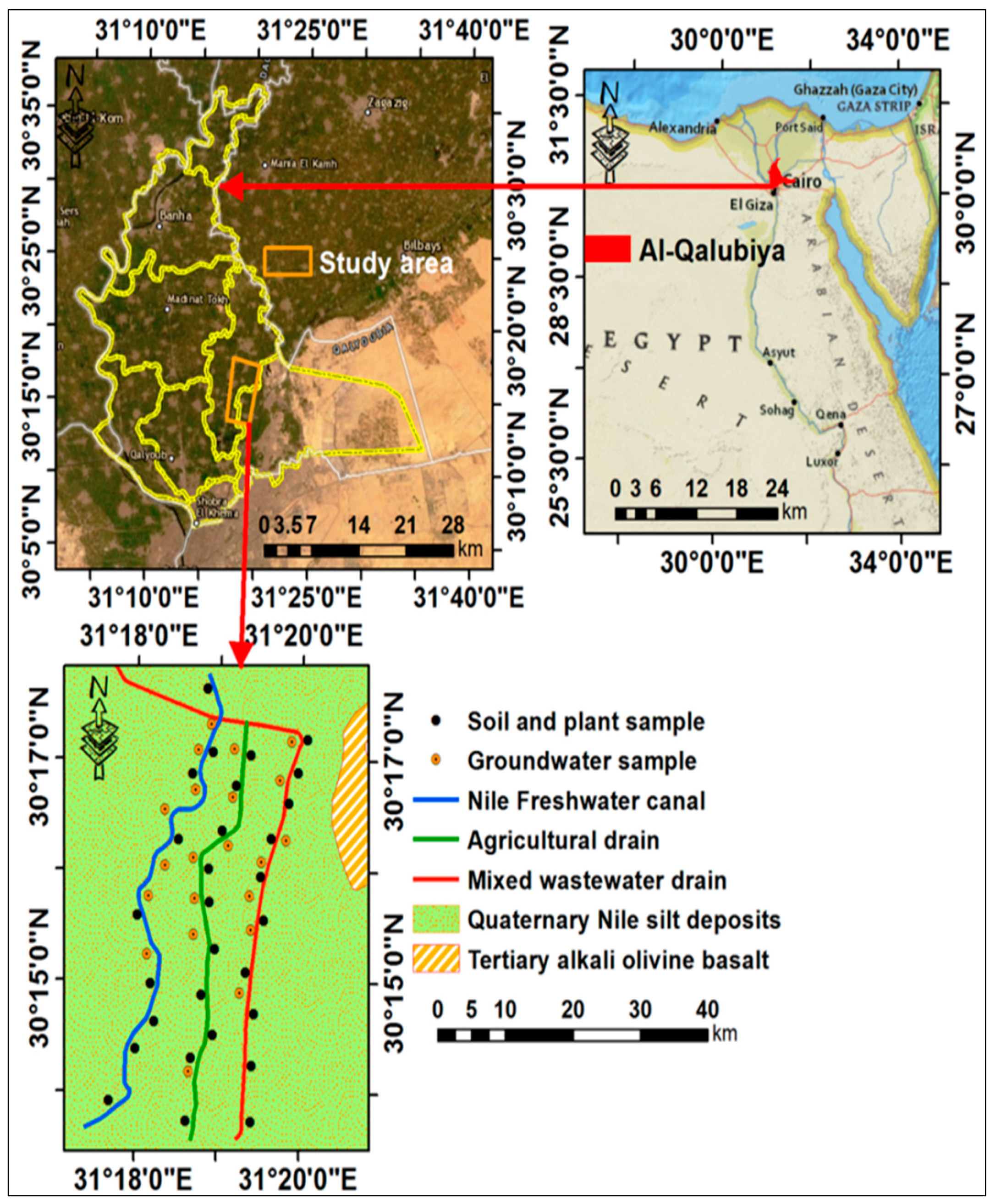
2.1. Site Description
- Nile freshwater (NFW) from the Al-Sharqua canal;
- Agricultural drainage water (ADW) from the Sendiwah drain;
- Mixed wastewater (MWW) from the Shebin Al-Kanater drain.
2.2. Collection of Samples
2.3. Laboratory Analyses
2.4. Assessment of Soil Contamination by PTMs
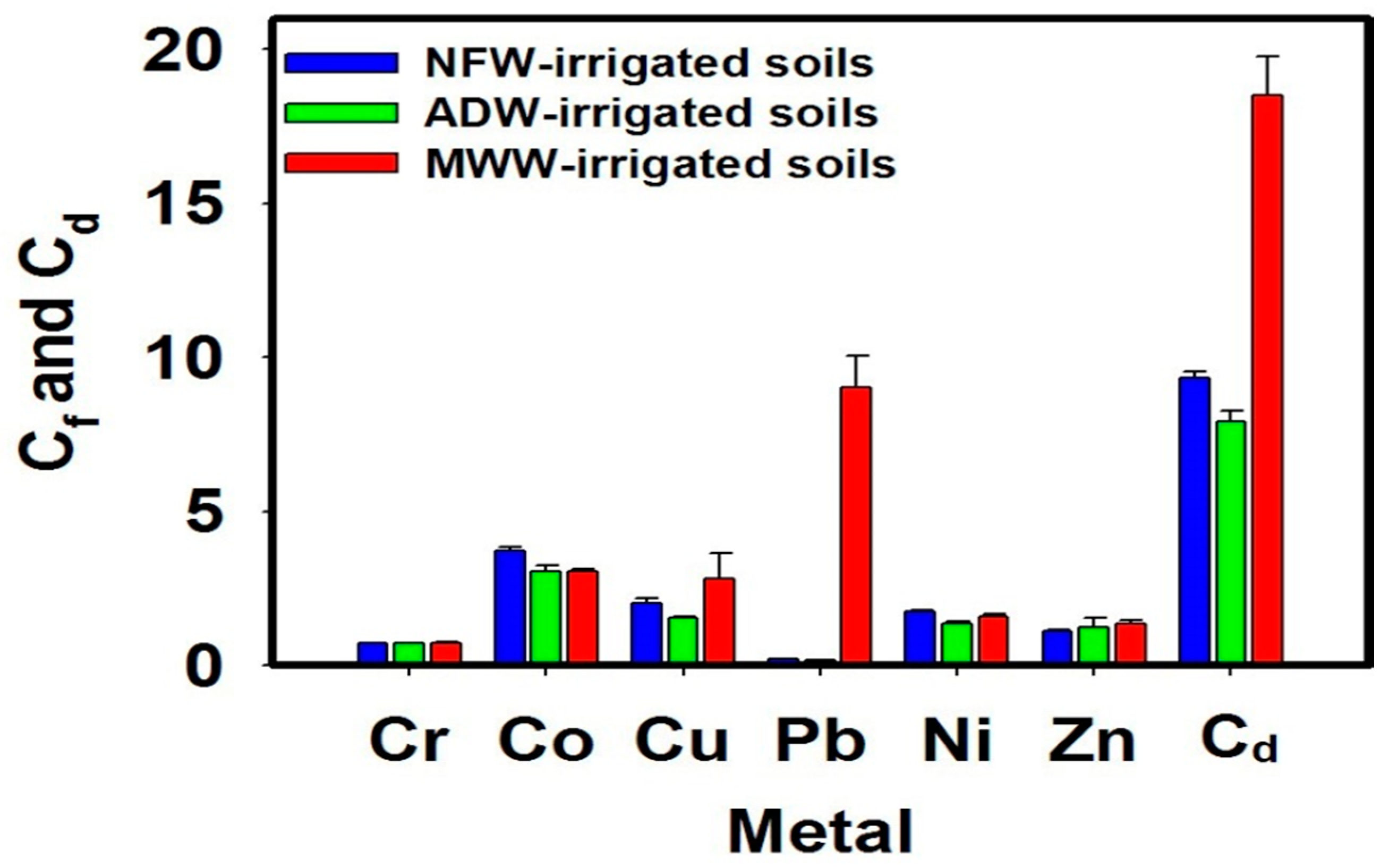
2.4.1. Contamination Factor (Cf)
2.4.2. Contamination Degree (Cd)
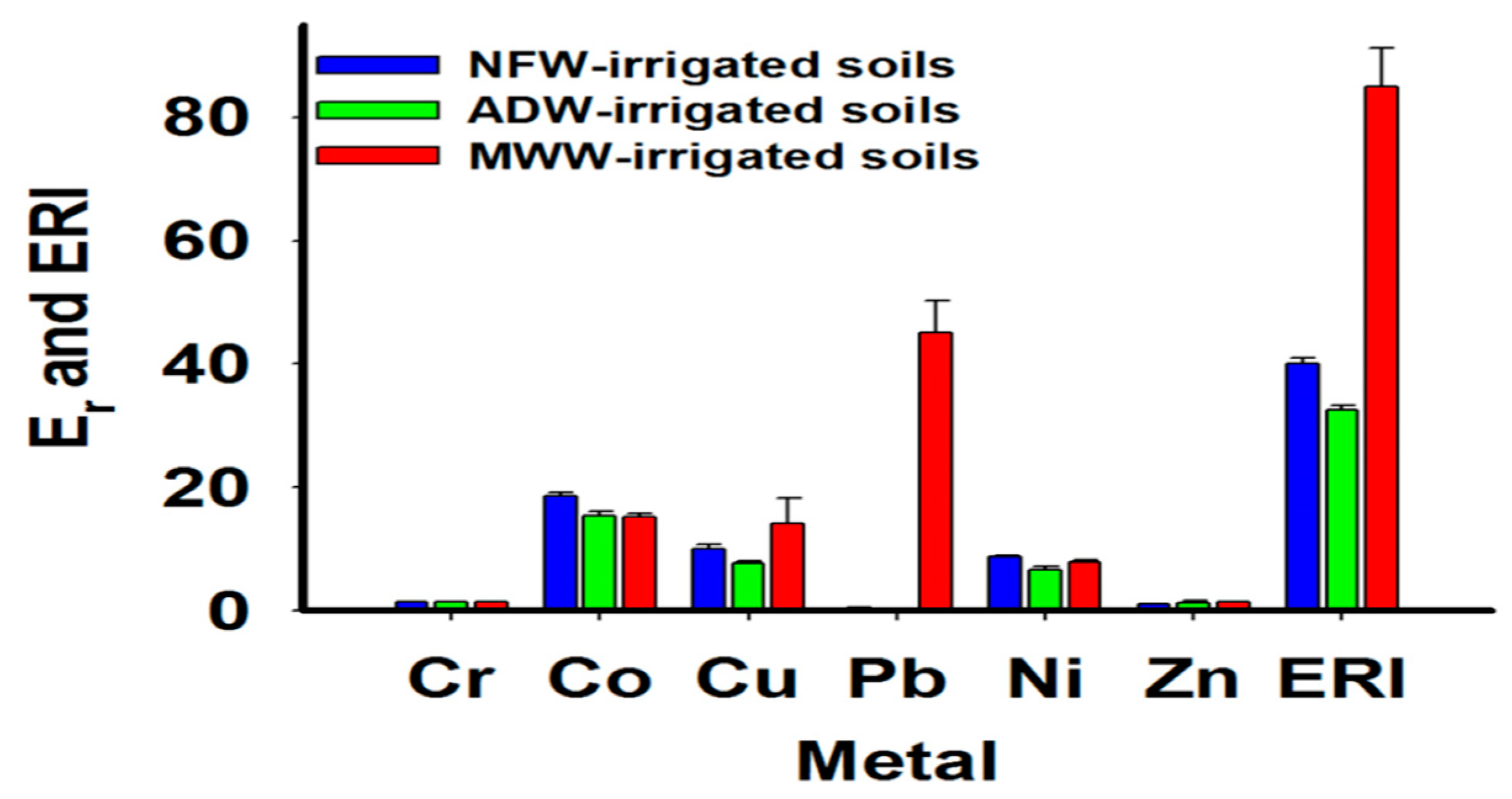
2.4.3. Potential Ecological Risk Index (PERI)
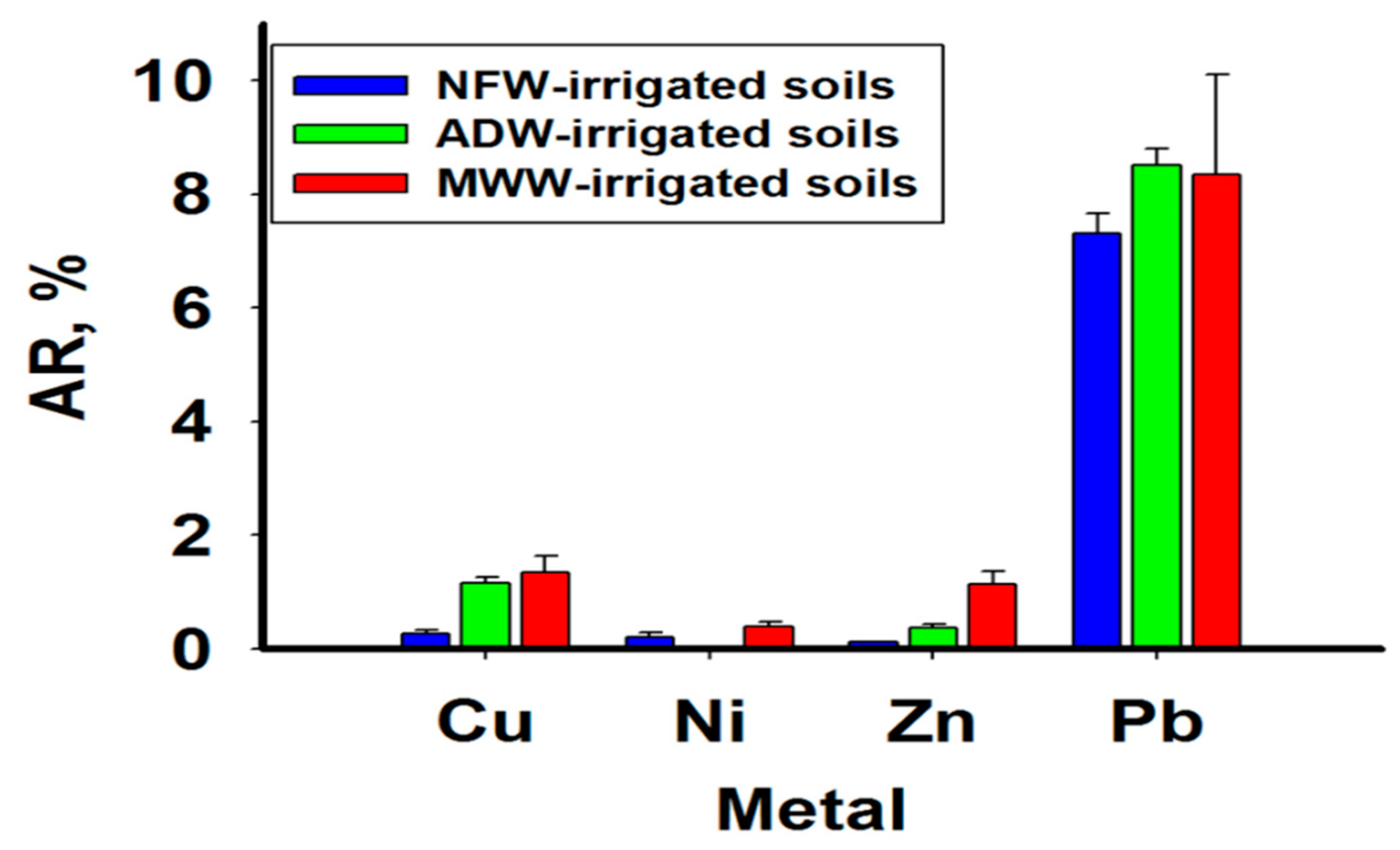
2.4.4. Availability Ratio (AR)
2.5. Accumulation and Translocation of PTMsin Berseem Plants
2.6. Data Analysis
2.7. Quality Control
3. Results
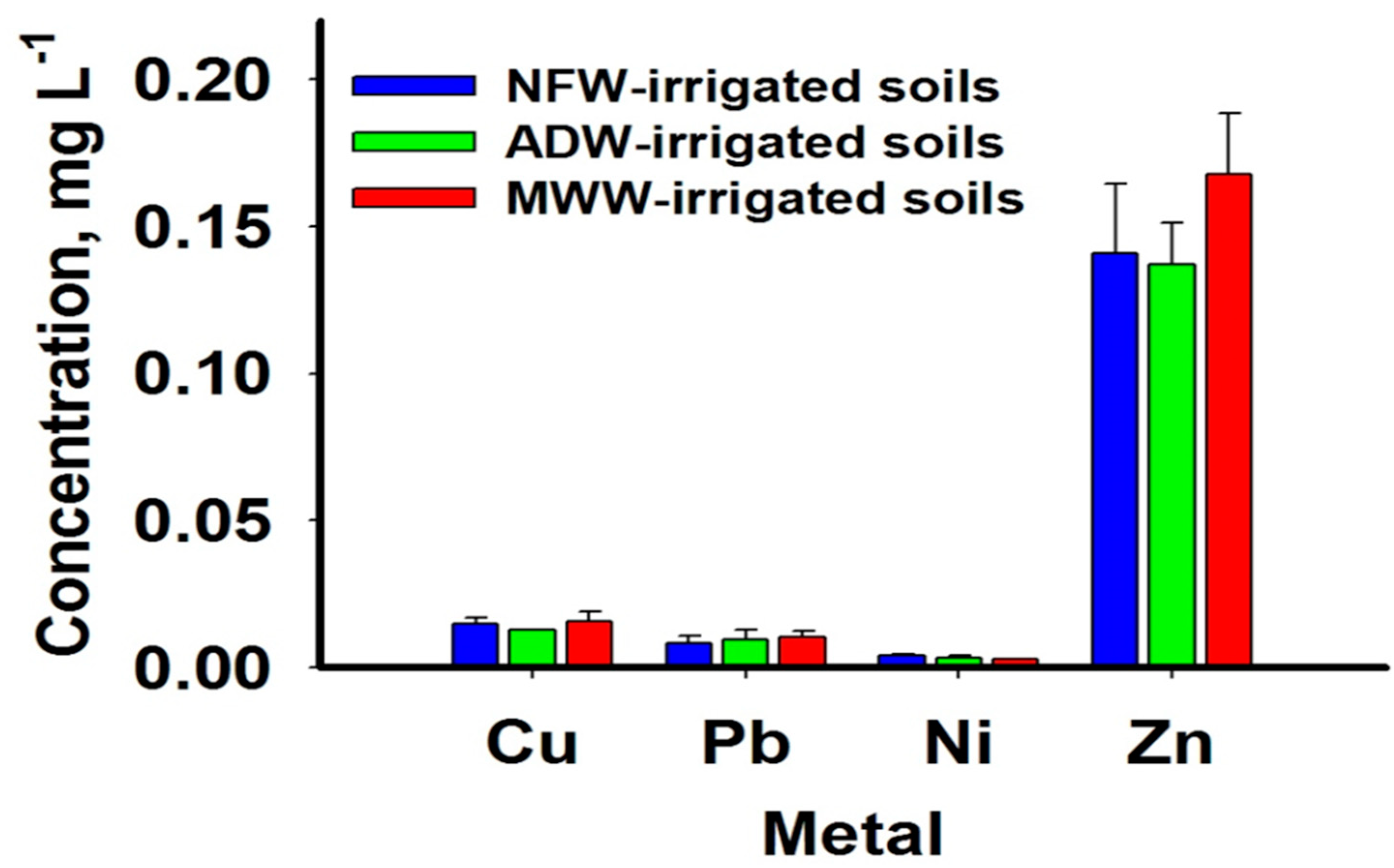
3.1. Concentrations of PTMs in Irrigation Water
3.2. Soil Contamination by PTMs
3.3. Availability Ratio (AR)
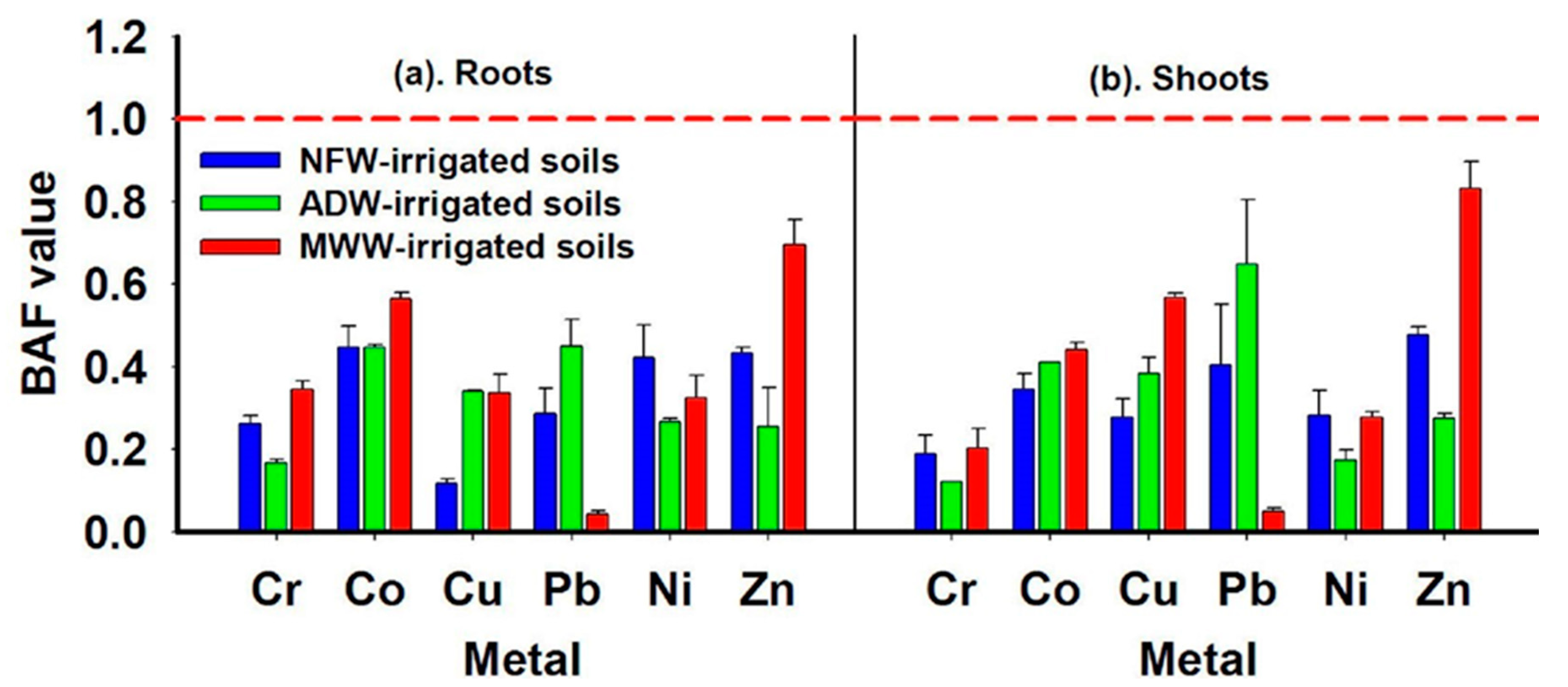
3.4. Berssem Plant Contamination by PTMs
3.5. Concentrations of PTMs in Groundwater
4. Discussion
4.1. Concentrations of PTMs in Irrigation Water
4.2. Soil Contamination by PTMs
4.3. Availability Ratio (AR)
4.4. Berseem Plant Contamination by PTMs
4.5. Concentrations of PTMs in Groundwater
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Neris, J.B.; Olivares, D.M.; Velasco, F.; Luzardo, F.; Correia, L.; González, L. HHRISK: A code for assessment of human health risk due to environmental chemical pollution. Ecotoxicol. Environ. Saf. 2019, 170, 538–547. [Google Scholar] [CrossRef]
- Latif, A.; Sheng, D.; Sun, K.; Si, Y.; Azeem, M.; Abbas, A.; Bilal, M. Remediation of heavy metals polluted environment using Fe-based nanoparticles: Mechanisms, influencing factors, and environmental implications. Environ. Pollut. 2020, 264, 114728. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Abuzaid, A.S.; Jahin, H. Profile Distribution and Source Identification of Potentially Toxic Elements in North Nile Delta, Egypt. Soil Sedim. Contam. Int. J. 2019, 28, 582–600. [Google Scholar] [CrossRef]
- Meli, S.; Porto, M.; Belligno, A.; Bufo, S.A.; Mazzatura, A.; Scopa, A. Influence of irrigation with lagooned urban wastewater on chemical and microbiological soil parameters in a citrus orchard under Mediterranean condition. Sci. Total. Environ. 2002, 285, 69–77. [Google Scholar] [CrossRef]
- Elbana, T.; Gaber, H.M.; Kishk, F.M. Soil chemical pollution and sustainable agriculture. In The Soils of Egypt; El-Ramady, H., Alshaal, T., Bakr, N., Elbana, T., Mohamed, E., Belal, A.-A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 187–200. [Google Scholar]
- Abbas, H.; Abuzaid, A.S.; Jahin, H.; Kasem, D. Assessing the quality of untraditional water sources for irrigation purposes in Al-Qalubiya Governorate, Egypt. Egypt. J. Soil Sci. 2020, 60, 157–166. [Google Scholar] [CrossRef]
- Loutfy, N.M. Reuse of wastewater in mediterranean region, Egyptian experience. In Waste Water Treatment and Reuse in the Mediterranean Region; Barceló, D., Petrovic, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 183–213. [Google Scholar]
- Elbana, T.A.; Bakr, N.; Elbana, M. Reuse of Treated Wastewater in Egypt: Challenges and Opportunities. In Unconventional Water Resources and Agriculture in Egypt; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 429–453. [Google Scholar]
- El-Quosy, D.E.D.A.H. The evolution of drainage water in Egypt. In Unconventional Water Resources and Agriculture in Egypt; Negm, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–16. [Google Scholar]
- El Hassan, W.H.A.; Allam, A. Management of the Integration between Irrigation and Drainage Water in the Nile Delta. In Unconventional Water Resources and Agriculture in Egypt; Negm, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 17–24. [Google Scholar]
- Abuzaid, A.S.; Fadl, M.E. Mapping potential risks of long-term wastewater irrigation in alluvial soils, Egypt. Arab. J. Geosci. 2018, 11, 433. [Google Scholar] [CrossRef]
- Abuzaid, A.S. Soil quality indicators in Al-Qalyubia Governorate as affected by long-term wastewater irrigation. Egypt. J. Soil Sci. 2018, 58, 1–11. [Google Scholar] [CrossRef]
- El-Rawy, M.; Abdelrahman, M.A.E.; Ismail, E. Integrated Use of Pollution Indices and Geomatics to Assess Soil Contamination and Identify Soil Pollution Source in El-Minia Governorate, Upper Egypt. J. Eng. Sci. Technol. 2020, 15, 2223–2238. [Google Scholar]
- Abuzaid, A.S. Sewage effluent as an alternative source for irrigation: Impact on soil properties and heavy metalstatus. Ann. Agric. Sci. Moshtohor 2016, 54, 387–396. [Google Scholar] [CrossRef]
- Elbana, T.; Bakr, N.; George, B.; Elbana, M. Assessment of Marginal Quality Water for Sustainable Irrigation Management: Case Study of Bahr El-Baqar Area, Egypt. Water Air Soil Pollut. 2017, 228, 214. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Goma, B.S.; Shuravilin, A.V. Assessment of heavy metal contamination in soils of eastern Nile Delta. Russ. Agric. Sci. 2014, 40, 454–458. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Awaad, H.; El-Naggar, N.; Hassan, H. Importance of Forage Mixtures in Increasing Sustainable Food Supply in Egypt. In Sustainability of Agricultural Environment in Egypt: Part II: Soil-Water-Plant Nexus; Negm, A.M., Abu-hashim, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 289–309. [Google Scholar]
- Ouda, S.; Noreldin, T.; Zohry, A.E.H. Field crops and deficit irrigation in Egypt. In Deficit Irrigation: A Remedy for Water Scarcity; Springer International Publishing: Cham, Switzerland, 2020; pp. 59–83. [Google Scholar]
- Bhatti, S.S.; Kumar, V.; Sambyal, V.; Singh, J.; Nagpal, A.K. Comparative analysis of tissue compartmentalized heavy metal uptake by common forage crop: A field experiment. Catena 2018, 160, 185–193. [Google Scholar] [CrossRef]
- Galal, T.M. Impact of environmental pollution on the growth and production of Egyptian clover. Int. J. Plant Prod. 2016, 10, 303–316. [Google Scholar]
- Salman, S.A.; Elnazer, A.; El Nazer, H.A. Integrated mass balance of some heavy metals fluxes in Yaakob village, south Sohag, Egypt. Int. J. Environ. Sci. Technol. 2017, 14, 1011–1018. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mohamed, N.N. Use of groundwater in Nile alluvial soils and their fringes. In Groundwater in the Nile Delta; Negm, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 107–140. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- EEAE (Egyptian Environmental Affairs Agency). Environmental Characterization of Al-Qalyubia Governorate (In Arabic); Ministry of Environment: Cairo, Egypt, 2007.
- EGPC/CONCO-Coral. Geologic Map of Egypt, Scale 1:500,000; EGPC/CONCO-Coral: Cairo, Egypt, 1987. [Google Scholar]
- Abd El-Hameed, A.H.; Naufal, E.H.; El-Husseiny, O.H.; Mohamed, M.K.; Abuzaid, A.S. Land suitability classification of some Qalubiya soils. Ann. Agric. Sci. Moshtohor 2013, 51, 147–158. [Google Scholar]
- Abuzaid, A.S. Pedological and Evaluation Studies on Some Soils of Middle Delta. Ph.D. Thesis, Faculty of Agriculture, Benha University, Benha, Egypt, 2013. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 23th ed.; APHA-AWWA-WEF: Washington, DC, USA, 2017. [Google Scholar]
- Soil Survey Staff. Soil survey field and laboratory methods manual. In Soil Survey Investigations Report No. 51, Version 2.0; Burt, R., Soil Survey Staff, Eds.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Test methods for evaluating solid waste. In Vol. Ia: Laboratory Manual Physical/Chemical Methods, SW 846, 3rd ed.; U.S. Government Publishing Office: Washington, DC, USA, 1995. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [Green Version]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Massas, I.; Gasparatos, D.; Ioannou, D.; Kalivas, D. Signs for secondary buildup of heavy metals in soils at the periphery of Athens International Airport, Greece. Environ. Sci. Pollut. Res. 2018, 25, 658–671. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water quality for agriculture. In FAO Irrigation and Drainage Paper 29; FAO: Rome, Italy, 1994. [Google Scholar]
- Yang, Z.; Jing, F.; Chen, X.; Liu, W.; Guo, B.; Lin, G.; Huang, R.; Liu, W. Spatial distribution and sources of seven available heavy metals in the paddy soil of red region in Hunan Province of China. Environ. Monit. Assess. 2018, 190, 611. [Google Scholar] [CrossRef]
- European Commission. Opinion of The Scientific Committee for Animal Nutrition on the Use of Copper in Feedingstuffs; Directive 70/524/EEC; European Commission: Brussel, Belgium, 2003. [Google Scholar]
- European Commission. Opinion of the Scientific Committee on Animal Nutrition on Undesirable Substances in Feed; Directive 1999/29/EC; European Commission: Brussel, Belgium, 2003. [Google Scholar]
- Khan, Z.I.; Ugulu, I.; Umar, S.; Ahmad, K.; Mehmood, N.; Ashfaq, A.; Bashir, H.; Sohail, M. Potential Toxic Metal Accumulation in Soil, Forage and Blood Plasma of Buffaloes Sampled from Jhang, Pakistan. Bull. Environ. Contam. Toxicol. 2018, 101, 235–242. [Google Scholar] [CrossRef]
- Christou, A.; Eliadou, E.; Michael, C.; Hapeshi, E.; Fatta-Kassinos, D. Assessment of long-term wastewater irrigation impacts on the soil geochemical properties and the bioaccumulation of heavy metals to the agricultural products. Environ. Monit. Assess. 2014, 186, 4857–4870. [Google Scholar] [CrossRef] [PubMed]
- Abuzaid, A.S.; Bassouny, M.A. Total and DTPA-extractable forms of potentially toxic metals in soils of rice fields, north Nile Delta of Egypt. Environ. Technol. Innov. 2020, 18, 100717. [Google Scholar] [CrossRef]
- Han, F.X.; Singer, A. Binding and distribution of trace rlements among solid-phase components in arid zone soils. In Biogeochemistry of Trace Elements in Arid Environments; Han, F.X., Singer, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 131–167. [Google Scholar]
- Gasparatos, D. Fe–Mn concretions and nodules to sequester heavy metals in soils. In Environmental Chemistry for A Sustainable World: Volume 2: Remediation of Air and Water Pollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 443–474. [Google Scholar]
- Young, S.D. Chemistry of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2013; pp. 51–96. [Google Scholar]
- Abuzaid, A.S.; Bassouny, M.A.; Jahin, H.; Abdelhafez, A.A. Stabilization of Lead and Copper in a Contaminated Typic Torripsament Soil Using Humic Substances. CLEAN Soil Air Water 2019, 47, 1800309. [Google Scholar] [CrossRef]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef] [PubMed]
- Liénard, A.; Colinet, G. Assessment of vertical contamination of Cd, Pb and Zn in soils around a former ore smelter in Wallonia, Belgium. Environ. Earth Sci. 2016, 75, 1322. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y. Effect of soil pH and organic matter content on heavy metals availability in maize (Zea mays L.) rhizospheric soil of non-ferrous metals smelting area. Environ. Monit. Assess. 2019, 191, 634. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.U.; Ayub, M.A.; Khalid, H.; Haq, M.A.U.; Rasul, A.; Rehman, M.Z.U.; Ali, S. Fate of micronutrients in alkaline Soils. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 577–613. [Google Scholar]
- Tahervand, S.; Jalali, M. Sorption, desorption, and speciation of Cd, Ni, and Fe by four calcareous soils as affected by pH. Environ. Monit. Assess. 2016, 188, 322. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Mineral nutrition. In Plant Physiological Ecology; Lambers, H., Oliveira, R.S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 301–384. [Google Scholar]
- Mahmud, J.A.; Borhannuddin Bhuyan, M.H.M.; Nahar, K.; Parvin, K.; Hasanuzzaman, M. Response and tolerance of Fabaceae plants to metal/metalloid toxicity. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Hasanuzzaman, M., Araújo, S., Gill, S.S., Eds.; Springer: Singapore, 2020; pp. 435–482. [Google Scholar]
- Ali, H.; Naseer, M.; Sajad, M.A. Phytoremediation of heavy metals by Trifolium alexandrinum. Int. J. Environ. Sci. 2012, 2, 1459–1469. [Google Scholar] [CrossRef] [Green Version]
- Jagetiya, B.; Kumar, S. Phytoremediation of Lead: A Review. In Lead in Plants and The Environment; Gupta, D.K., Chatterjee, S., Walther, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–202. [Google Scholar]
- Singh, N.P.; Santal, A.R. Phytoremediation of heavy metals: The use of green approaches to clean the environment. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 2, pp. 115–129. [Google Scholar]
- Blume, H.-P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Soil-Plant Relations. In Scheffer/Schachtschabel Soil Science; Blume, H.-P., Brümmer, G.W., Fleige, H., Horn, R., Kandeler, E., Kögel-Knabner, I., Kretzschmar, R., Stahr, K., Wilke, B.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 409–484. [Google Scholar]
- Shaheen, S.M.; Rinklebe, J.; Tsadilas, C.D. Fractionation and mobilization of toxic elements in floodplain soils from Egypt, Germany, and Greece: A comparison study. Eurasian Soil Sci. 2015, 48, 1317–1328. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Jahin, H.S. Implications of irrigation water quality on shallow groundwater in the Nile Delta of Egypt. Environ. Technol. Innov. 2021, 22, 101383. [Google Scholar] [CrossRef]
- Abu Salem, H.; Gemail, K.S.; Nosair, A.M. A multidisciplinary approach for delineating wastewater flow paths in shallow groundwater aquifers: A case study in the southeastern part of the Nile Delta, Egypt. J. Contam. Hydrol. 2020, 236, 103701. [Google Scholar] [CrossRef] [PubMed]
- Bassouny, M.A.; Abuzaid, A.S. Impact of Biogas Slurry on Some Physical Properties in Sandy and Calcareous Soils, Egypt. Int. J. Plant Soil Sci. 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]

| Property | Unit | NFW-Irrigated Soils | ADW-Irrigated Soils | MWW-Irrigated Soils |
|---|---|---|---|---|
| pH | --- | 7.08 ± 0.03 b | 7.22 ± 0.01 a | 7.09 ± 0.03 b |
| EC | dS m−1 | 1.26 ± 0.31 a | 2.45 ± 0.55 a | 1.41 ± 0.23 a |
| ESP | --- | 5.44 ± 0.49 b | 10.98 ± 0.82 a | 9.72 ± 0.55 a |
| OM | g kg−1 | 15.77 ± 1.11 a | 16.84 ± 2.61 a | 17.95 ± 2.21 a |
| Sand | % | 62.98 ± 3.08 a | 42.95 ± 2.77 b | 55.03 ± 2.88 a |
| Silt | % | 19.09 ± 2.44 c | 40.74 ± 2.63 a | 29.12 ± 2.11 b |
| Clay | % | 17.93 ± 3.52 a | 16.31 ± 1.75 a | 15.85 ± 1.58 a |
| Al | g kg−1 | 45.32 ± 0.94 a | 39.89 ± 2.96 ab | 37.83 ± 1.55 b |
| Fe | 41.95 ± 1.41 a | 36.68 ± 2.04 b | 33.68 ± 1.09 b | |
| Mn | 0.81 ± 0.02 a | 0.74 ± 0.05 a | 0.72 ± 0.03 a |
| PTM | Nile Freshwater | Agricultural Drainage Water | Mixed Wastewater | MAC | |||
|---|---|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | ||
| Cr | Bdl–0.006 | 0.001 ± 0.001 a | bdl–0.008 | 0.003 ± 0.001 a | bdl–0.008 | 0.004 ± 0.001 a | 0.100 |
| Co | bdl–0.006 | 0.002 ± 0.001 a | bdl–0.008 | 0.002 ± 0.001 a | bdl–0.008 | 0.003 ± 0.001 a | 0.050 |
| Cu | bdl–0.073 | 0.021 ± 0.007 a | 0.032–0.068 | 0.049 ± 0.005 a | 0.029–0.413 | 0.087 ± 0.037 a | 0.200 |
| Pb | bdl–0.007 | 0.001± 0.001 b | bdl–0.016 | 0.004 ± 0.002 ab | 0.005–0.014 | 0.008 ± 0.001 a | 5.000 |
| Ni | bdl–0.017 | 0.007 ± 0.002 ab | bdl–0.008 | 0.004 ± 0.001 b | 0.004–0.034 | 0.022 ± 0.003 a | 0.200 |
| Zn | bdl–0.020 | 0.003 ± 0.002 a | 0.010–0.394 | 0.063 ± 0.047a | 0.012–0.088 | 0.029 ± 0.008 a | 5.000 |
| PTM | NFW-Irrigated Soils | ADW-Irrigated Soils | MWW-Irrigated Soils | Earth’s Crust | MAC | |||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | |||
| Total content | ||||||||
| Cr | 61.11–75.32 | 71.65 ± 1.61 a | 56.51–85.52 | 69.19 ± 74.56 a | 67.13–89.51 | 74.65 ± 2.85 a | 100 | 50–200 |
| Co | 32.51–41.55 | 36.88 ± 0.97 a | 24.53–40.61 | 30.63 ± 1.64 b | 27.41–36.01 | 30.88 ± 1.12 b | 10 | 20–50 |
| Cu | 84.11–141.21 | 106.25 ± 8.44 a | 71.11–95.52 | 84.56 ± 2.81 a | 76.56–206.12 | 110.63 ± 4.57 a | 55 | 60–150 |
| Pb | 5.11–12.51 | 6.13 ± 0.61 b | 0.67–4.11 | 1.81 ± 0.13 b | 55.63–193.42 | 136.63 ± 9.14 a | 15 | 20–300 |
| Ni | 30.35–39.12 | 34.06 ± 0.86 a | 20.15–35.61 | 26.81 ± 1.77 b | 25.61–40.12 | 32.25 ± 1.89 ab | 20 | 20–60 |
| Zn | 61.51–89.12 | 74.88 ± 3.29 a | 54.53–288.21 | 86.13 ± 6.62 a | 67.56–155.67 | 94.97 ± 4.93 a | 70 | 100–300 |
| DTPA-extractable content | ||||||||
| Cr | <0.002 | 0.05–0.26 | 0.06 ± 0.02 | 0.50 | ||||
| Co | <0.003 | 0.50 | ||||||
| Cu | 0.11–0.45 | 0.35 ± 0.04 b | 0.67–1.54 | 0.98 ± 0.11 a | 0.36–2.74 | 1.42 ± 0.22 a | --- | 0.20 |
| Pb | 0.05–0.46 | 0.22 ± 0.05 b | 0.31–43 | 0.35 ± 0.01 b | 1.58–36.71 | 12.21 ± 2.65 a | --- | 10.00 |
| Ni | 0.02–0.03 | 0.03 ± 0.01 b | <0.004 | 0.07–0.24 | 0.15 ± 0.01 a | --- | 1.00 | |
| Zn | 0.02–0.13 | 0.05 ± 0.01 b | 0.17–0.44 | 0.27 ± 0.03 b | 0.31–2.72 | 1.01 ± 0.25 a | --- | 0.50 |
| PTM | Content | NFW-Irrigated Sites | ADW-Irrigated Sites | MWW-Irrigated Sites | |||
|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | ||
| Cr | Total | −0.005 | ns | 0.521 | * | 0.580 | * |
| Available | --- | --- | --- | --- | −0.318 | ns | |
| Co | Total | −0.018 | ns | 0.069 | ns | −0.376 | ns |
| Available | --- | --- | --- | --- | --- | --- | |
| Cu | Total | −0.171 | ns | 0.080 | ns | 0.546 | * |
| Available | 0.176 | ns | −0.401 | ns | 0.537 | * | |
| Pb | Total | 0.176 | ns | 0.515 | * | −0.507 | * |
| Available | 0.771 | ** | −0.092 | ns | −0.293 | ns | |
| Ni | Total | −0.463 | ns | 0.171 | ns | 0.064 | ns |
| Available | 0.998 | ** | --- | --- | −0.686 | * | |
| Zn | Total | 0.167 | ns | −0.149 | ns | −0.041 | ns |
| Available | −0.073 | ns | −0.390 | ns | −0.291 | ns | |
| Soil Property | Cr | Cu | Pb | Ni | Zn |
|---|---|---|---|---|---|
| Nile freshwater-irrigated soils | |||||
| pH | --- | −0.193 | −0.107 | −0.132 | −0.272 |
| Organic matter | --- | 0.328 | 0.633 * | 0.024 | 0.278 |
| Clay | --- | 0.530 | 0.831 ** | 0.586 | 0.753 * |
| Al | --- | 0.095 | 0.030 | 0.053 | 0.124 |
| Fe | --- | −0.148 | 0.019 | −0.158 | 0.063 |
| Mn | --- | −0.318 | −0.050 | 0.041 | 0.119 |
| Agricultural drainage water-irrigated soils | |||||
| pH | --- | −0.479 | −0.054 | --- | −0.481 |
| Organic matter | --- | 0.159 | −0.232 | --- | 0.500 |
| Clay | --- | 0.108 | −0.167 | --- | 0.503 |
| Al | --- | −0.156 | −0.451 | --- | −0.240 |
| Fe | --- | −0.190 | −0.434 | --- | −0.282 |
| Mn | --- | −0.117 | −0.193 | --- | −0.204 |
| Mixed wastewater-irrigated soils | |||||
| pH | −0.025 | −0.581 | −0.224 | −0.692 * | −0.439 |
| Organic matter | −0.060 | 0.368 | −0.031 | 0.424 | 0.206 |
| Clay | −0.160 | 0.082 | 0.171 | −0.032 | −0.159 |
| Al | 0.057 | −0.210 | −0.377 | 0.015 | −0.250 |
| Fe | 0.106 | −0.154 | −0.296 | 0.055 | −0.096 |
| Mn | −0.317 | 0.155 | 0.322 | 0.014 | 0.470 |
| PTM | NFW-Irrigated Soils | ADW-Irrigated Soils | MWW-Irrigated Soils | MAC | |||
|---|---|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | ||
| Roots | |||||||
| Cr | 17.11–21.62 | 18.38 ± 1.09 ab | 10.51–13.62 | 12.75 ± 0.14 b | 21.62–25.65 | 23.05 ± 1.30 a | --- |
| Co | 16.12–19.25 | 16.51 ± 0.29 a | 12.65–14.63 | 13.44 ± 0.29 b | 15.32–17.98 | 16.22 ± 0.45 a | --- |
| Cu | 17.43–22.51 | 19.33 ± 1.59 b | 26.51–28.11 | 26.54 ± 0.29 a | 24.62–33.31 | 28.76 ± 1.88 a | --- |
| Pb | 1.11–2.96 | 1.61 ± 0.27 b | 1.33–2.96 | 1.93 ± 0.34 b | 3.87–7.51 | 5.96 ± 0.87 a | --- |
| Ni | 13.34–19.11 | 16.96 ± 1.51 a | 6.36–9.84 | 8.23 ± 0.29 b | 7.32–12.69 | 9.62 ± 1.44 b | --- |
| Zn | 39.62–48.32 | 43.16 ± 1.91 a | 24.94–31.23 | 29.31 ± 1.15 b | 46.74–55.61 | 51.25 ± 1.88 a | --- |
| Shoots | |||||||
| Cr | 11.51–19.75 | 14.33 ±1.71 a | 8.44–10.93 | 9.26 ± 0.43 a | 8.76–10.29 | 9.11 ± 0.14 a | 15 |
| Co | 12.66–14.27 | 13.67 ± 0.46 a | 11.33–12.66 | 12.54 ± 0.05 a | 11.75–13.73 | 12.75 ± 0.58 a | 0.50 |
| Cu | 36.52–54.25 | 45.46 ± 3.12 ab | 25.63–35.71 | 30.38 ± 2.96 b | 44.73–53.81 | 49.38 ± 1.96 a | 15 |
| Pb | 0.95–3.78 | 2.17 ± 0.84 b | 1.52–4.11 | 2.81± 0.75 b | 5.11–8.78 | 6.95 ± 0.77 a | 5 |
| Ni | 7.33–13.47 | 10.67 ± 1.74 a | 3.62–7.01 | 5.91 ± 0.43 b | 6.91–9.72 | 8.13 ± 0.51 ab | 3 |
| Zn | 45.62–54.31 | 48.29 ± 1.39 a | 17.51–60.19 | 38.75 ± 2.91 a | 48.75–65.14 | 61.38 ± 1.44 a | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzaid, A.S.; Jahin, H.S.; Asaad, A.A.; Fadl, M.E.; AbdelRahman, M.A.E.; Scopa, A. Accumulation of Potentially Toxic Metals in Egyptian Alluvial Soils, Berseem Clover (Trifolium alexandrinum L.), and Groundwater after Long-Term Wastewater Irrigation. Agriculture 2021, 11, 713. https://doi.org/10.3390/agriculture11080713
Abuzaid AS, Jahin HS, Asaad AA, Fadl ME, AbdelRahman MAE, Scopa A. Accumulation of Potentially Toxic Metals in Egyptian Alluvial Soils, Berseem Clover (Trifolium alexandrinum L.), and Groundwater after Long-Term Wastewater Irrigation. Agriculture. 2021; 11(8):713. https://doi.org/10.3390/agriculture11080713
Chicago/Turabian StyleAbuzaid, Ahmed S., Hossam S. Jahin, Amany A. Asaad, Mohamed E. Fadl, Mohamed A. E. AbdelRahman, and Antonio Scopa. 2021. "Accumulation of Potentially Toxic Metals in Egyptian Alluvial Soils, Berseem Clover (Trifolium alexandrinum L.), and Groundwater after Long-Term Wastewater Irrigation" Agriculture 11, no. 8: 713. https://doi.org/10.3390/agriculture11080713









