Effect of Calcium Cyanamide on Soil Fungal Community in Successive Tea-Cuttings Nursery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Soil Physicochemical Properties
2.3. Soil DNA Extraction, PCR Amplification and Illumina Sequencing
2.4. Data Analysis
3. Results
3.1. General Characteristics of Soil Properties
3.2. Assessment of Sequencing Data
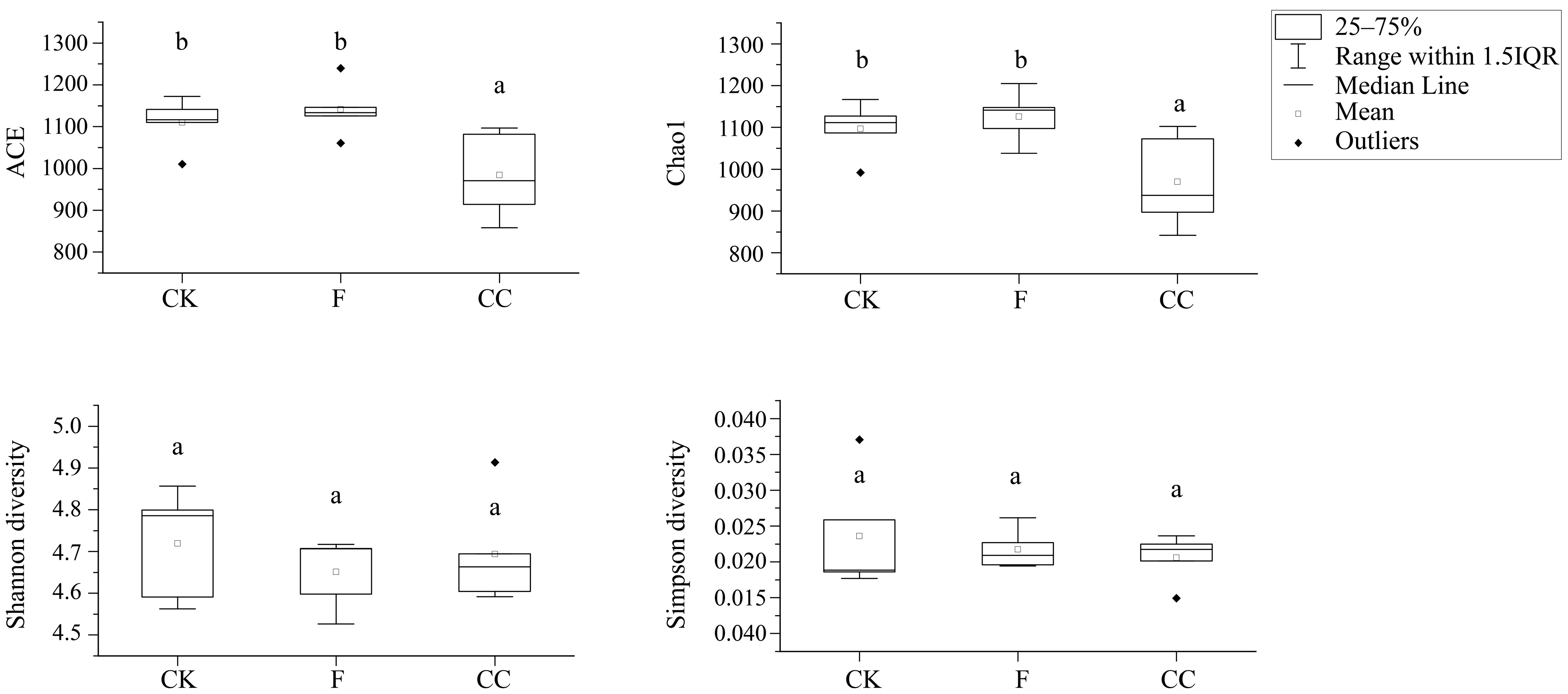
3.3. Alpha Diversity Analysis
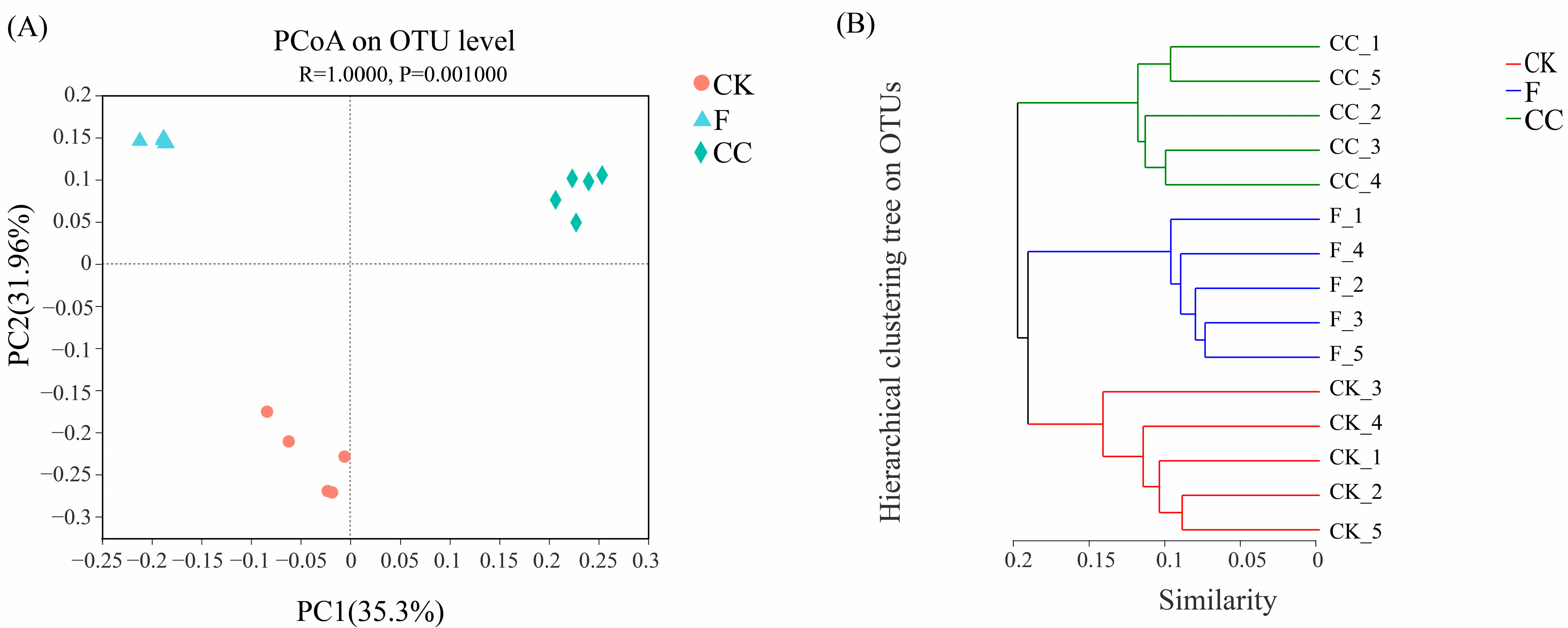
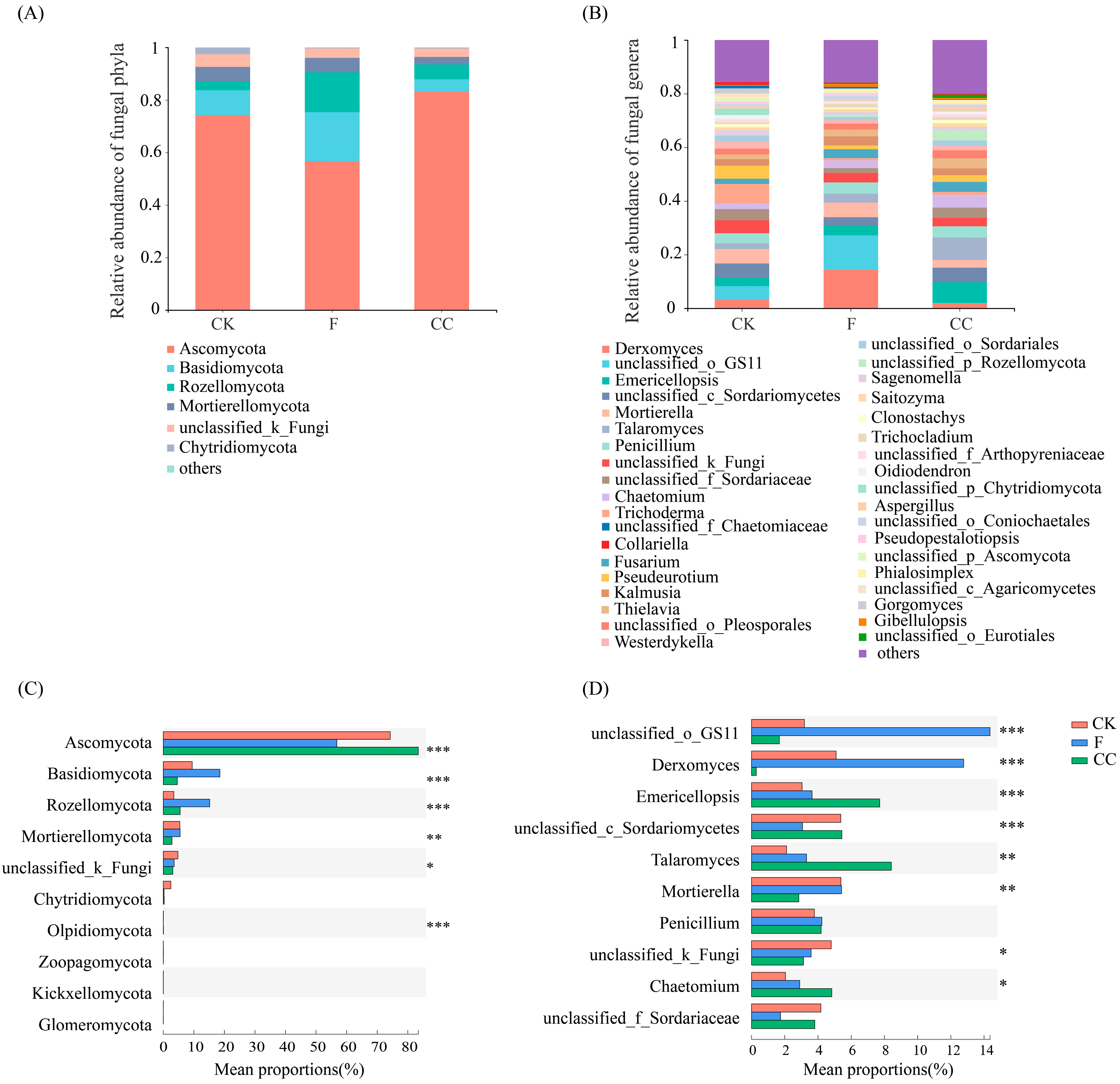
3.4. Fungal Community Structure and Composition
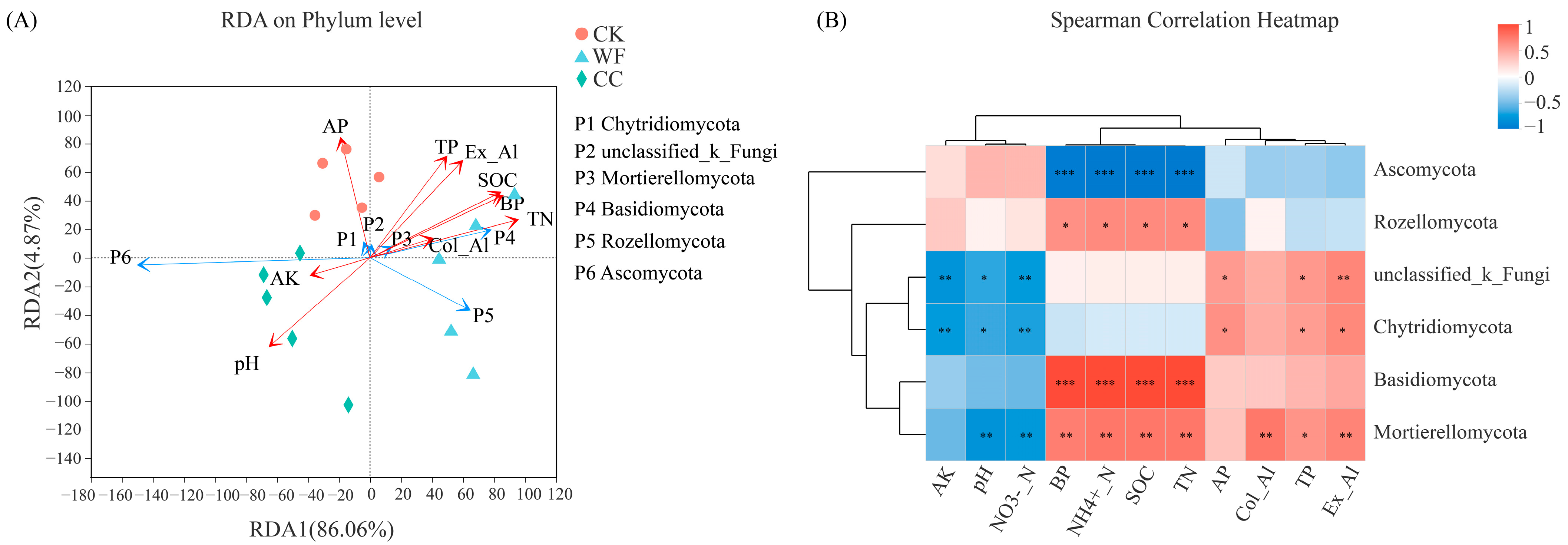
3.5. Correlation among Soil Physicochemical Properties and Abundance of Fungal Taxa
3.6. Molecular Ecological Network Analysis
3.7. Functional Prediction of Soil Fungal Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Amjad, H.; Awais, S.; Lin, X.M.; Lin, W.X.; Lin, S. Long-Term Monoculture Negatively Regulates Fungal Community Composition and Abundance of Tea Orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Li, Z.W.; Arafat, Y.; Lin, W.X. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, R.K.; Wang, N.; Li, X.H. Soil Acidification of Alfisols as Influenced by Tea Cultivation in Eastern China. Pedosphere 2010, 20, 799–806. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.H.; Wang, H.B.; Yang, X.Y.; Zhang, Q.; Li, J.Y.; Jia, X.L.; Kong, X.H.; He, H.B. Autotoxicity of the soil of consecutively cultured tea plantations on tea (Camellia sinensis) seedlings. Acta Physiol. Plant. 2016, 38, 195. [Google Scholar] [CrossRef]
- Fan, D.M.; Fan, K.; Yu, C.P.; Lu, Y.T.; Wang, X.C. Tea polyphenols dominate the short-term tea (Camellia sinensis) leaf litter decomposition. J. Zhejiang Univ. Sci. B 2017, 18, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wu, F. p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f.sp. cucumerinum Owen. PLoS ONE 2012, 7, e48288. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Motta, A.C.; Reeves, D.W.; Burmester, C.H.; van Santen, E.; Osborne, J.A. Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biol. Biochem. 2003, 35, 1693–1703. [Google Scholar] [CrossRef]
- Lu, L.; Yin, S.; Liu, X.; Zhang, W.; Gu, T.; Shen, Q.; Qiu, H. Fungal networks in yield-invigorating and -debilitating soils induced by prolonged potato monoculture. Soil Biol. Biochem. 2013, 65, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Nicola, L.; Turco, E.; Albanese, D.; Donati, C.; Thalheimer, M.; Pindo, M.; Insam, H.; Cavalieri, D.; Pertot, I. Fumigation with dazomet modifies soil microbiota in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 113, 71–79. [Google Scholar] [CrossRef]
- Benizri, E.; Piutti, S.; Verger, S.; Pagès, L.; Vercambre, G.; Poessel, J.L.; Michelot, P. Replant diseases: Bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol. Biochem. 2005, 37, 1738–1746. [Google Scholar] [CrossRef]
- Lin, W.; Wu, L.; Lin, S.; Zhang, A.; Zhou, M.; Lin, R.; Wang, H.; Chen, J.; Zhang, Z.; Lin, R. Metaproteomic analysis of ratoon sugarcane rhizospheric soil. BMC Microbiol. 2013, 13, 135. [Google Scholar] [CrossRef] [Green Version]
- Dixon, G.R. Managing clubroot disease (caused by Plasmodiophora brassicae Wor.) by exploiting the interactions between calcium cyanamide fertilizer and soil microorganisms. J. Agric. Sci. 2016, 155, 527–543. [Google Scholar] [CrossRef]
- Ma, J.; Sun, W.; Hu, Q.; Yu, Q.; Wang, Q.; Fu, J. Effects of cyanamide fertiliser on microbial community structure of continuous cropping soil. J. Zhejiang Univ. Agric. Life Sci. 2013, 39, 281–290. [Google Scholar]
- Oh, K.; Kato, T.; Li, Z.-P.; Li, F.-Y. Environmental Problems From Tea Cultivation in Japan and a Control Measure Using Calcium Cyanamide. Pedosphere 2006, 16, 770–777. [Google Scholar] [CrossRef]
- Chai, A.L.; Xie, X.W.; Shi, Y.X.; Li, B.J. Research status of clubroot (Plasmodiophora brassicae) on cruciferous crops in China. Can. J. Plant Pathol. 2014, 36 (Suppl. 1), 142–153. [Google Scholar] [CrossRef]
- Bourbos, V.A.; Skoudridakis, M.T.; Darakis, G.A.; Koulizakis, M. Calcium cyanamide and soil solarization for the control of Fusarium solani f.sp. cucurbitae in greenhouse cucumber. Crop Prot. 1997, 16, 383–386. [Google Scholar] [CrossRef]
- Liu, S.C.; Tang, L.Z.; Shi, Q.; Yi, Z.X.; Zhou, W.X. Progress on application of calcium cyanamide in agricultural production. Soil Fertil. Sci. China 2017, 4, 1–6. [Google Scholar]
- Hou, Y.L. Experimental Study of Potassium Nitrate and Calcium Cyanamide on Breaking Natural Dormancy of Apple and Grape. J. Anhui Agric. Sci. 2008, 36, 13612–13617. [Google Scholar]
- Dixon, G.R. Calcium Cyanamide—A Synoptic Review of an Environmentally Benign Fertiliser Which Enhances Soil Health. Acta Hortic. 2012, 938, 211–217. [Google Scholar] [CrossRef]
- Wu, Q.F.; Xu, Q.F.; Qin, H.; Zhang, J.L.; Qian, M.; Qian, J.W. Effects of calcium cyanamide on soil microbial properties of intensively managed Phyllostachys violascens stands. J. Zhejiang Univ. 2014, 31, 352–357. [Google Scholar]
- Belec, C.; Tremblay, N.; Coulombe, J. Liming and calcium cyanamid for clubroot control in cauliflower. Acta Hortic. 2004, 635, 41–46. [Google Scholar] [CrossRef]
- Strauss, S.L.; Greenhut, R.F.; McClean, A.E.; Kluepfel, D.A. Effect of anaerobic soil disinfestation on the bacterial community and key soilborne phytopathogenic agents under walnut tree-crop nursery conditions. Plant Soil 2017, 415, 493–506. [Google Scholar] [CrossRef]
- Khadka, R.B.; Marasini, M.; Rawal, R.; Testen, A.L.; Miller, S.A. Effects of anaerobic soil disinfestation carbon sources on soilborne diseases and weeds of okra and eggplant in Nepal. Crop Prot. 2020, 135, 104846. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.D.; Forn, I.; Gadelha, C.; Egan, M.J.; Bass, D.; Massana, R.; Richards, T.A. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 2011, 474, 200–203. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- De Gannes, V.; Eudoxie, G.; Hickey, W.J. Insights into fungal communities in composts revealed by 454-pyrosequencing: Implications for human health and safety. Front. Microbiol. 2013, 4, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Wang, K.; Yin, X.B.; Yin, X.Q.; Du, M.F.; Gao, Y.Z.; Antwi, P.; Ren, N.Q.; Wang, A.J. Trophic mode and organics metabolic characteristic of fungal community in swine manure composting. Front. Environ. Sci. Eng. 2019, 13, 93. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M. Functional groups of soil fungi decline under grazing. Plant Soil 2018, 426, 51–60. [Google Scholar] [CrossRef]
- Anthony, M.A.; Frey, S.D.; Stinson, K.A. Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion. Ecosphere 2017, 8, e01951. [Google Scholar] [CrossRef] [Green Version]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Che, R.; Wang, S.; Wang, Y.; Xu, Z.; Wang, W.; Rui, Y.; Wang, F.; Hu, J.; Tao, J.; Cui, X. Total and active soil fungal community profiles were significantly altered by six years of warming but not by grazing. Soil Biol. Biochem. 2019, 139, 107611. [Google Scholar] [CrossRef]
- Li, P.F.; Liu, J.; Jiang, C.Y.; Wu, M.; Liu, M.; Wei, S.P.; Qiu, C.P.; Li, G.L.; Xu, C.X.; Li, Z.P. Trade-off between potential phytopathogenic and non-phytopathogenic fungi in the peanut monoculture cultivation system. Appl. Soil Ecol. 2020, 148, 103508. [Google Scholar] [CrossRef]
- Roper, W.R.; Robarge, W.P.; Osmond, D.L.; Heitman, J.L. Comparing Four Methods of Measuring Soil Organic Matter in North Carolina Soils. Soil Sci. Soc. Am. J. 2019, 83, 466–474. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, D.M.; Zhu, Q.; Luo, Y.P.; Wang, X.C. Contribution of High Accumulated Polyphenols to C Stabilization in Soil of Tea Gardens. In Functions of Natural Organic Matter in Changing Environment; Springer: Dordrecht, The Netherlands, 2013; pp. 397–400. [Google Scholar]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2, e00122-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafat, Y.; Din, I.U.; Tayyab, M.; Jiang, Y.; Chen, T.; Cai, Z.; Zhao, H.; Lin, X.; Lin, W.; Lin, S. Soil Sickness in Aged Tea Plantation Is Associated with a Shift in Microbial Communities as a Result of Plant Polyphenol Accumulation in the Tea Gardens. Front. Plant Sci. 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Xu, T.-L.; Veresoglou, S.D.; Hu, H.-W.; Hao, Z.-P.; Hu, Y.-J.; Liu, L.; Deng, Y.; Rillig, M.C.; Chen, B.-D. Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol. Biochem. 2017, 110, 12–21. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N.; Neff, J.C.; Bowman, W.D.; Schadt, C.W.; Weintraub, M.N.; Schmidt, S.K. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ. Microbiol. 2008, 10, 3093–3105. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.; Lonhienne, T.G.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, K.; Wang, M.H. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiol. Mol. Plant Pathol. 2020, 109, 101458. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yong, Z.; Oh, K.T.; Nguyen, V.N.; Park, R.D. Enzymatic deacetylation of chitin by extracellular chitin deacetylase from a newly screened Mortierella sp. DY-52. J. Microbiol. Biotechnol. 2008, 18, 759–766. [Google Scholar]
- Zhang, H.S.; Wu, X.H.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, F.S.; Wu, X.Q.; Luan, F.G.; Zhang, L.P.; Fang, X.M.; Wan, S.Z.; Hu, X.F.; Ye, J.R. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar] [CrossRef] [Green Version]
- Tayyab, M.; Islam, W.; Lee, C.G.; Pang, Z.Q.; Khalil, F.; Lin, S.; Lin, W.X.; Zhang, H. Short-Term Effects of Different Organic Amendments on Soil Fungal Composition. Sustainability 2019, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Zhang, Y.C.; Wang, K.; Hao, X.L.; Chen, W.L.; Huang, Q.Y. Complexity of bacterial and fungal network increases with soil aggregate size in an agricultural Inceptisol. Appl. Soil Ecol. 2020, 154, 103640. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Jiliberto, R.; Valdovinos, F.S.; de Espanes, P.M.; Flores, J.D. Topological plasticity increases robustness of mutualistic networks. J. Anim. Ecol. 2012, 81, 896–904. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Muff, S.; Memmott, J.; Muller, C.B.; Caflisch, A. The robustness of pollination networks to the loss of species and interactions: A quantitative approach incorporating pollinator behaviour. Ecol. Lett. 2010, 13, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Peura, S.; Bertilsson, S.; Jones, R.I.; Eiler, A. Resistant microbial cooccurrence patterns inferred by network topology. Appl. Environ. Microbiol. 2015, 81, 2090–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Shi, K.; Wang, L.; Zhou, Y.H.; Yu, Y.L.; Yu, J.Q. Effects of calcium cyanamide on soil microbial communities and Fusarium oxysporum f. sp. cucumberinum. Chemosphere 2009, 75, 872–877. [Google Scholar] [CrossRef]
- Martinelli, L.; Zalar, P.; Gunde-Cimerman, N.; Azua-Bustos, A.; Sterflinger, K.; Pinar, G. Aspergillus atacamensis and A. salisburgensis: Two new halophilic species from hypersaline/arid habitats with a phialosimplex-like morphology. Extremophiles 2017, 21, 755–773. [Google Scholar] [CrossRef]
- Havenga, M.; Gatsi, G.M.; Halleen, F.; Spies, C.F.J.; van der Merwe, R.; Mostert, L. Canker and Wood Rot Pathogens Present in Young Apple Trees and Propagation Material in the Western Cape of South Africa. Plant Dis. 2019, 103, 3129–3141. [Google Scholar] [CrossRef]
- Sampaio, J.P.; Fell, L.; Statzell, I.L. Hunter & Phaff (1969). In The Yeasts; Elsevier: London, UK, 2011; pp. 1485–1494. [Google Scholar]
- Zheng, F.; Xu, G.; Zheng, F.Q.; Ding, X.F.; Xie, C.P. Neocosmospora rubicola Causing Stem Rot of Pitaya (Hylocereus costaricensis) in China. Plant Dis. 2018, 102, 2653. [Google Scholar] [CrossRef]
- de Medeiros, L.S.; Abreu, L.M.; Nielsen, A.; Ingmer, H.; Larsen, T.O.; Nielsen, K.F.; Rodrigues-Filho, E. Dereplication-guided isolation of depsides thielavins S-T and lecanorins D-F from the endophytic fungus Setophoma sp. Phytochemistry 2015, 111, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Ji, Y.-P.; Qu, Y.-Y.; Qi, Y.-K.; Li, D.-W.; Liu, Z.-Y.; Wu, X.-Q. The response strategies of Colletotrichum gloeosporioides s.s. due to the stress caused by biological control agent Bacillus amyloliquefaciens deciphered by transcriptome analyses. Biol. Control 2020, 150, 104372. [Google Scholar] [CrossRef]
- Xu, D.; Pang, X.J.; Zhao, T.; Xu, L.L.; Yang, X.L. New alkenylated tetrahydropyran derivatives from the marine sediment-derived fungus Westerdykella dispersa and their bioactivities. Fitoterapia 2017, 122, 45–51. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, M.; Zhu, R.X.; Zhang, J.Z.; Xie, F.; Li, X.B.; Chang, W.Q.; Wang, X.N.; Zhao, Z.T.; Lou, H.X. Heptaketides from an Endolichenic Fungus Biatriospora sp. and Their Antifungal Activity. J. Nat. Prod. 2016, 79, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Bartíková, M.; Holková, L.; Šafránková, I. Occurrence of boxwood blight (Calonectria pseudonaviculata and C. henricotiae) in historical gardens in the Czech Republic. Eur. J. Plant Pathol. 2020, 158, 135–142. [Google Scholar] [CrossRef]
- Mendez-Liter, J.A.; de Eugenio, L.I.; Nieto-Dominguez, M.; Prieto, A.; Martinez, M.J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2021, 324, 124623. [Google Scholar] [CrossRef]
- Aduroja, E.D.; Moore, G.G.; Beltz, S.B.; Soares, C.; Lima, N.; Fapohunda, S.O. Induction of biodeterioration on vegetables by three fungal species. J. Plant Pathol. 2018, 101, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, L.; Jimenez, C.; Rodriguez, J.; Areche, C.; Chavez, R.; Henriquez, M.; de la Cruz, M.; Diaz, C.; Segade, Y.; Vaca, I. 3-Nitroasterric Acid Derivatives from an Antarctic Sponge-Derived Pseudogymnoascus sp. Fungus. J. Nat. Prod. 2015, 78, 919–923. [Google Scholar] [CrossRef]

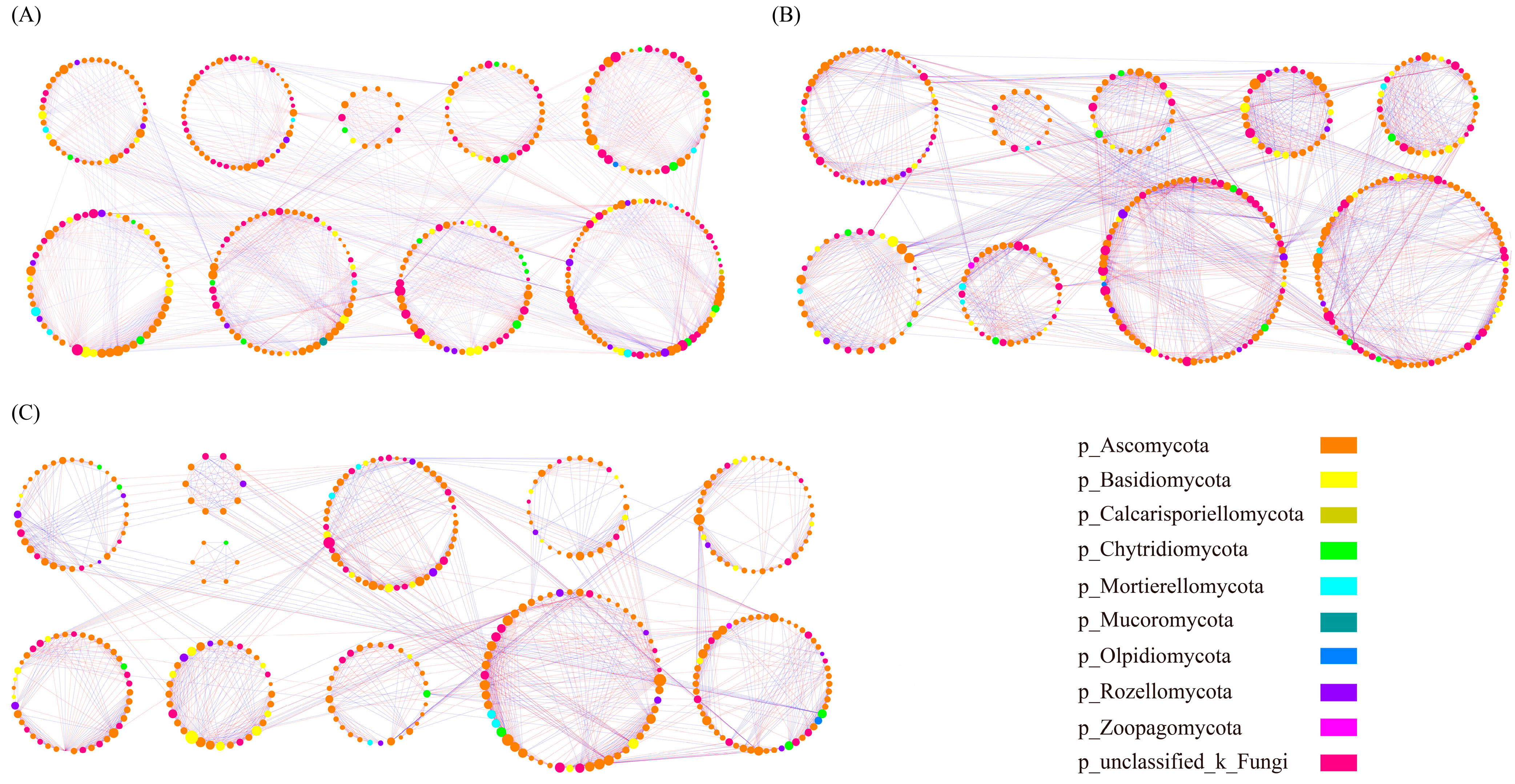

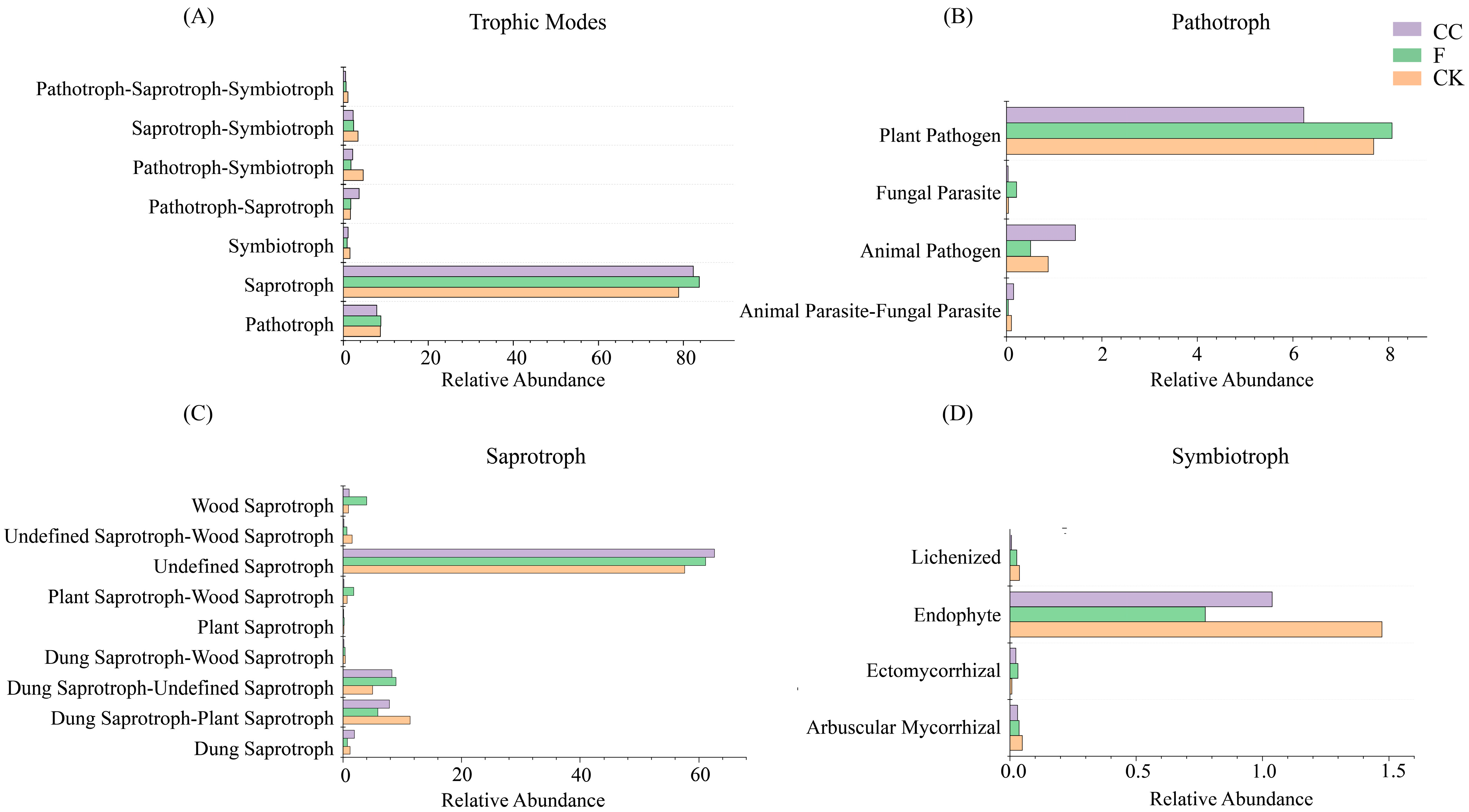
| Treatment | pH | TP (mg/g) | BP (mg/g) | AP (mg/kg) | AK (mg/kg) | Ex-Al (mg/kg) | Col-Al (g/kg) | SOC (g/kg) | TN (g/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 3.96 ± 0.04 a | 10.06 ± 0.45 c | 1.10 ± 0.04 b | 212.27 ± 4.21 b | 218.28 ± 16.25 a | 180.99 ± 1.30 c | 3.11 ± 0.02 a | 21.76 ± 0.05 b | 2.43 ± 0.006 b | 62.77 ± 0.064 a | 38.37 ± 0.32 a |
| F | 4.07 ± 0.01 a | 8.80 ± 0.15 b | 1.16 ± 0.01 b | 157.64 ± 6.88 a | 292.22 ± 3.88 ab | 152.17 ± 2.31 b | 3.04 ± 0.66 a | 22.08 ± 0.07 b | 2.47 ± 0.006 c | 69.06 ± 0.055 b | 42.1 ± 0.1 b |
| CC | 4.93 ± 0.08 b | 5.50 ± 0.002 a | 0.981 ± 0.05 a | 154 ± 2.38 a | 303.29 ± 42.23 b | 20.66 ± 3.88 a | 2.59 ± 0.10 a | 20.30 ± 0.27 a | 2.38 ± 0.006 a | 76.94 ± 0.055 c | 38 ± 0.1 a |
| Survival Rate (%) | |
|---|---|
| CK | 69.64 |
| F | 82.64 |
| CC | 83.65 |
| Non-successive tea nursery | 86.97 |
| Empirical Networks | Random Networks | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Similarity Threshold (St) | Network Size (n) | Average Connectivity (avgK) | Average Clustering Coefficient (avgCC) | Average Path Distance (GD) | Modularity (No. of Modules) | Average Clustering Coefficient (avgCC) | Average Path Distance (GD) | Modularity (M) |
| CK | 0.91 | 437 | 12.43 | 0.824 | 4.594 | 0.751(12) | 0.037 ± 0.003 | 2.694 ± 0.005 | 0.242 ± 0.004 |
| F | 0.92 | 437 | 12.252 | 0.785 | 4.742 | 0.722(18) | 0.034 ± 0.002 | 2.725 ± 0.009 | 0.243 ± 0.004 |
| CC | 0.91 | 387 | 10.098 | 0.827 | 5.607 | 0.772(12) | 0.035 ± 0.003 | 2.828 ± 0.006 | 0.273 ± 0.005 |
| Treatment | Keystones OTU Name | Classification | Related Functions | References |
|---|---|---|---|---|
| CK | OTU757 | s_Aspergillus_chlamydosporus | halophilic and halotolerant fungi | [60] |
| OTU953 | s_Coniochaeta_fasciculata | Canker and wood rot pathogens | [61] | |
| OTU221 | s_Leucosporidium_golubevii | psychrotolerant | [62] | |
| OTU1295 | s_Neocosmospora_rubicola | Causing stem rot | [63] | |
| OTU1363 | s_Setophoma_sp | Some depsides showed moderate antibacterial activity against Gram-positive bacteria | [64] | |
| OTU973 | s_unclassified_g_Colletotrichum | anthracnose pathogen | [65] | |
| OTU2761 | s_unclassified_o_Pleosporales | Function unknown | ||
| OTU21 | s_unclassified_k_Fungi | Function unknown | ||
| OTU1123 | s_unclassified_f_Cyphellaceae | Function unknown | ||
| F | OTU1706 | s_Westerdykella_purpurea | Antagonist to agricultural pathogenic fungi | [66] |
| OTU1843 | s_Arachnomyces_sp | Function unknown | ||
| OTU1791 | s_Biatriospora_sp | antifungal activity | [67] | |
| OTU1204 | s_Rozellomycota_sp | Function unknown | ||
| OTU1279 | s_unclassified_g_Calonectria | blight and crown and root rot pathogens | [68] | |
| OTU1813 | s_unclassified_k_Fungi | promising sources of hemicellulases | [69] | |
| OTU26 | s_unclassified_o_Onygenales | Function unknown | ||
| OTU2420 | s_unclassified_g_Talaromyces | Function unknown | ||
| OTU1594 | s_unclassified_k_Fungi | Function unknown | ||
| OTU1893 | s_unclassified_g_Acaulium | Function unknown | ||
| OTU451 | s_unclassified_p_Ascomycota | Function unknown | ||
| OTU66 | s_unclassified_k_Fungi | Function unknown | ||
| OTU614 | s_unclassified_o_Hymenochaetales | Function unknown | ||
| CC | OTU837 | s_Neocosmospora_ramosa | ability of biodeterioration | [70] |
| OTU503 | s_Setophoma_sp | Some depsides showed moderate antibacterial activity against Gram-positive bacteria | [64] | |
| OTU2712 | s_Pseudogymnoascus_sp | some metabolites showed antibacterial and antifungal activities | [71] | |
| OTU2127 | s_unclassified_g_Westerdykella | Antagonist to agricultural pathogenic fungi | [66] | |
| OTU1586 | s_unclassified_k_Fungi | Function unknown | ||
| OTU2046 | s_unclassified_c_Agaricomycetes | Function unknown | ||
| OTU2577 | s_unclassified_o_Sordariales | Function unknown | ||
| OTU88 | s_unclassified_o_Xylariales | Function unknown | ||
| OTU451 | s_unclassified_p_Ascomycota | Function unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Fan, D.; Wang, Y.; Huang, D.; Wang, Y.; Ma, J.; Wang, X. Effect of Calcium Cyanamide on Soil Fungal Community in Successive Tea-Cuttings Nursery. Agriculture 2021, 11, 716. https://doi.org/10.3390/agriculture11080716
Qiu Q, Fan D, Wang Y, Huang D, Wang Y, Ma J, Wang X. Effect of Calcium Cyanamide on Soil Fungal Community in Successive Tea-Cuttings Nursery. Agriculture. 2021; 11(8):716. https://doi.org/10.3390/agriculture11080716
Chicago/Turabian StyleQiu, Qinli, Dongmei Fan, Yinmao Wang, Danyi Huang, Yu Wang, Junhui Ma, and Xiaochang Wang. 2021. "Effect of Calcium Cyanamide on Soil Fungal Community in Successive Tea-Cuttings Nursery" Agriculture 11, no. 8: 716. https://doi.org/10.3390/agriculture11080716
APA StyleQiu, Q., Fan, D., Wang, Y., Huang, D., Wang, Y., Ma, J., & Wang, X. (2021). Effect of Calcium Cyanamide on Soil Fungal Community in Successive Tea-Cuttings Nursery. Agriculture, 11(8), 716. https://doi.org/10.3390/agriculture11080716






