Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatments
2.3. Weight Loss
2.4. Color Measurements
2.5. Texture Measurement
2.6. Total Phenolic Content (TPC)
2.7. Total Antioxidant Activity (TAA)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Assessment of Mushrooms Weight Loss during Storage
3.2. Color Measurements
3.3. Firmness
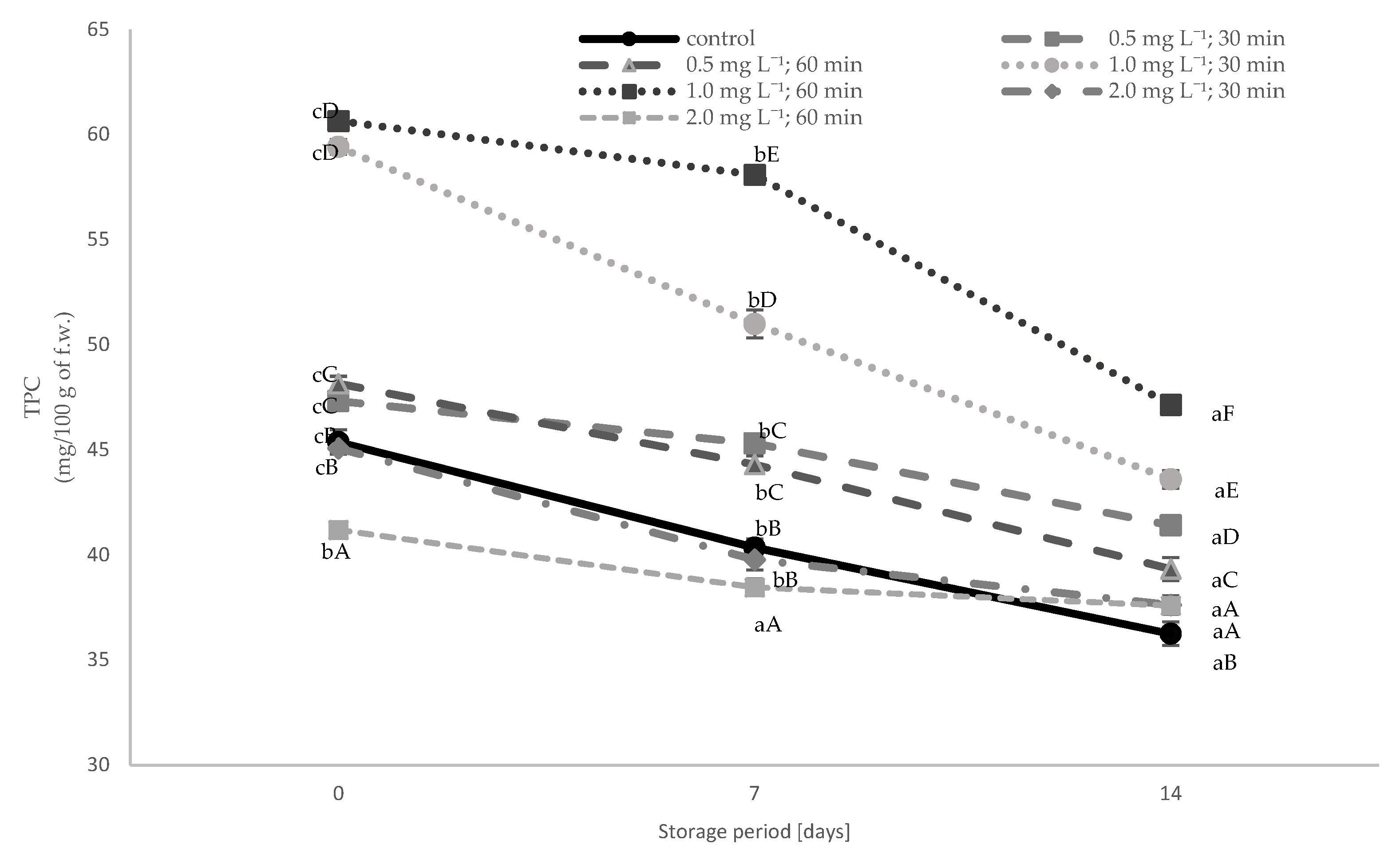
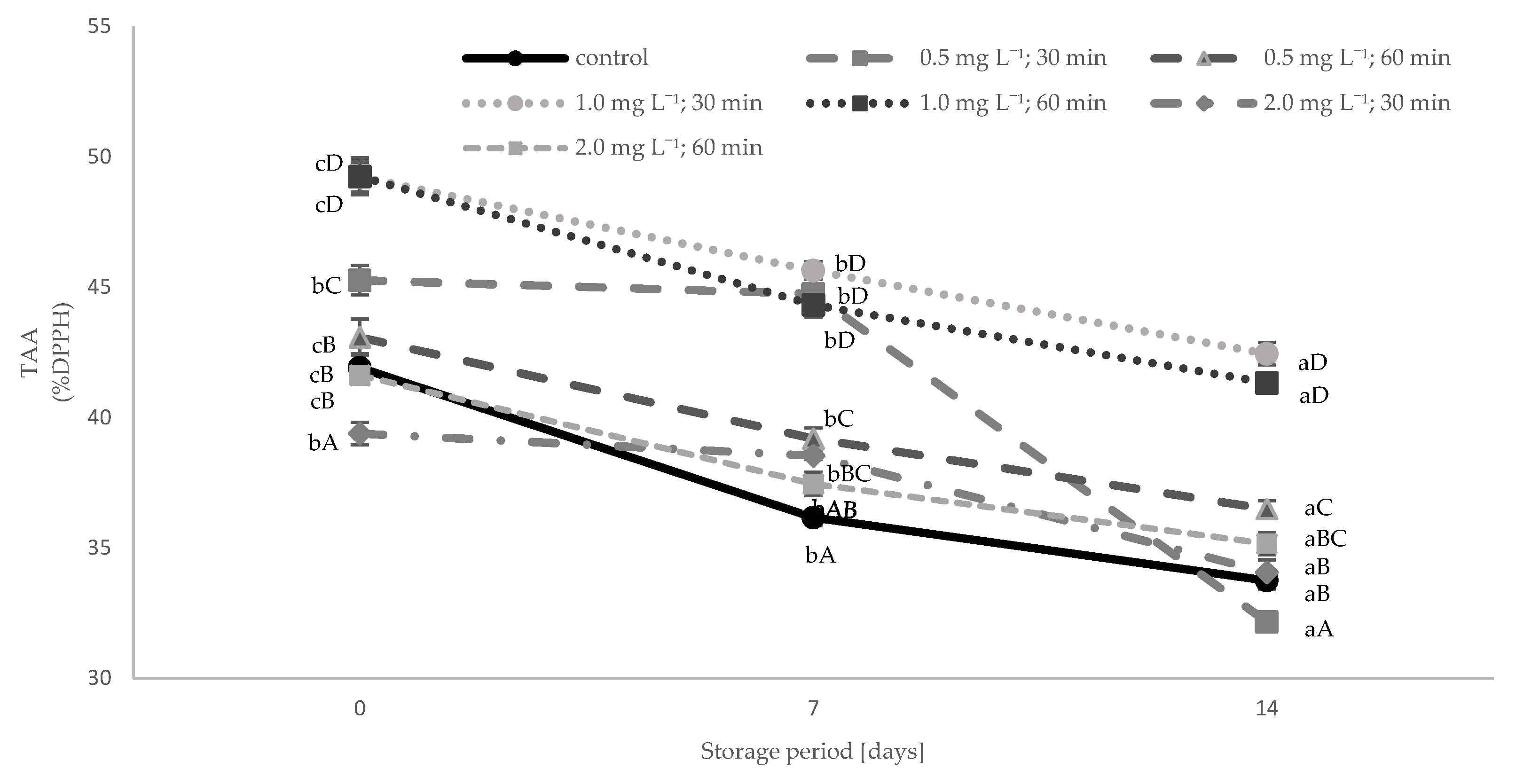
3.4. Total Phenolic Content (TPC) and Total Antioxidant Activity (TAA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, B.K.; Jain, S.K.; Sharma, G.P.; Doshi, A.; Jain, H.K. Cultivation of button mushroom and its processing: A techno-economic feasibility. Int. J. Adv. Biotech. Res. 2011, 2, 201–207. [Google Scholar]
- Chen, S.Y.; Yu, H.T.; Kao, J.P.; Yang, C.C.; Chiang, S.S.; Mishchuk, D.O.; Slupsky, C.M. Consumption of vitamin D2 enhanced mushrooms is associated with improved bone health. J. Nutr. Biochem. 2015, 26, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Ban, Z.; Li, L.; Guan, J.; Feng, J.; Wu, M.; Xu, X.; Li, J. Modified atmosphere packaging (MAP) and coating for improving preservation of whole and sliced Agaricus bisporus. J. Food Sci. Technol. 2014, 51, 3894–3901. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Langowski, H.-C.; Wani, A.; Saengerlaub, S. Recent advances in extending the self life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef]
- Niksic, M.; Klaus, A.; Argyropoulos, D. Safety of foods based on mushrooms. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martin-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 421–439. [Google Scholar] [CrossRef]
- Lagnika, C.; Zhang, M.; Nsor-Atindana, J.; Bashari, M. Effects of ultrasound and chemical treatments on white mushroom (Agaricus bisporus) prior to modified atmosphere packaging in extending shelf-life. J. Food Sci. Technol. 2014, 51, 3749–3757. [Google Scholar] [CrossRef] [Green Version]
- Skog, L.J.; Chu, C.L. Effect of ozone on qualities of fruits and vegetables in cold storage. Can. J. Plant Sci. 2001, 81, 73–778. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Sunoj, S.; Manikantan, M.R.; Anjineyulu Kothakota; Hebbar, K.B. Application and kinetics of ozone in food preservation. Ozone Sci. Eng. 2017, 39, 115–126. [Google Scholar] [CrossRef]
- Salu, A.K.; Yadav, B.K.; Santhakumaran, A. Effect of temperature and ozone treatment on the respiration of oyster mushroom. Int. J. Agric. Sci. Res. 2016, 6, 377–388. [Google Scholar]
- Onopiuk, A.; Półtorak, A.; Wyrwisz, J.; Moczkowska, M.; Stelmasiak, A.; Lipińska, A.; Szpicer, A.; Zalewska, M.; Zaremba, R.; Kuboń, M.; et al. Impact of ozonisation on pro-health properties and antioxidant capacity of ‘Honeoye’ strawberry fruit. CYTA J. Food 2017, 15, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Escriche, I.; Serra, J.A.; Gómez, M.; Galotto, M.J. Effect of ozone treatment and storage temperature on physicochemical properties of mushrooms (Agaricus bisporus). Food Sci. Technol. Int. 2001, 7, 251–258. [Google Scholar] [CrossRef]
- Onopiuk, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Impact of ozonisation time and dose on health related and microbiological properties of rapanui tomatoes. Agriculture 2021, 11, 428. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Brunton, N.P.; Cullen, P.J. Effect of ozone processing on anthocyanins and ascorbic acid degradation of strawberry juice. Food Chem. 2009, 113, 1119–1126. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Sehab, A.F.; Hassanien, F.R.; El-Nemr, S.E.; Amra, H.A.; Abdel-Alim, H.A. Efficacy of ozone to reduce fungal spoilage and aflatoxin contamination in peanuts. J. Nuts Relat. Sci. 2011, 2, 01–14. [Google Scholar]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of ozone application during cold storage of kiwifruit on the development of stem-end caused by Botrytis cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Gabler, F.M.; Smilanick, J.L.; Mansour, M.F.; Karaca, H. Influence of fumigation with high concentrations of ozone gas on postharvest gray mold and fungicide residues on table grapes. Postharvest Biol. Technol. 2010, 55, 85–90. [Google Scholar] [CrossRef]
- De Souza, L.P.; Faroni, L.; Heleno, F.F.; Cecon, P.R.; Gonçalves, T.D.C.; da Silva, G.J.; Prates, L.H.F. Effects of ozone treatment on postharvest carrot quality. LWT 2018, 90, 53–60. [Google Scholar] [CrossRef]
- Zalewska, M.; Marcinkowska-Lesiak, M.; Onopiuk, A. Physicochemical properties of Agaricus bisporus as affected by coating. J. Food Process. Pres. 2018, 42, e13419. [Google Scholar] [CrossRef]
- Eissa, H.A.; Zohair, A. Quality and safety of halawa modified with mushroom. J. Sci. Food Agric. 2006, 86, 2551–2559. [Google Scholar] [CrossRef]
- Zalewska, M.; Marcinkowska-Lesiak, M.; Onopiuk, A.; Stelmasiak, A.; Półtorak, A. Modified atmosphere packaging for extending the shelf life of fresh Agaricus bisporus. J. Food Process Pres. 2018, 42, e13839. [Google Scholar] [CrossRef]
- Kim, K.M.; Ko, J.A.; Lee, J.S.; Park, H.J.; Hanna, M.A. Effect of modified atmosphere packaging on shelf-life of coated, whole and sliced mushrooms. LWT 2006, 39, 364–371. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, L.; Zhang, Z.; Li, J.; Zhao, C. Impact of ozone on a quality of strawberry during cold storage. Front. Agric. China 2011, 5, 356–360. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Anjineyulu Kothakota; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Andrés, A.I.; Timón, M.L.; Molina, G.; González, N.; Petrón, M.J. Effect of MAP storage on chemical, physical and sensory characteristics of “níscalos” (Lactarius delicious). Food Packag. Shelf Life 2014, 1, 179–189. [Google Scholar] [CrossRef]
- Oliveira, F.; Sousa-Gallagher, M.J.; Mahajan, P.V.; Teixeira, J.A. Evaluation of MAP engineering design parameters on quality of fresh-sliced mushrooms. J. Food Eng. 2012, 108, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Yun, J.; Zhang, Y.; Bi, Y.; Zhao, F.; Niu, Y. Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2021, 176, 111501. [Google Scholar] [CrossRef]
- Prabha, V.; Barma, R.D.; Singh, R.; Madan, A. Ozone Technology in Food Processing: A Review. Trends Biosci. 2015, 8, 4031–4047. [Google Scholar]
- Kying, O.M.; Ali, A. Effect of ozone exposure on microbial flora and quality attributes of papaya (Carica papaya L.) fruit. J. Agron. Agric. Asp. 2016, JAAA-104. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Effects of ozone and chlorine postharvest treatments on quality of fresh-cut red bell peppers. Int. J. Food Sci. Technol. 2012, 47, 1935–1943. [Google Scholar] [CrossRef]
- Dhamodharan, G.; Mirunalini, S. A detail study of phytochemical screening, antioxidant potential and acute toxicity of Agaricus bisporus extract and its chitosan loaded nanoparticles. J. Pharm. Res. 2013, 6, 818–822. [Google Scholar] [CrossRef]
- Basile, A.; Ferrara, L.; Del Pozzo, M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Martínez, N.R.; González Aguilar, G.A. Total phenols and antioxidant activity of commercial and wild mushrooms from Chihuahua, Mexico. Cienc. Tecnol. Aliment. 2007, 5, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Radzki, W.; Sławińska, A.; Jabłońska-Ryś, E.; Gustaw, W. Antioxidant capacity and polyphenolics content in dried wild growing edible mushrooms. Int. J. Med. Mushrooms 2014, 16, 65–75. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Ozone Applications in fruit and vegetable processing. Food Rev. Int. 2007, 23, 91–106. [Google Scholar] [CrossRef]
- Beltran, D.; Selma, M.V.; Marin, A.; Gil, M.I. Ozonated water extends the shelf life of fresh-cut lettuce. J. Agric. Food Chem. 2005, 53, 5654–5663. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concellon, A.; Alicia, A.R.C.; Vicente, A.R. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. Review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef]
| Item | Effect | Interaction | |||||
|---|---|---|---|---|---|---|---|
| Storage Period (day) SP | Ozone Dose (ml/L) OD | Time of Ozonization (min) OT | SP × OD | SP × OT | OD × OT | SP × OD × OT | |
| Weight loss (%) | *** | * | * | *** | *** | NS | *** |
| firmness | *** | *** | *** | *** | *** | *** | *** |
| L* external | *** | *** | *** | *** | *** | *** | *** |
| a* external | *** | *** | *** | *** | *** | *** | *** |
| b* external | *** | *** | *** | *** | *** | *** | *** |
| L* internal | *** | *** | *** | *** | *** | *** | *** |
| a* internal | *** | *** | *** | *** | *** | *** | *** |
| b* internal | *** | *** | ** | *** | *** | *** | *** |
| BI external | *** | *** | *** | *** | *** | *** | *** |
| BI internal | *** | *** | *** | *** | *** | *** | *** |
| ΔE external | *** | *** | *** | *** | *** | *** | *** |
| ΔE internal | *** | *** | *** | *** | *** | *** | *** |
| TAA | *** | *** | NS | *** | *** | *** | *** |
| TPC | ** | ** | NS | *** | NS | *** | *** |
| Group | Days of Storage | WL (%) |
|---|---|---|
| Control | 4 | 0.17 aA ± 0.02 |
| 7 | 0.43 bA ± 0.03 | |
| 11 | 0.53 bA ± 0.02 | |
| 14 | 0.91 cA ± 0.02 | |
| 0.5 mg L−1; 30 min | 4 | 0.48 aB ± 0.03 |
| 7 | 0.58 aB ± 0.06 | |
| 11 | 0.73 bB ± 0.02 | |
| 14 | 1.06 cB ± 0.07 | |
| 0.5 mg L−1; 60 min | 4 | 0.58 aB ± 0.02 |
| 7 | 0.78 bC ± 0.03 | |
| 11 | 0.96 cC ± 0.05 | |
| 14 | 1.08 cAB ± 0.02 | |
| 1.0 mg L−1; 30 min | 4 | 0.75 aC ± 0.05 |
| 7 | 0.77 aCD ± 0.03 | |
| 11 | 0.95 bC ± 0.06 | |
| 14 | 1.27 cC ± 0.05 | |
| 1.0 mg L−1; 60 min | 4 | 0.79 aC ± 0.03 |
| 7 | 0.79 aCD ± 0.04 | |
| 11 | 0.94 bC ± 0.06 | |
| 14 | 1.19 bC ± 0.03 | |
| 2.0 mg L−1; 30 min | 4 | 0.86 aC ± 0.07 |
| 7 | 0.84 aCD ± 0.04 | |
| 11 | 0.94 aC ± 0.03 | |
| 14 | 1.45 bd ± 0.07 | |
| 2.0 mg L−1; 60 min | 4 | 0.83 aC ± 0.04 |
| 7 | 0.83 aCD ± 0.03 | |
| 11 | 0.97 bC ± 0.03 | |
| 14 | 1.18 cBC ± 0.07 |
| Group | Storage Period (days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 11 | 14 | |||
| L* (%) | Control | 94.31 c ± 0.74 | 92.12 bc ± 0.88 | 89.55 a ± 1.42 | 85.39 a ± 2.76 | 83.15 aB ± 2.23 | |
| 0.5 mg/L | 30 min | 94.31 c ± 0.74 | 75.86 bBC ± 3.43 | 68.54 aA ± 3.21 | 69.86 aAB ± 1.58 | 69.29 aA ± 1.55 | |
| 60 min | 94.31 c ± 0.74 | 75.72 bB ± 2.84 | 71.71 aBC ± 1.48 | 72.96 abC ± 1.23 | 70.28 aA ± 1.27 | ||
| 1 mg/L | 30 min | 94.31 c ± 0.74 | 73.81 bAB ± 2.37 | 71.95 abC ± 1.44 | 72.32 abBC ± 1.03 | 70.49 aA ± 1.10 | |
| 60 min | 94.31 d ± 0.74 | 75.76 cB ± 2.25 | 69.02 aAB ± 2.04 | 72.36 bBC ± 1.32 | 68.09 aA ± 1.79 | ||
| 2 mg/L | 30 min | 94.31 c ± 0.74 | 78.74 bC ± 1.76 | 73.41 aC ± 1.35 | 72.93 aC ± 1.10 | 70.70 aA ± 1.15 | |
| 60 min | 94.31 c ± 0.74 | 72.65 bA ± 2.35 | 73.88 bC ± 1.38 | 68.01 aA ± 1.63 | 68.46 aA ± 1.40 | ||
| a* (−) | Control | −0.66 a ± 0.10 | −0.03 aA ± 0.39 | 1.17 bA ± 0.42 | 2.39 cA ± 0.76 | 2.93 cA ± 0.62 | |
| 0.5 mg/L | 30 min | −0.66 a ± 0.10 | 5.67 bCD ± 1.49 | 7.59 cC ± 1.19 | 7.28 cE ± 0.68 | 6.67 bcB ± 0.72 | |
| 60 min | −0.66 a ± 0.10 | 5.81 bCD ± 0.94 | 6.88 bC ± 0.81 | 6.16 bCDE ± 0.49 | 6.25 bB ± 0.82 | ||
| 1 mg/L | 30 min | −0.66 a ± 0.10 | 6.58 bD ± 0.67 | 6.77 bC ± 0.55 | 6.03 bBCD ± 0.63 | 6.38 bB ± 0.82 | |
| 60 min | −0.66 a ± 0.10 | 5.38 bC ± 1.19 | 6.77 cC ± 0.62 | 4.95 bB ± 0.47 | 5.80 bcB ± 0.64 | ||
| 2 mg/L | 30 min | −0.66 a ± 0.10 | 4.10 bB ± 0.99 | 5.43 cB ± 0.46 | 5.13 bcBC ± 0.33 | 6.01 cB ± 0.51 | |
| 60 min | −0.66 a ± 0.10 | 6.38 cCD ± 0.78 | 4.85 bB ± 0.77 | 6.48 cDE ± 0.94 | 6.71 cB ± 0.76 | ||
| b* (−) | Control | 8.48 a ± 0.25 | 12.71 bA ± 1.37 | 14.05 bcA ± 0.66 | 14.58 bcA ± 1.35 | 15.67 cA ± 1.70 | |
| 0.5 mg/L | 30 min | 8.48 a ± 0.25 | 18.44 bBC ± 3.18 | 22.12 cC ± 2.56 | 23.41 cC ± 1.86 | 21.02 cBC ± 2.06 | |
| 60 min | 8.48 a ± 0.25 | 18.74 bBC ± 1.27 | 20.78 bBC ± 1.42 | 21.09 bBC ± 1.26 | 20.70 bBC ± 1.46 | ||
| 1 mg/L | 30 min | 8.48 a ± 0.25 | 20.44 bC ± 1.12 | 22.45 bcC ± 1.55 | 21.23 bcBC ± 1.08 | 23.01 cC ± 1.73 | |
| 60 min | 8.48 a ± 0.25 | 18.33 bBC ± 1.87 | 21.03 cBC ± 1.44 | 19.45 bcB ± 1.07 | 19.26 bcB ± 1.14 | ||
| 2 mg/L | 30 min | 8.48 a ± 0.25 | 16.61 bB ± 1.69 | 19.33 cB ± 0.70 | 18.97 bcB ± 0.90 | 19.84 cB ± 1.00 | |
| 60 min | 8.48 a ± 0.25 | 20.24 bcC ± 1.20 | 18.70 bB ± 1.24 | 21.25 cB ± 1.40 | 21.62 cBC ± 2.28 | ||
| ΔE (−) | Control | 9.02 a ± 0.25 | 13.78 bA ± 1.50 | 16.25 bcA ± 1.24 | 19.22 cdA ± 2.66 | 21.52 dA ± 2.58 | |
| 0.5 mg/L | 30 min | 9.02 a ± 0.25 | 29.10 bC ± 4.76 | 37.35 cE ± 3.78 | 37.05 cC ± 2.02 | 35.88 cB ± 2.17 | |
| 60 min | 9.02 a ± 0.25 | 29.44 bCD ± 2.86 | 33.94 cCD ± 1.72 | 33.02 cB ± 1.59 | 34.81 cB ± 1.76 | ||
| 1 mg/L | 30 min | 9.02 a ± 0.25 | 32.11 bCD ± 2.32 | 34.79 bcCDE ± 1.63 | 33.54 bcB ± 1.29 | 36.12 cB ± 1.36 | |
| 60 min | 9.02 a ± 0.25 | 29.03 bC ± 2.87 | 36.12 cDE ± 1.94 | 32.16 bB ± 1.56 | 35.62 cB ± 1.82 | ||
| 2 mg/L | 30 min | 9.02 a ± 0.25 | 25.44 bB ± 2.49 | 31.41 cBC ± 1.30 | 31.47 cB ± 1.38 | 33.91 cB ± 1.47 | |
| 60 min | 9.02 a ± 0.25 | 32.78 bD ± 2.45 | 30.53 bB ± 1.82 | 36.95 cC ± 2.01 | 36.90 cB ± 1.92 | ||
| BI (−) | Control | 8.57 a ± 0.26 | 14.33 bA ± 1.80 | 17.47 bcA ± 1.41 | 20.25 bcA ± 2.99 | 22.89 cA ± 3.47 | |
| 0.5 mg/L | 30 min | 8.57 a ± 0.26 | 32.96 bBC ± 7.72 | 46.41 cD ± 8.76 | 47.34 cD ± 5.09 | 42.27 cBC ± 5.53 | |
| 60 min | 8.57 a ± 0.26 | 33.35 bBC ± 4.24 | 40.29 cCD ± 3.79 | 39.34 bcBC ± 3.28 | 40.44 cBC ± 4.14 | ||
| 1 mg/L | 30 min | 8.57 a ± 0.26 | 38.10 bC ± 3.60 | 43.24 bcD ± 3.88 | 39.84 bcBC ± 2.89 | 44.97 cC ± 4.11 | |
| 60 min | 8.57 a ± 0.26 | 32.22 bBC ± 4.98 | 42.54 cD ± 3.86 | 35.42 bB ± 2.90 | 38.56 bcBC ± 3.28 | ||
| 2 mg/L | 30 min | 8.57 a ± 0.26 | 26.87 bB ± 4.09 | 35.09 cBC ± 2.04 | 34.41 cB ± 2.37 | 38.23 cB ± 2.88 | |
| 60 min | 8.57 a ± 0.26 | 38.25 bcC ± 3.88 | 33.18 bB ± 3.49 | 43.45 cCD ± 4.58 | 44.12 cBC ± 5.49 | ||
| Group | Storage Period (days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 11 | 14 | |||
| L* (%) | Control | 91.92 c ± 0.76 | 90.64 cAB ± 1.20 | 84.17 bA ± 2.62 | 85.01 bA ± 2.60 | 78.34 aA ± 1.70 | |
| 0.5 mg/L | 30 min | 91.92 bc ± 0.76 | 89.61 bAB ± 1.80 | 89.68 bB ± 1.46 | 85.68 aA ± 1.90 | 83.11 aB ± 1.03 | |
| 60 min | 91.92 b ± 0.76 | 92.21 bB ± 1.11 | 91.47 bB ± 3.25 | 87.09 aA ± 2.46 | 86.55 aC ± 1.53 | ||
| 1 mg/L | 30 min | 91.92 c ± 0.76 | 88.55 bA ± 1.91 | 90.42 bcB ± 2.10 | 91.34 bcB ± 0.89 | 86.29 aC ± 1.19 | |
| 60 min | 91.92 b ± 0.76 | 89.81 bAB ± 1.76 | 83.95 aA ± 1.86 | 89.21 bB ± 1.91 | 89.74 bD ± 2.06 | ||
| 2 mg/L | 30 min | 91.92 ± 0.76 | 89.46 AB ± 1.91 | 91.20 B ± 2.00 | 90.13 B ± 2.45 | 91.76 DE ± 1.73 | |
| 60 min | 91.92 b ± 0.76 | 90.54 bAB ± 2.24 | 89.60 bB ± 2.16 | 91.05 bB ± 2.03 | 82.30 aB ± 2.3 | ||
| a* (−) | Control | −0.39 a ± 0.12 | 0.23 aAB ± 0.36 | 2.22 bD ± 0.87 | 1.53 bD ± 0.59 | 3.12 cC ± 0.43 | |
| 0.5 mg/L | 30 min | −0.39 ab ± 0.12 | 1.01 cdB ± 0.59 | 0.31 bcBC ± 0.53 | 1.38 dCD ± 0.44 | −0.82 aA ± 0.16 | |
| 60 min | −0.39 a ± 0.12 | −0.10 abA ± 0.43 | −0.64 aA ± 0.62 | 0.55 bBC ± 0.51 | 1.82 cB ± 0.93 | ||
| 1 mg/L | 30 min | −0.39 a ± 0.12 | 0.81 bB ± 0.67 | −0.45 aAB ± 0.61 | −0.57 aA ± 0.29 | 4.13 cC ± 0.67 | |
| 60 min | −0.39 a ± 0.12 | 0.22 abAB ± 0.69 | 1.05 bC ± 0.34 | −0.27 aAB ± 0.46 | −0.36 aA ± 0.46 | ||
| 2 mg/L | 30 min | −0.39 ab ± 0.12 | 0.57 cAB ± 0.67 | −0.85 aA ± 0.38 | 0.22 bcAB ± 0.74 | −0.79 aA ± 0.40 | |
| 60 min | −0.39 ab ± 0.12 | 0.42 bAB ± 0.85 | −0.74 aA ± 0.40 | −0.64 aA ± 0.53 | 1.57 cB ± 0.45 | ||
| b* (−) | Control | 8.58 a ± 0.41 | 10.29 abAB ± 1.04 | 13.95 cB ± 2.38 | 11.98 bAB ± 1.18 | 15.75 cD ± 1.81 | |
| 0.5 mg/L | 30 min | 8.58 a ± 0.41 | 10.24 aAB ± 0.98 | 9.99 aA ± 0.85 | 12.62 bB ± 1.23 | 12.85 aB ± 0.66 | |
| 60 min | 8.58 a ± 0.41 | 9.45 abA ± 0.48 | 9.33 abA ± 0.83 | 10.59 bA ± 1.30 | 13.10 cC ± 1.17 | ||
| 1 mg/L | 30 min | 8.58 a ± 0.41 | 11.47 cB ± 1.46 | 9.39 abA ± 1.07 | 10.52 bcA ± 1.16 | 19.89 dE ± 1.01 | |
| 60 min | 8.58 a ± 0.41 | 10.70 AB ± 1.21 | 13.32 cB ± 0.95 | 11.63 bcAB ± 1.13 | 12.24 bcBC ± 1.20 | ||
| 2 mg/L | 30 min | 8.58 a ± 0.41 | 10.57 bAB ± 1.11 | 10.03 abA ± 1.27 | 10.85 bAB ± 1.37 | 10.41 abAB ± 0.99 | |
| 60 min | 8.58 a ± 0.41 | 10.45 abAB ± 1.46 | 10.55 bA ± 1.21 | 10.32 abA ± 1.32 | 14.80 cCD ± 1.36 | ||
| ΔE (−) | Control | 10.11 a ± 0.63 | 12.33 aAB ± 1.38 | 19.46 bC ± 3.39 | 17.37 bB ± 2.49 | 24.99 cD ± 2.07 | |
| 0.5 mg/L | 30 min | 10.11 a ± 0.63 | 13.03 aAB ± 1.83 | 12.63 aA ± 1.46 | 17.31 bB ± 2.13 | 16.90 aB ± 1.00 | |
| 60 min | 10.11 a ± 0.63 | 10.81 aA ± 0.81 | 11.12 aA ± 2.61 | 14.83 bAB ± 2.18 | 17.21 bB ± 1.96 | ||
| 1 mg/L | 30 min | 10.11 a ± 0.63 | 14.58 bB ± 2.10 | 11.66 abA ± 1.91 | 12.06 abA ± 1.21 | 29.38 cE ± 1.24 | |
| 60 min | 10.11 a ± 0.63 | 13.13 abAB ± 1.89 | 18.93 cB ± 1.74 | 14.14 bA ± 1.95 | 14.37 bB ± 2.09 | ||
| 2 mg/L | 30 min | 10.11 a ± 0.63 | 13.28 bAB ± 2.03 | 11.71 abA ± 2.03 | 13.12 abA ± 2.43 | 11.77 abAB ± 1.66 | |
| 60 min | 10.11 a ± 0.63 | 12.60 aAB ± 2.39 | 13.01 aA ± 2.17 | 12.07 aA ± 2.02 | 21.19 bC ± 2.53 | ||
| BI (−) | Control | 9.14 a ± 0.57 | 11.84 aAB ± 1.52 | 19.62 cB ± 4.59 | 16.06 bBC ± 2.35 | 26.35 dD ± 3.71 | |
| 0.5 mg/L | 30 min | 9.14 a ± 0.57 | 12.58 aAB ± 1.77 | 11.80 aA ± 1.59 | 16.64 bC ± 2.34 | 17.17 aC ± 0.91 | |
| 60 min | 9.14 a ± 0.57 | 10.36 abA ± 0.87 | 9.69 abA ± 2.16 | 13.02 bAB ± 2.24 | 17.45 cC ± 2.65 | ||
| 1 mg/L | 30 min | 9.14 a ± 0.57 | 14.11 bB ± 2.24 | 10.26 aA ± 1.91 | 11.36 abA ± 1.34 | 33.25 cE ± 2.44 | |
| 60 min | 9.14 a ± 0.57 | 12.46 abAB ± 2.00 | 17.65 cB ± 1.65 | 13.29 bABC ± 1.81 | 13.92 bB ± 2.17 | ||
| 2 mg/L | 30 min | 9.14 a ± 0.57 | 12.65 bAB ± 2.11 | 10.61 abA ± 1.98 | 12.63 abAB ± 2.57 | 11.02 abAB ± 1.67 | |
| 60 min | 9.14 a ± 0.57 | 12.24 aAB ± 2.67 | 11.54 aA ± 2.11 | 11.14 aA ± 1.99 | 20.65 bC ± 2.73 | ||
| Group | Days of Storage | Firmness (N) |
|---|---|---|
| Control | 0 | 15.25 ab ± 1.35 |
| 4 | 19.59 bAB ± 1.99 | |
| 7 | 18.15 bBCD ± 2.98 | |
| 11 | 10.93 aAB ± 2.25 | |
| 14 | 10.95 aBC ± 1.75 | |
| 0.5 mg L−1; 30 min | 0 | 15.25 b ± 1.35 |
| 4 | 16.39 bA ± 1.37 | |
| 7 | 14.20 bAB ± 2.57 | |
| 11 | 9.25 aA ± 1.48 | |
| 14 | 5.89 aA ± 1.03 | |
| 0.5 mg L−1; 60 min | 0 | 15.25 ab ± 1.35 |
| 4 | 18.02 bcAB ± 3.33 | |
| 7 | 21.60 cD ± 1.03 | |
| 11 | 12.67 aABC ± 2.02 | |
| 14 | 14.25 abC ± 2.58 | |
| 1.0 mg L−1; 30 min | 0 | 15.25 b ± 1.35 |
| 4 | 17.66 bAB ± 1.53 | |
| 7 | 13.23 abA ± 2.73 | |
| 11 | 14.02 bBCD ± 0.74 | |
| 14 | 9.51 aAB ± 2.47 | |
| 1.0 mg L−1; 60 min | 0 | 9.51 aAB ± 2.47 |
| 4 | 21.35 bB ± 2.34 | |
| 7 | 16.65 aABC ± 3.15 | |
| 11 | 16.53 aCD ± 1.39 | |
| 14 | 16.50 aC ± 1.78 | |
| 2.0 mg L−1; 30 min | 0 | 15.25 ab ± 1.35 |
| 4 | 16.49 abA ± 2.56 | |
| 7 | 18.90 bCD ± 3.46 | |
| 11 | 13.02 aABC ± 3.51 | |
| 14 | 17.46 abC ± 3.34 | |
| 2.0 mg L−1; 60 min | 0 | 15.25 b ± 1.35 |
| 4 | 17.26 AB ± 3.41 | |
| 7 | 19.23 CD ± 3.66 | |
| 11 | 18.44 D ± 2.11 | |
| 14 | 17.21 C ± 4.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, M.; Górska-Horczyczak, E.; Marcinkowska-Lesiak, M. Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus). Agriculture 2021, 11, 748. https://doi.org/10.3390/agriculture11080748
Zalewska M, Górska-Horczyczak E, Marcinkowska-Lesiak M. Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus). Agriculture. 2021; 11(8):748. https://doi.org/10.3390/agriculture11080748
Chicago/Turabian StyleZalewska, Magdalena, Elżbieta Górska-Horczyczak, and Monika Marcinkowska-Lesiak. 2021. "Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus)" Agriculture 11, no. 8: 748. https://doi.org/10.3390/agriculture11080748






