Abstract
Parthenium weed is an invasive weed species of economic importance worldwide. Native to the American tropics, the infestation ability of Parthenium weed to a new habitat is largely influenced by environmental factors. Despite Parthenium weed invasion in Malaysia dated back to 2013, investigation on its ecological behavior is still lacking. Hence, extensive studies on the ecological behavior of two predominant Malaysian Parthenium weed populations were executed. In the Petri dish seed bioassay, germination of Parthenium weed seeds was evident at temperatures up to 80 °C. Parthenium weed was also germinable in saline condition of up to 250 mM, osmotic pressure ranging from −1.2 to 0 MPa, and a wide range of pH (4–9), thus these abiotic conditions are by no means the limiting factors for the Parthenium weed. The pot trial observed that this invasive weed grew readily in various Malaysian soil textures. Parthenium weed successfully emerged from 0 cm to not beyond 2 cm soil burial and retained its emergence capacity under different submergence periods in water. The most favorable soil moisture condition for Parthenium weed emergence was saturated (0 kPa), followed by field capacity (−30–−50 kPa), while no emergence occurred in drought (−70 kPa) as well as flooded soils. These indicate that both Parthenium weed populations possess high tolerance to various abiotic conditions in Malaysia. Results obtained in the current study have crucially become guidelines for the local government authorities in predicting wide spread of Parthenium weed in diverse ecological zones, to further manage this pernicious weed efficiently.
1. Introduction
Exotic species invasion is listed as one of the significant worldwide problems encountered by natural ecosystems [1]. Many negative impacts generated by invasive species toward native species communities and ecosystems have been well known for decades [2,3]. Parthenium hysterophorus (L.), globally known as Parthenium weed is regarded as one of the aggressive exotic species that have invaded almost the entire world [4]. The weed causes adverse significant negative impacts on flora composition and diversity [3], severe economic losses [5], and health issues in animals and humans [6,7,8]. Parthenium weed holds allelopathic chemicals such as parthenin and hysterin which makes it a strong colonizer in various habitats [4]. Whole parts of Parthenium weed are covered by small multicellular white trichomes that can cause dermatitis in various animals including cattle and horses [4]. The weed is toxic to humans that can lead to various allergies such as dermatitis, asthma, hay fever, and bronchitis [9].
Parthenium weed is native to South, North, and Central America, and to date has broadened its territory to 48 countries in Asia, Middle East, Australia, Oceania, and Africa [10]. The invasion of Parthenium weed into Malaysia was first recorded in 2013 [11], although some locals had eye-witnessed the existence of this weed species dated back in 2008. The wide spread has since then extended to over 70.4 hectares comprising Kedah, Perak, Negeri Sembilan, Melaka, Johor, Pulau Pinang, Perlis, Selangor, Pahang, and Sabah [12]. This transition annual-perennial fast maturing weed is a high prolific seed producer [4], where a single plant is capable of producing around 25,000–28,000 seeds [13]. The black wedged shape seeds are 2 mm small and commonly dispersed by environmental occurrences (e.g., water and wind) but most long-distance dispersal is through vehicles, farm machinery, and as contaminants in animal feed and pasture or crop seeds lots [4].
Parthenium weed has the ability to adapt to a wide range of environmental conditions and possesses a fast growth performance, strongly related to its high reproductive potential [14,15]. The rapid spread of Parthenium weed in the environment depends on the ability to accustom to a broad range of climatic conditions, and on the short life cycle with prolific nature which contributes to huge seed bank occurrence [6]. It is well understood that the key success of an invasive species is strongly related to the seed germination process, establishment, and competition in different ecological niches [16,17]. All plant species need a specific range of environmental conditions to initiate the germination [18,19] governed by different environmental conditions such as temperature, water potential, and pH [20,21].
In order to prevent serious health cases, economic losses and ecological diversity imbalance, it is necessary to determine the tolerant and susceptible habitats to Parthenium weed invasion, further taking appropriate actions to prevent the additional infestation of this alien plant species into susceptible areas. Successful establishment of the plant depends heavily on seed germination ability, in which the germination can be influenced by various environmental conditions [20]. However, the fundamental knowledge of basic biology and ecology of Parthenium weed responding to different environmental conditions in Malaysia is still inadequate, thus limiting the options for its efficient control. Thus, this study embarked on determining germination and seedling emergence of Parthenium weed under various Malaysia ecological conditions, crucial for predicting its potential spread into new areas and allows mechanical, cultural, chemical, and biological options to be properly timed, providing better insight to develop effective control measures.
2. Materials and Methods
2.1. Seed Collection and Preparation
The study represents two Parthenium weed populations collected from different invaded areas, namely Sg. Pasir (5.5969° N, 100.4728° E), Sungai Petani, Kedah (herein after regarded as Pop 1) and Department of Veterinary Services Infoternak Farm (4.7844° N, 101.1089° E), Sg. Siput, Perak (Pop 2). Sg. Pasir is a small town surrounded by villages, while Infoternak Farm in Sg. Siput is a semi-recreational animal farm owned by the Perak State Government.
The seeds were harvested carefully from mature plants, placed in a paper bags, then wrapped with a translucent plastic bag, labeled accordingly, and sealed. Seeds were immediately brought to Weed Science Laboratory, Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia and oven-dried at 30 °C for a 12-hour period for 3 days. The seeds were cleaned and stored in a refrigerator at 4 °C until use, since cold storage of below 0 °C substantially reduced the germination up to 70% (data not shown). Seed germination test was conducted prior to the experiment using 100 seeds in four replicates and 93% germination was recorded (data not shown).
2.2. Soil Sampling
Soil samples were obtained from Parthenium weed-free areas in several locations in Selangor, Malaysia, representing different soil textures. Confirmation on soil profile and collection of the samples was completed according to the Soil Correlation Tour Kuala Langat, Selangor prepared by Department of Agriculture Malaysia (DOA) and Soil Resources Management and Conservation Division, Soil Survey Section, Shah Alam, Selangor.
The soil samples then were crushed to break up the soil aggregates, air-dried for 3 days in the glasshouse, and sieved using a 2 mm sieve following Food and Agriculture Organization (FAO) guidelines [22]. All soil samples were sent to the Soil Physics Laboratory, Department of Land Management, Faculty of Agriculture, Universiti Putra Malaysia, Selangor for analysis on texture and the soil classification, while soil chemical analysis was performed at the Soil Fertility Laboratory, Department of Land Management, Faculty of Agriculture, Universiti Putra Malaysia.
2.3. General Experimental Procedure in Petri Dish Bioassay
Twenty Parthenium weed seeds from each population were placed randomly in a 9 cm diameter Petri dish containing two pieces of Whatman No.1 filter papers, later moistened with 4 mL of experimental solutions or distilled water [23]. Following treatments as described below, the Petri dishes were sealed with parafilm to minimize water evaporation before placed in a plant incubator for 14 days at a constant temperature of 26 °C, as demonstrated by Li et al. [24].
2.3.1. Heat Stress
Seeds of each population were wrapped in an aluminum foil before placed in an oven with temperatures of 40, 60, 80, 100, 120, 140, 160, 180, and 200 °C for 5 min. Seeds without heat treatment (room temperature: 24–28 °C) were considered as control. These temperatures were selected to simulate the temperatures occurring in the top of soil layer during vegetation burning [25].
2.3.2. Salt Stress
Parthenium weed seeds were placed in the Petri dishes containing different concentrations of sodium chloride (NaCl) solutions: 0, 10, 20, 40, 60, 80, 100, 150, 250, and 350 mM. A serial concentration of NaCl was prepared by diluting NaCl with distilled water.
2.3.3. Effect of pH
The germination responses of Parthenium weed seeds from the two local populations on different levels of pH were determined using buffer solutions of pH 3–9. The buffer solutions were prepared as described by Chachalis and Reddy [26]. Petri dish with distilled water was used as a control. The solutions were adjusted to the appropriate pH level using 1 M HCl and 1 M NaOH.
2.3.4. Effect of Osmotic Pressure
A series of osmotic pressure solutions of 0, −0.1, −0.2, −0.4, −0.6, −0.8, −1.0, and −1.2 MPa were prepared according to Michel and Kaufmann [27] by diluting polyethylene glycol 6000 in distilled water.
2.4. General Pot Trial Procedure
The pot trials were conducted in a quarantine glasshouse at Faculty of Agriculture, Universiti Putra Malaysia. The Parthenium weed seeds were placed randomly in a 9 cm diameter Petri dish containing two pieces of Whatman No. 1 filter papers. The seeds were soaked with potassium nitrate to enhance germination. After 24 h, seeds were drained and placed in a plant incubator at a constant temperature of 26 °C until hypocotyl emerged. These pre-germinated seeds were used in the pot trial treatments. The treatments comprised types of soil texture, burial depth, flooding duration, and soil moisture condition, as detailed below.
2.4.1. Soil Texture
In this experiment, a sum of 72 plastic pots with 15 cm diameter and 12 cm height were filled with different types of soil textures up to three quarters full. The soil textures were sandy loam, loam, silt clay, silt clay loam, loamy sand, sand, clay loam, silt loam, and sand beach. A total of 20 pre-germinated Parthenium weed seeds from each population were placed on the top of soil surface and covered with a thin layer of each soil texture. The soils were pressed equally and gently to avoid soil compaction and resistance for seedling development. The plants were watered twice a day to maintain the soil humidity.
2.4.2. Burial Depth
Twenty pre-germinated seeds from each population were placed in different burial depths and then covered with soils. The burial depths treatment started from 0 cm (soil surface), and descended to 1, 2, 4, 6, 8, and 10 cm below soil surface, respectively. The base and uppermost layer of soils were levelled and gently pressured with a similar force before and after sowing to standardize the burial depths treatment and enhance seed–soil contact. Pots were watered twice daily.
2.4.3. Submerging Duration
Twenty mature seeds from both populations were submerged/immersed in a container filled with tap water of 10 cm depth at room temperature for 1, 3, 5, 10, 15, 20, 25, and 30 days, separately. At the end of each flooding duration treatment, seeds were taken out from the container, tossed and sown evenly in a 10 cm diameter plastic pot containing a third quarter of sandy loam soil.
2.4.4. Soil Moisture
The different soil moisture conditions were prepared by using tensiometer (Irrometer Co., Riverside, CA, USA). Four soil moisture condition treatments, namely flooded (5 cm water level above the soil), saturated (0 kPa), field capacity (−30–−50 kPa), and drought (−70 kPa). A total of 20 pre-germinated seeds from both populations were sown evenly on the clay loam soil with different soil moisture conditions.
2.5. Parameters Measured
Observation on the Petri dish bioassays was made at 14 days following treatments. Percentage of germination was calculated as follows:
Germination was considered to have occurred when the radicle of length greater than 2 mm protruded from the seed coat was discerned [28]. The length of the shoot was determined by measuring the emerged upright epicotyls (shoot) from the cotyledons, while the length of the root was determined by measuring the length of the protruded radicle (root) using a regular centimeter ruler.
For pot trial experiments, seedling emergence, plant height, and dry weight were recorded at 30 days after treatments application. The seedling emergence was defined as the full appearance of cotyledons from the seed coat or exceeding 3 mm height above the soil surface [29]. All seedlings that emerged from the soils were counted and removed manually by hand. Percentage of seedling emergence in each pot was calculated using Equation (1), with slight modification. Plant height was measured at 1 cm from the soil to the top of seedlings using a measurement tape or ruler. Parthenium weed seedlings were harvested at 1 cm aboveground, dried in an oven at 60 °C for 72 h and the dry weight was taken using analytical balance.
2.6. Statistical Analysis
The Petri dish bioassays were operated independently and laid out in a factorial complete randomized design (CRD) while the trial pots were arranged in a factorial randomized complete block design (RCBD), each with four replicates and conducted twice. The values attained from the experiments were subjected to analysis of variance (ANOVA) using Statistical Analysis System Software (SAS version 9.1, SAS Institute, Inc. Cary, NC, USA) then proceeded with mean separation via post hoc-test of Tukey’s studentized range test (HSD) with a considered significant difference when p < 0.05. Data parameters were subjected to non-linear regression analysis using least squares method in SigmaPlot version 14.0 software (Systat Inc., GmbH, Germany) to obtain the best-fit curves. R2 values were used to determine ‘goodness of fit’ for the selected equation.
3. Results
3.1. Petri Dish Bioassay
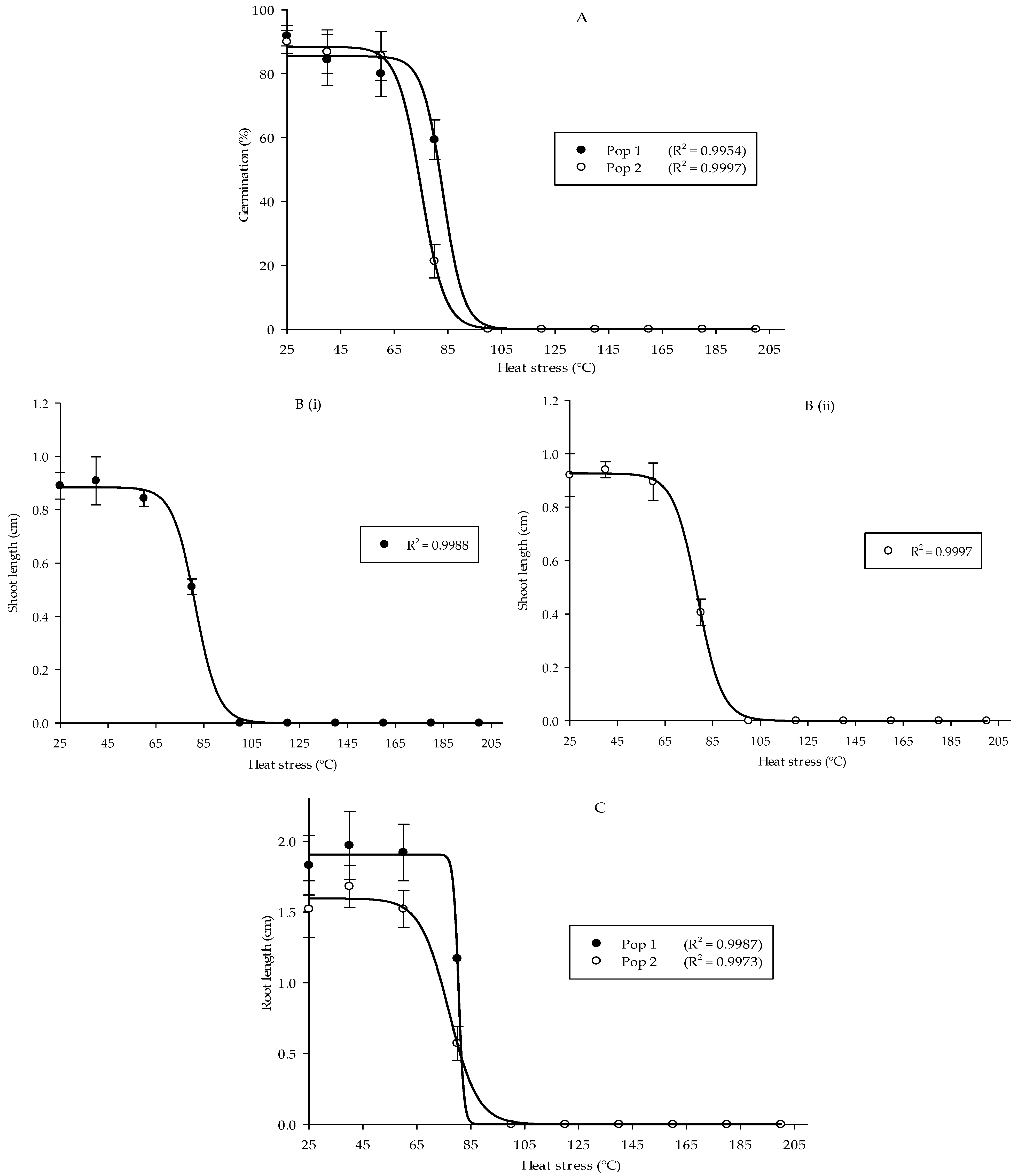
In general, heat stress significantly influenced germination and early growth development of local Parthenium weed populations. A significant interaction was evident between the population and treatment. Heat stress significantly reduced Parthenium weed germination by the increment of temperature (p < 0.001) (the mean squares from analysis of variance and p values tables of all Petri dish experiments are provided in Supplementary Table S1). Both populations exhibited similar germination behavior, although Pop 1 significantly exhibited higher germination percentage as compared to Pop 2. In all tested heat treatments, the highest germination occurred at room temperature (RT) followed by 40 and 60 °C, namely 91.88, 84.38, and 80.00% in Pop 1 and 90.00, 86.88, and 85.63% in Pop 2, respectively. Germination of Parthenium weed seeds was evident after 5 min heat treatment up to 80 °C: 59.38% in Pop 1 and 21.25% in Pop 2. Ultimately, the germination in both populations was totally inhibited at 100 °C and above as shown in Figure 1A.
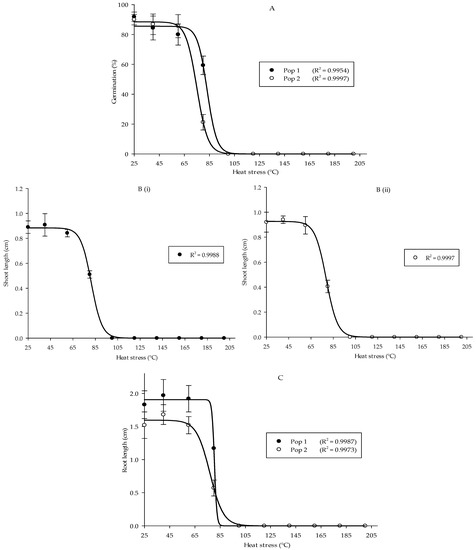
Figure 1.
Percentage of seed germination of Pop 1 and Pop 2 (A), shoot length of Pop 1 (Bi) and Pop 2 (Bii), and root length of Pop 1 and Pop 2 (C) of Parthenium weed under different heat stress treatments.
Similarly, heat stress distorted the shoot length in both populations (Figure 1(Bi,Bii)) (p < 0.001). In Pop 1, optimum shoot growth was obtained at 40 °C, followed by room temperature (RT) and 60 °C: 0.91, 0.89, and 0.84 cm. The results attained were similar in Pop 2, in which shoot lengths of 0.94, 0.92, and 0.89 cm were gained at 40, RT, and 60 °C, and then the length of the shoot was greatly reduced to 0.41 cm when the temperature was raised to 80 °C. In both populations, there was zero value for the shoot length at temperature of 100 °C and above due to the absence of germinated seeds in those heat treatments. However, no significant difference in shoot length was observed between the populations and in the interaction between population and treatment. A similar pattern was evident for root length, where substantial reduction was recorded on the increment in the temperature in both populations (p < 0.001). Maximum root length was observed at 40 °C: 1.97 cm in Pop 1 and 1.68 cm in Pop 2 (Figure 1C). The root length began to greatly reduce at 80 °C and it was prominently suppressed when the temperature rose above 100 °C. It was also noticed that greater root length was observed in Pop 1 over Pop 2 (p < 0.001).
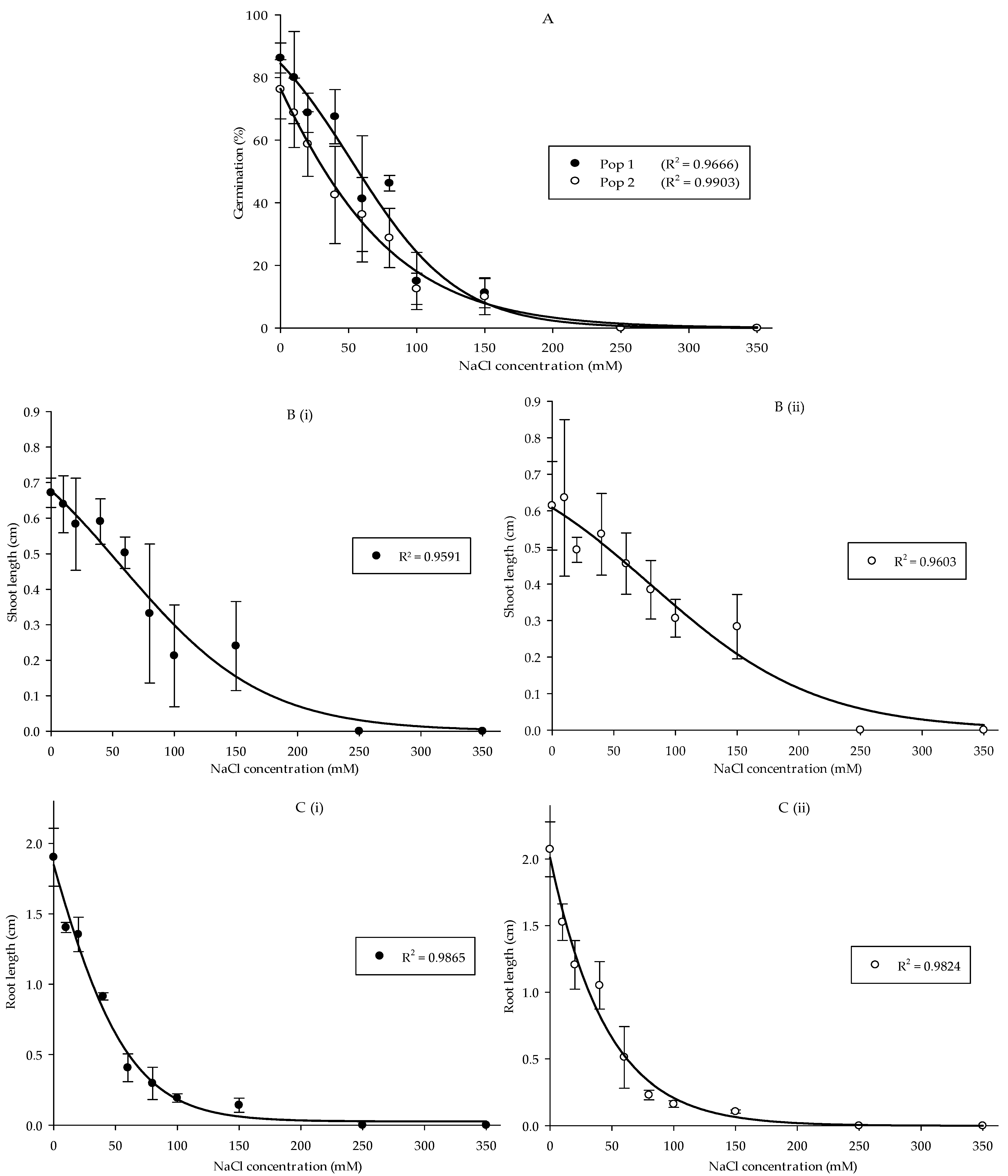
Overall, salinity treatments significantly affected the germination, as well as development of shoots and roots in Parthenium weed seedlings (p < 0.001). Nonetheless, the interaction between salt stress and population only influenced the germination (p < 0.05) but not the growth of shoots and roots. Germination in Pop 1 was greater than Pop 2 at 40 and 80 mM. The maximum germination was observed in the control treatment, viz. 86.25% in Pop 1 and 76.25% in Pop 2, and germination of more than 60% was still recorded at low concentrations (10 and 20 mM). Apparent reduction in germination began to take place at 40 mM and complete inhibition was observed from 250 mM (Figure 2A). The pattern continued in shoot length reduction corresponding to the increase in salinity (Figure 2(Bi,Bii)). In both Pop 1 and Pop 2, control treatment, 10, 20, and 40 mM exhibited no difference in shoot length. The shoot growth was starting to decline in 80 mM, and absolutely repressed at 250 mM. A slightly different pattern was noticed in root length, where a significant reduction already happened at 10 mM and the root formation was entirely inhibited at the concentrations of 250 and 350 mM (Figure 2(Ci,Cii)).
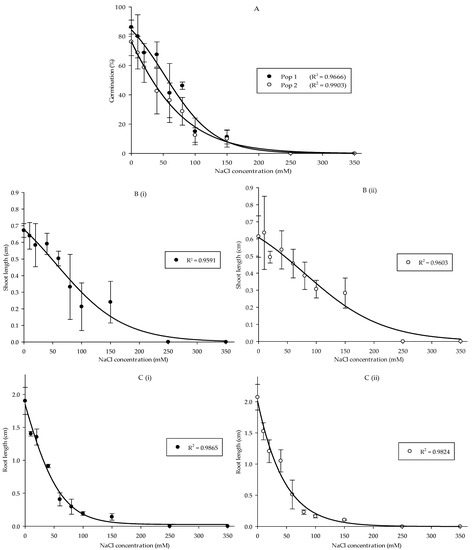
Figure 2.
Percentage of seed germination of Pop 1 and Pop 2 (A), shoot length of Pop 1 (Bi) and Pop 2 (Bii), and root length of Pop 1 (Ci) and Pop 2 (Cii) of Parthenium weed under different salinity treatments.
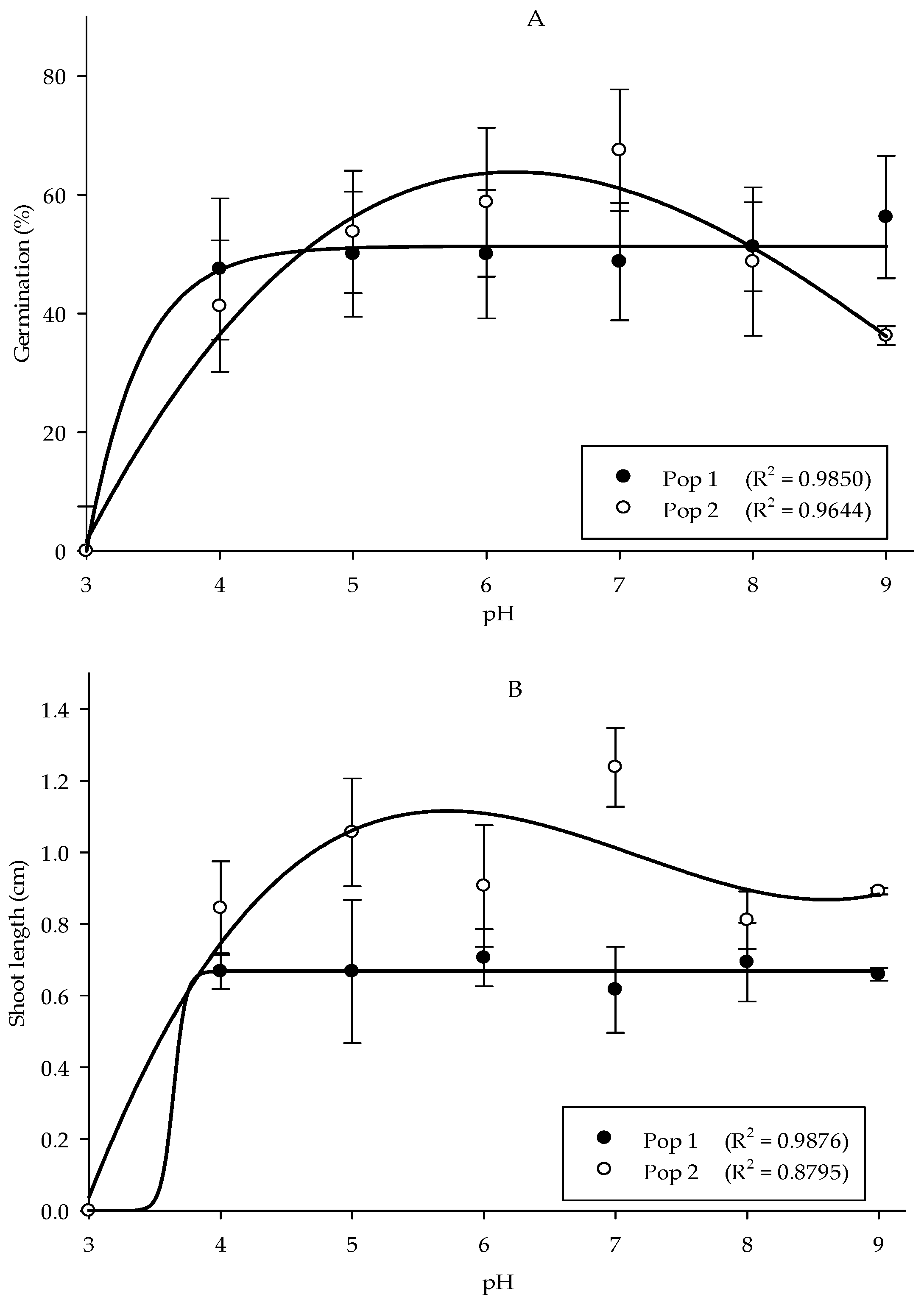
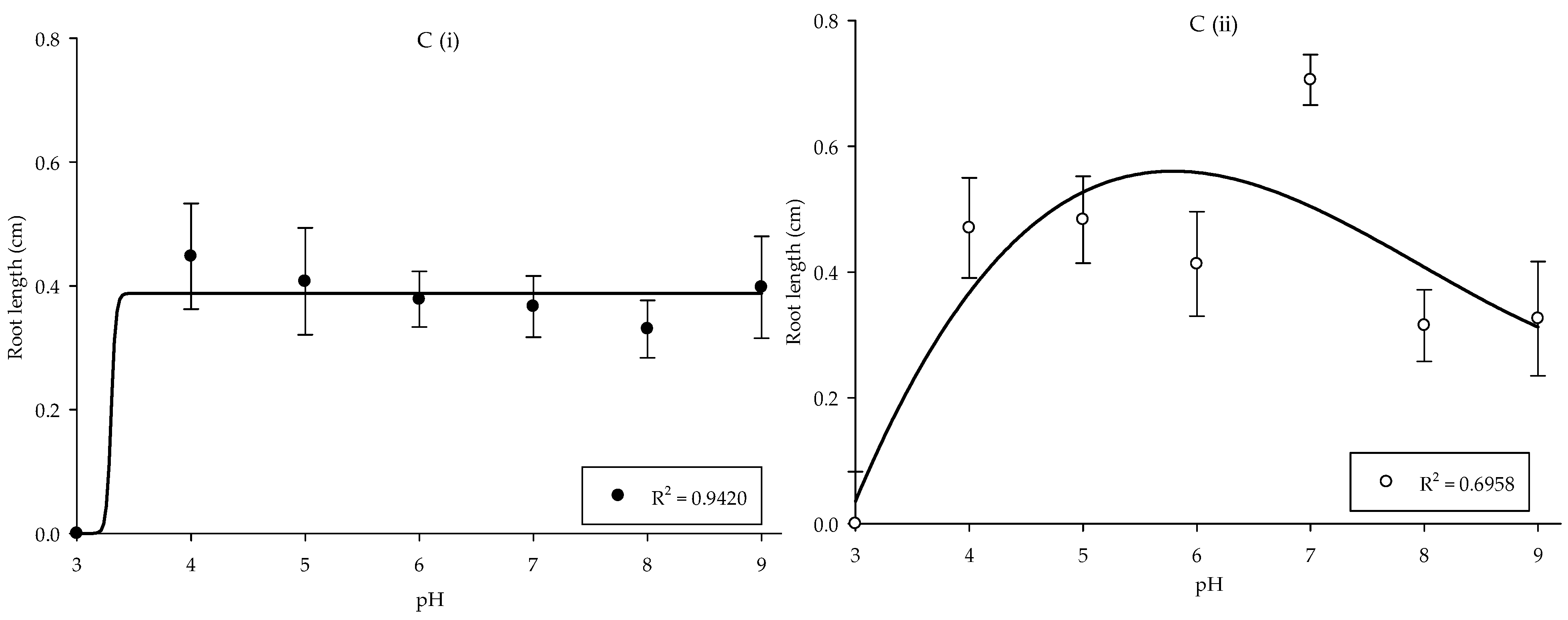
Growth responses of the Parthenium weed populations were least affected by the differences in pH, with the exception of pH 3. Meanwhile, the germination and root length showed no significant differences between populations, but shoot length was highly significant between populations (p < 0.001). In Pop 1, the maximum germination was found in pH 9 (56.25%), while in Pop 2, pH 7 yielded optimum germination of 67.50% (Figure 3A). The effect of pH treatment on shoot and root length of Parthenium weed in both Malaysia populations exhibited similar response (p < 0.001), where the total reduction was recorded in pH 3 (Figure 3B,(Ci,Cii)). The maximum shoot length was acquired in pH 6 in Pop 1 and pH 7 in Pop 2, respectively. As for the root length, the optimum root length was observed in pH 4 (0.45 cm). On the other hand, root length in Pop 2 was at its peak at pH 7 (0.71 cm).
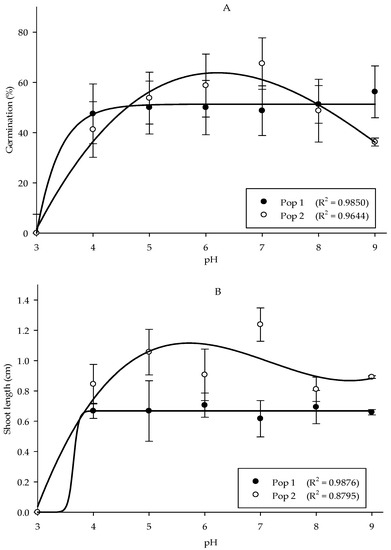
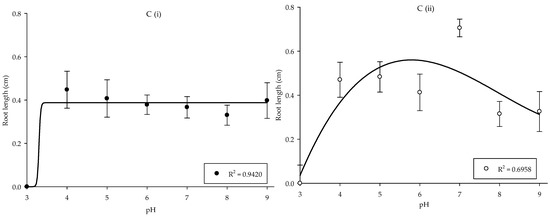
Figure 3.
Percentage of seed germination of Pop 1 and Pop 2 (A), shoot length of Pop 1 and Pop 2 (B), and root length of Pop 1 (Ci) and Pop 2 (Cii) of Parthenium weed under different pH treatments.
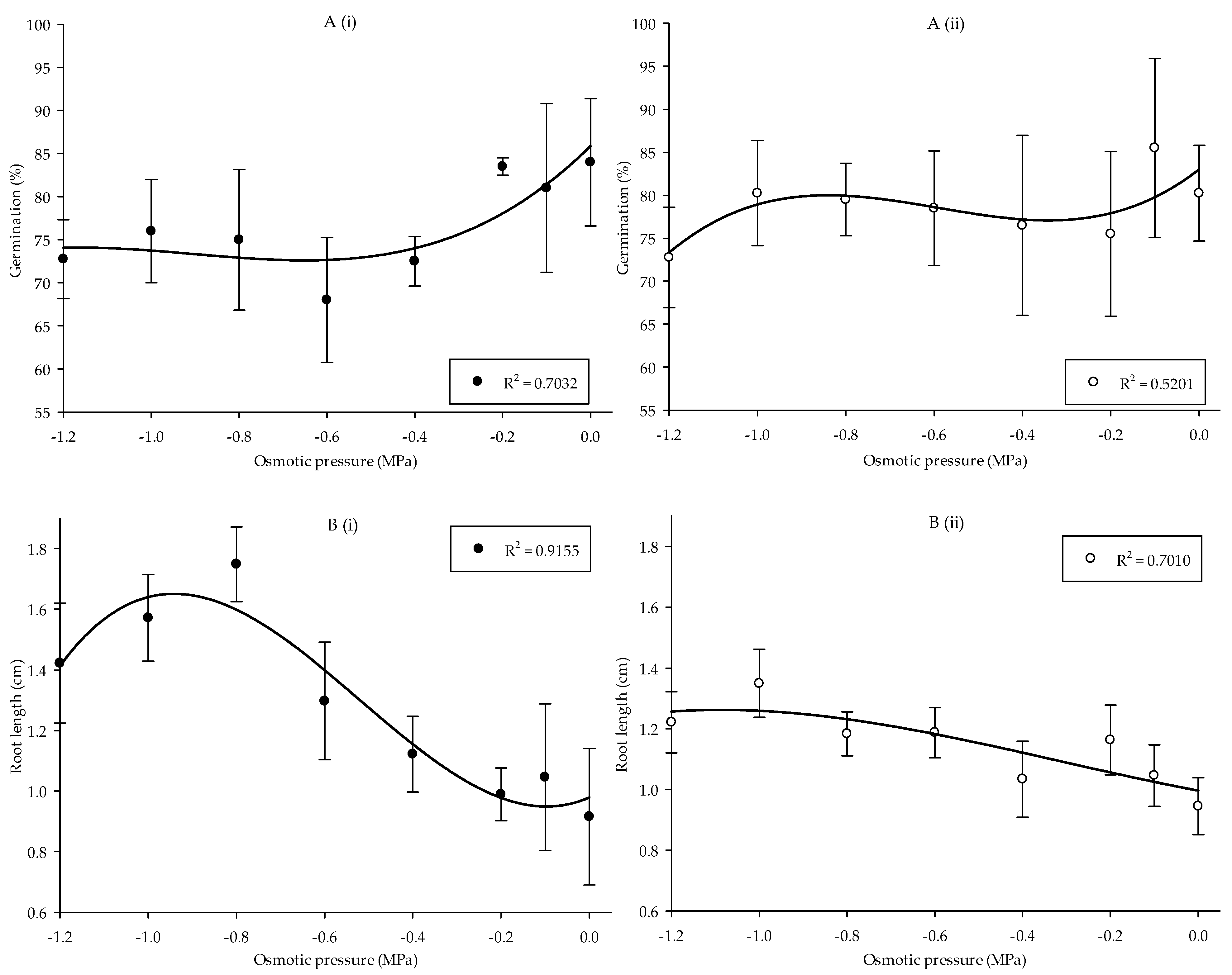
The overall result stipulates that germination and root length of the Parthenium weed exhibited fluctuating patterns corresponding to the differences in osmotic pressure (Figure 4(Ai,Aii,Bi,Bii)) (p < 0.05 and p < 0.01, respectively). In Pop 1, germination was at optimum (84%) in −0.2 MPa. Germination was substantially reduced in −0.6 MPa, resulting in the least germination of 68% (Figure 4(Ai)). Conversely, in Pop 2 germination was at maximum in −0.1 MPa and minimum in −1.2 MPa with the germination of 86 and 73%, respectively (Figure 4(Aii)). Osmotic pressure significantly affected the root formation of Parthenium weed in the two populations (Figure 4(Bi,Bii)). In Pop 1, the highest root length was observed in −0.8 MPa (1.75 cm) and the least root length was found in 0 MPa with 0.92 cm. In Pop 2, root length was the highest in −1.0 MPa (1.35 cm). The effect of osmotic pressure on shoot length is not presented since no significant result was observed.
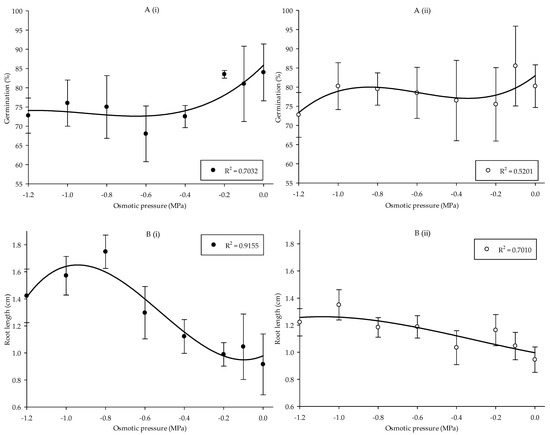
Figure 4.
Percentage of seed germination of Pop 1 (Ai) and Pop 2 (Aii), and root length of Pop 1 (Bi) and Pop 2 (Bii) of Parthenium weed under different osmotic pressures.
3.2. Pot Trial
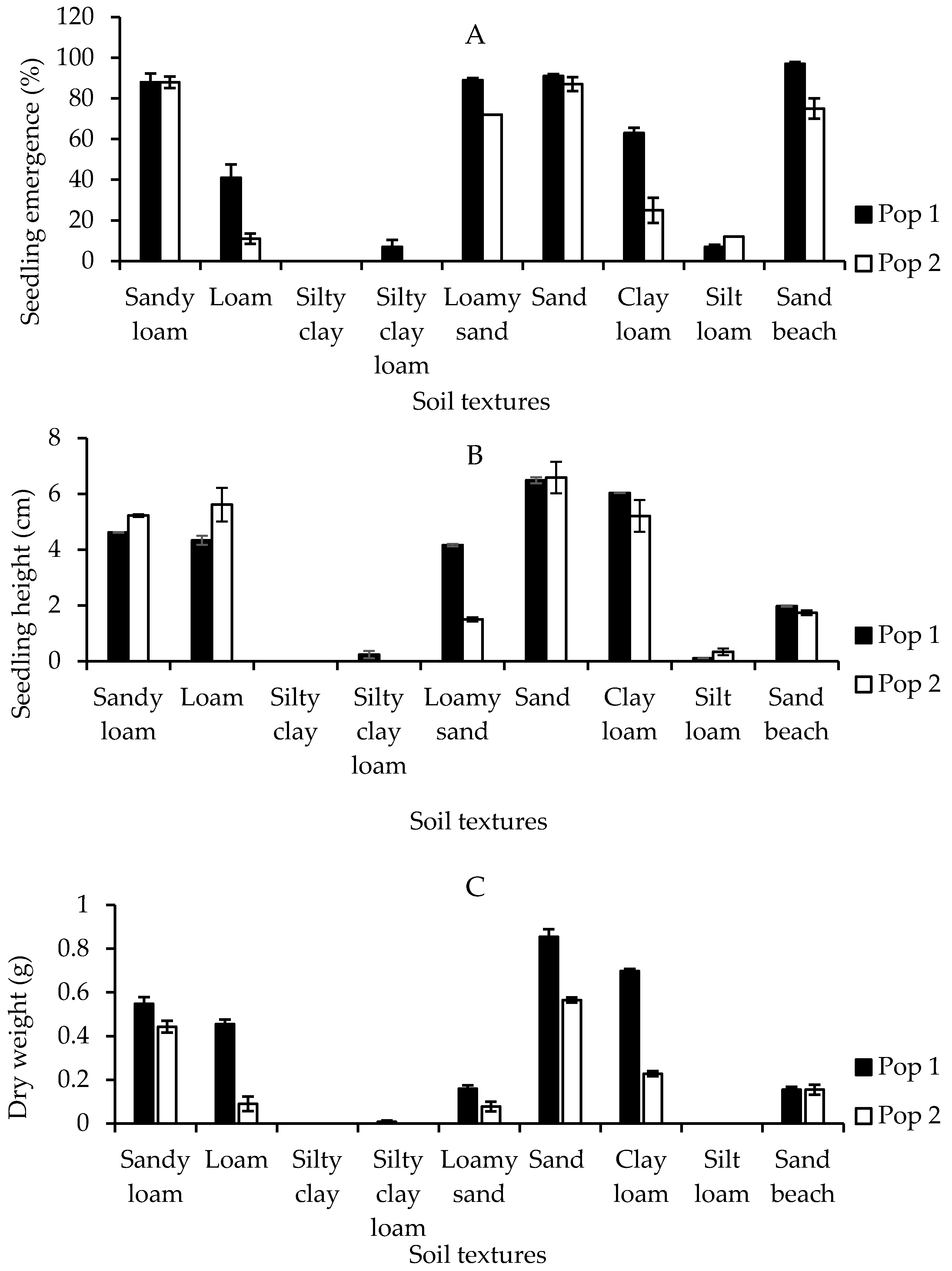
The seedling emergence and growth performance of the Malaysian Parthenium weed responded distinctively depending on the types of soil textures (p < 0.001) (the mean squares from analysis of variance and p values tables of all pot trials experiments are provided in Supplementary Table S1). In general, the seeds from both populations grew readily on all types of soil textures with the exception of silty clay type (Figure 5A). The highest percentage of seedling emergence from Pop 1 occurred in sand beach soil (97%), insignificantly followed by sand (91%), loamy sand (89%), and sandy loam (88%). A similar pattern, but with lower emergence was observed in Pop 2. Sandy loam (88%), followed by sand (86%), sand beach (75%), and loamy sand (72%) provided the most favorable conditions for Parthenium weed seeds to germinate over the other soil textures. Meanwhile, no germination was recorded in silty clay soil for both populations.
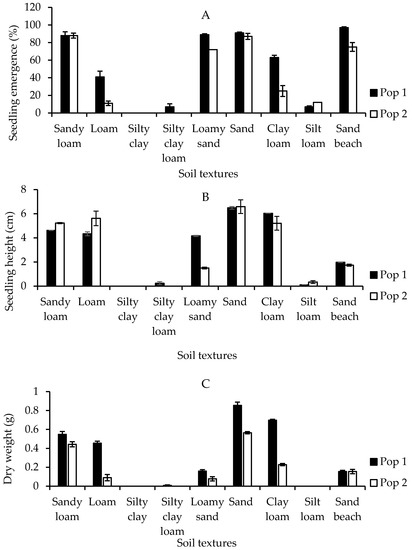
Figure 5.
Percentage of seedling emergence (A), seedling height (B), and seedling dry weight (C) of different Parthenium weed populations grown on different soil textures.
Seedling height and dry weight were measured to determine the effect of the different soil textures on the early growth of Parthenium weed populations. The seedlings from Pop 1 grew best on sand (6.50 cm), followed by clay loam (6.03 cm), sandy loam (4.62 cm), loam (4.33 cm), loamy sand (4.16 cm), and sand beach (1.97 cm). Similarly, for Pop 2, the optimum plant height was observed on sand (6.59 cm), loam (5.61 cm), sandy loam (5.22 cm), clay loam (5.21 cm), and sand beach (1.73 cm). Meanwhile, the heights of the weed grown on silt loam, silty clay loam and silty clay type of soil textures were less than 1 cm for both populations (Figure 5B), indicating that these types of soil textures were less suitable for Parthenium growth. The greatest dry weight was observed in sand (0.86 g), followed by clay loam (0.70 g) for Pop 1, and sand (0.57 g) and sandy loam (0.44 g) for Pop 2 (Figure 5C). Comparatively, the lowest Parthenium weed dry weight was from sandy beach soil (0.15 g) for Pop 1, while loamy sand (0.08 g) for Pop 2. Noticeably, even though observation on number of leaves was not taken, Pop 1 always produced a greater number of leaves as compared to Pop 2 under all soil textures. This perhaps contributed to higher biomass.
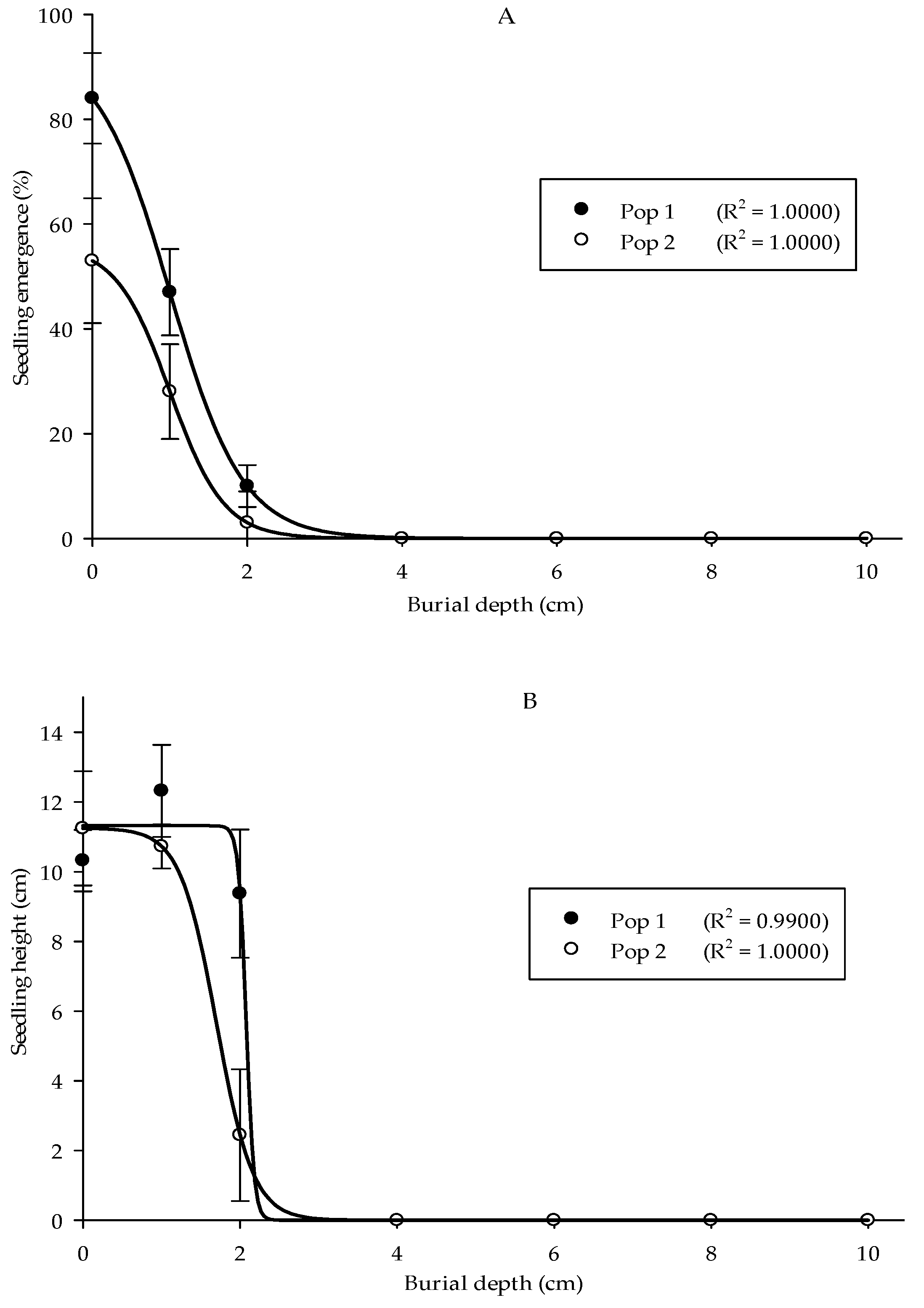
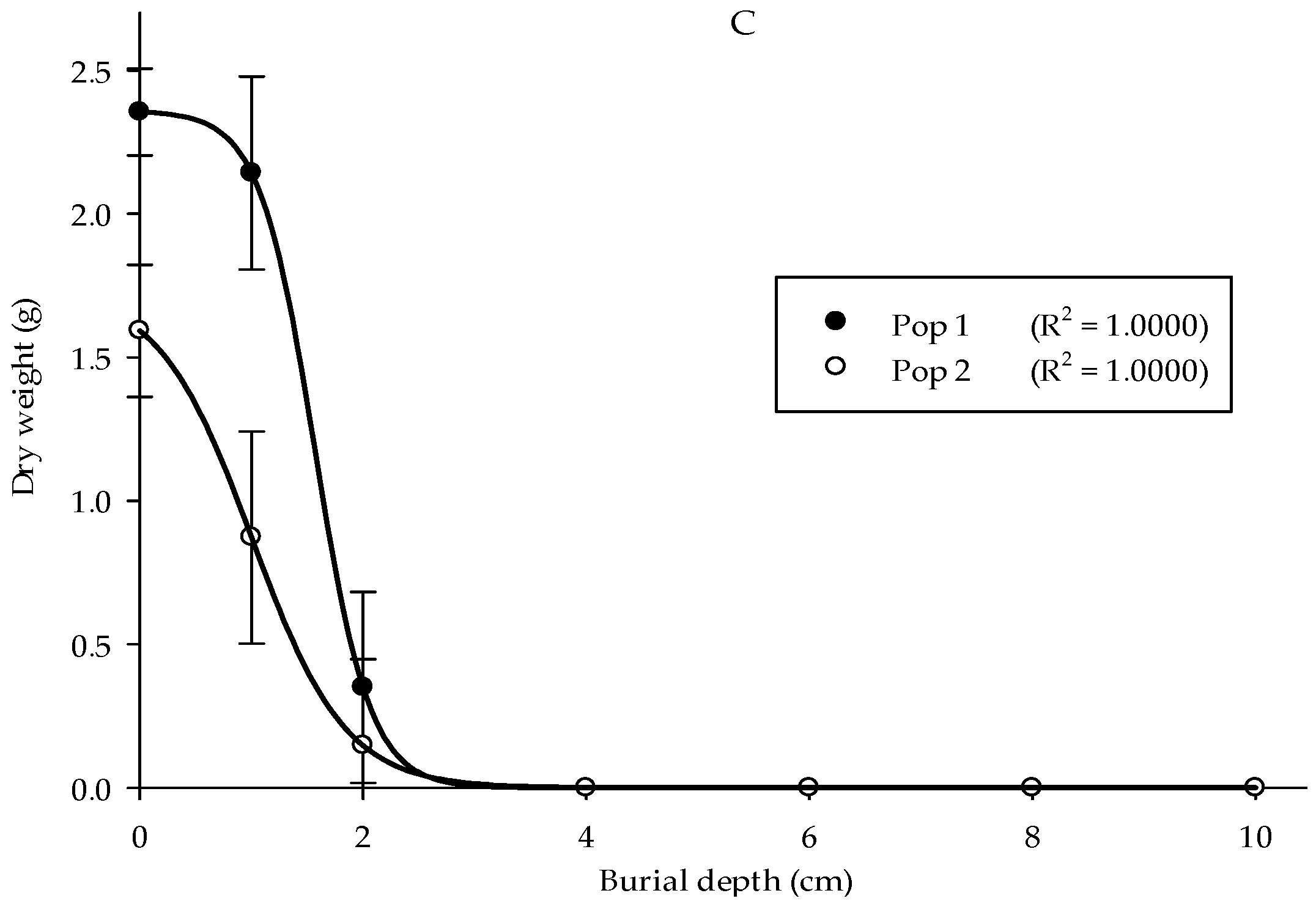
A burial depth experiment was carried out to understand Parthenium weed performance at different soil depths. The optimal seedling emergence in the two populations was seen at 0 cm, followed by 1 and 2 cm of burial depth with emergence values of 84%, 47%, and 10% for Pop 1, while 53%, 28%, and 3% for Pop 2, respectively (Figure 6A). No emergence was observed starting from 4 cm of burial depth and deeper for both populations. In general, the height of Parthenium weed seedlings in both populations significantly reduced as the burial depth increased (Figure 6B) (p < 0.001). Pop 1 emerged at 1 cm of burial depth and acquired the highest seedling height (12.32 cm). As for the Pop 2, maximum plant height was evident at 0 cm (11.6 cm). Minimal seedling height was observed at 2 cm burial depth for both populations. Thus, deep burial debilitated the development of the weed seeds. Similarly, the seeds that emerged from the soil surface (0 cm) showed the highest dry weight (2.36 g), followed by 1 cm of burial depth (2.13 g) for Pop 1, while 1.59 and 0.87 g for Pop 2, respectively (Figure 6C).
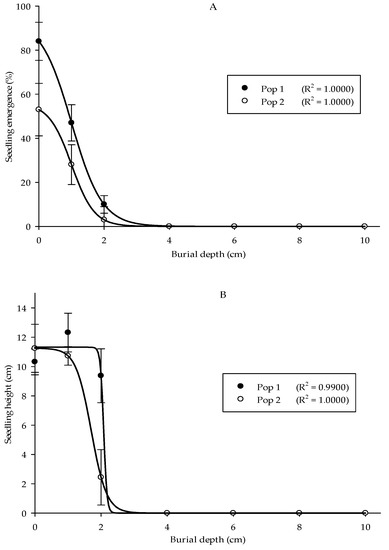
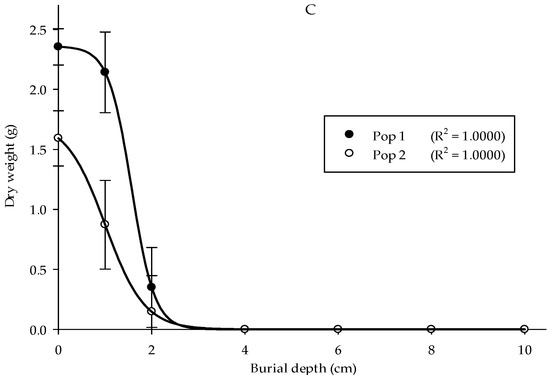
Figure 6.
Effect of different burial depths on percentage of seedling emergence (A), seedling height (B), and seedling dry weight (C) of different Parthenium weed populations 30 days after sowing.
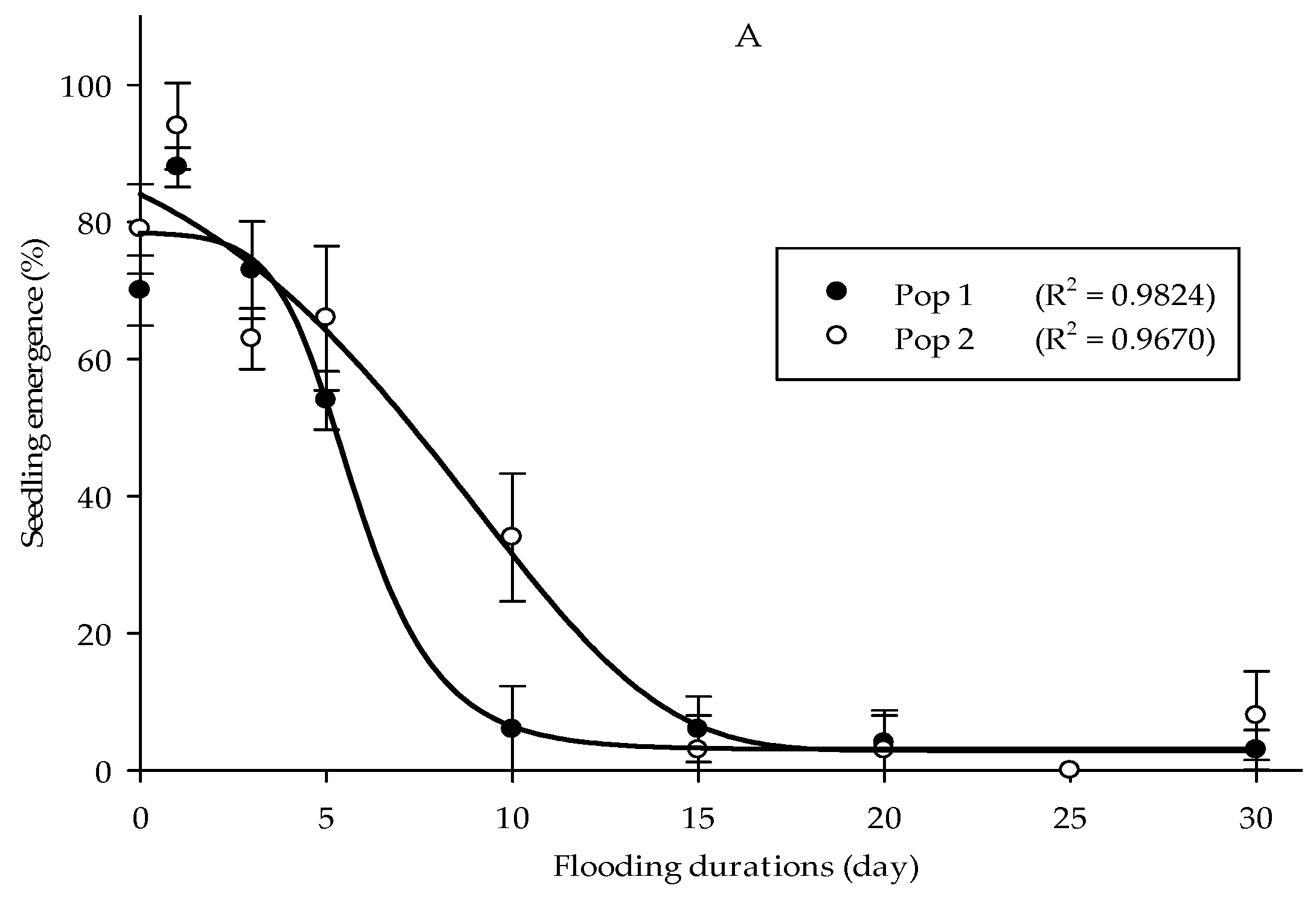
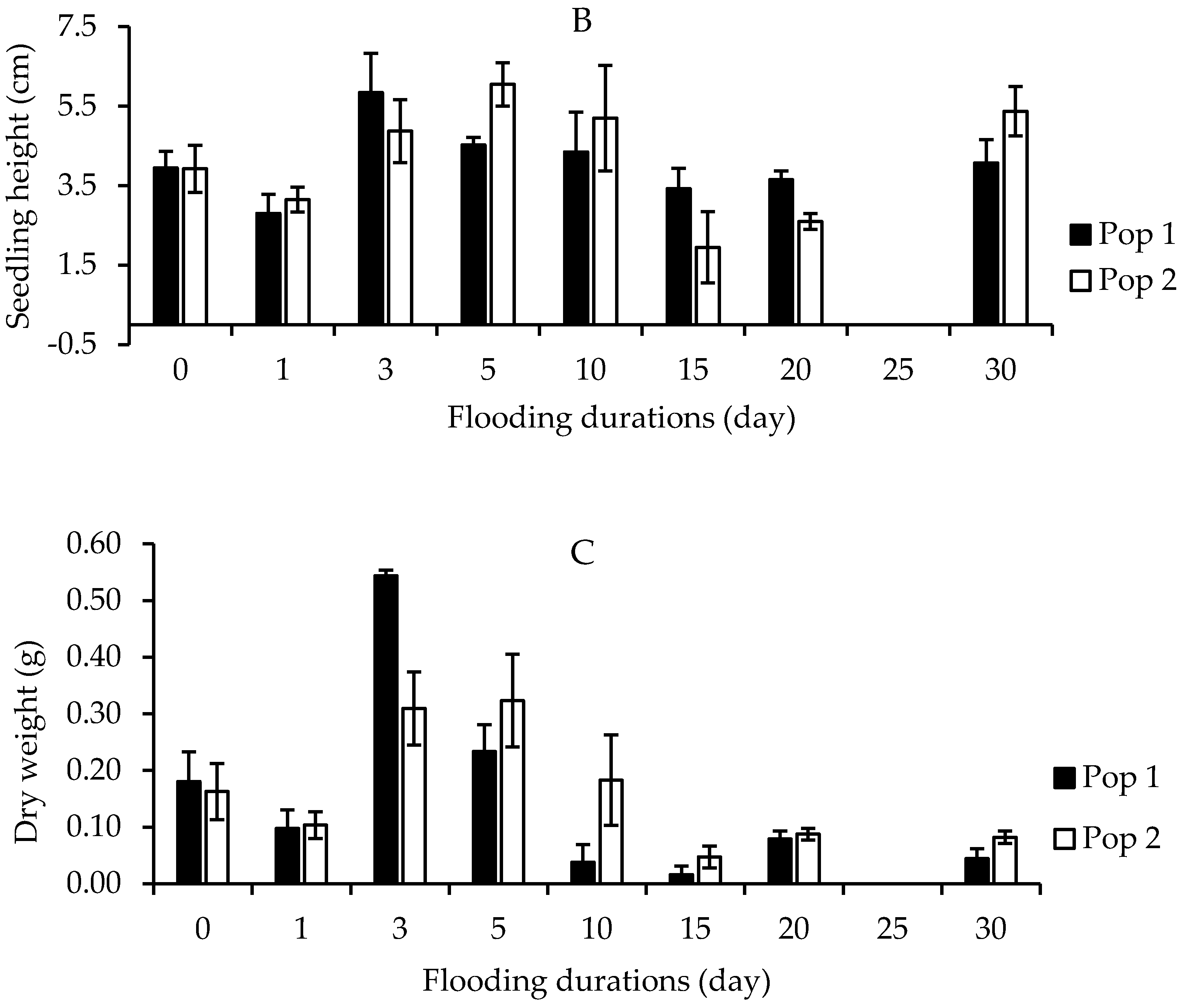
It was found that Parthenium weed seeds had maintained their capacity to emerge following submergence up to 30 days. Both populations exhibited optimal emergence at 0 and 1 days of submerging duration treatment, with germination higher than 80% (Figure 7A). At the longest submerging duration (30 days), some Parthenium weed seeds were still able to emerge, even though it was greatly reduced (<10%). The height of Parthenium weed seedlings populations was significantly affected by submerging duration treatments (p < 0.01). However, the responses of the seedling height towards the flooding duration treatment were not consistent for both populations (Figure 7B). The maximum seedling height was obtained at 3 days of submerging duration with 5.84 cm height in Pop 1, while in Pop 2 5 days of flooding period achieved the highest plant height (6.05 cm). A similar trend was observed in the dry weight, in which the highest dry weight was seen following 3 (0.54 g) and 5 (0.32 g) days of seed submergence, in Pop 1 and Pop 2, respectively (Figure 7C).
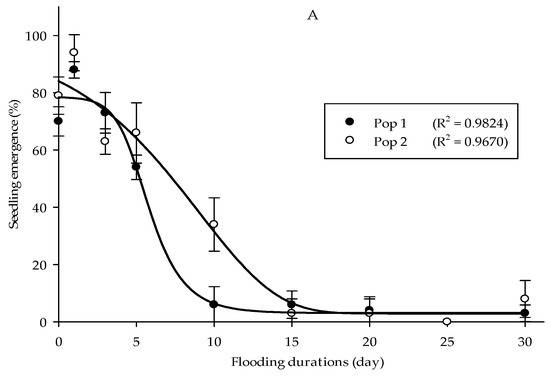
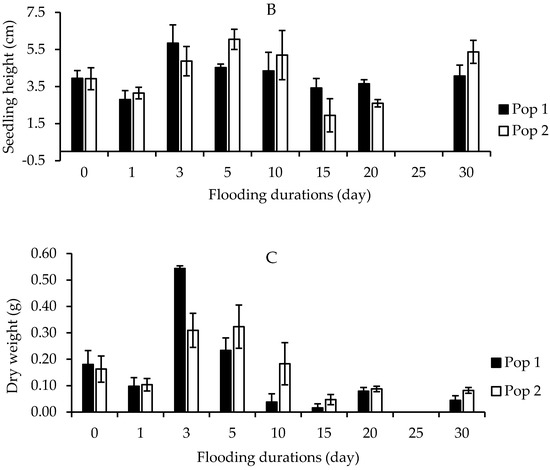
Figure 7.
Effect of flooding duration treatments on percentage of seedling emergence (A), seedling height (B), and seedling dry weight (C) of different Parthenium weed populations 30 days after sowing.
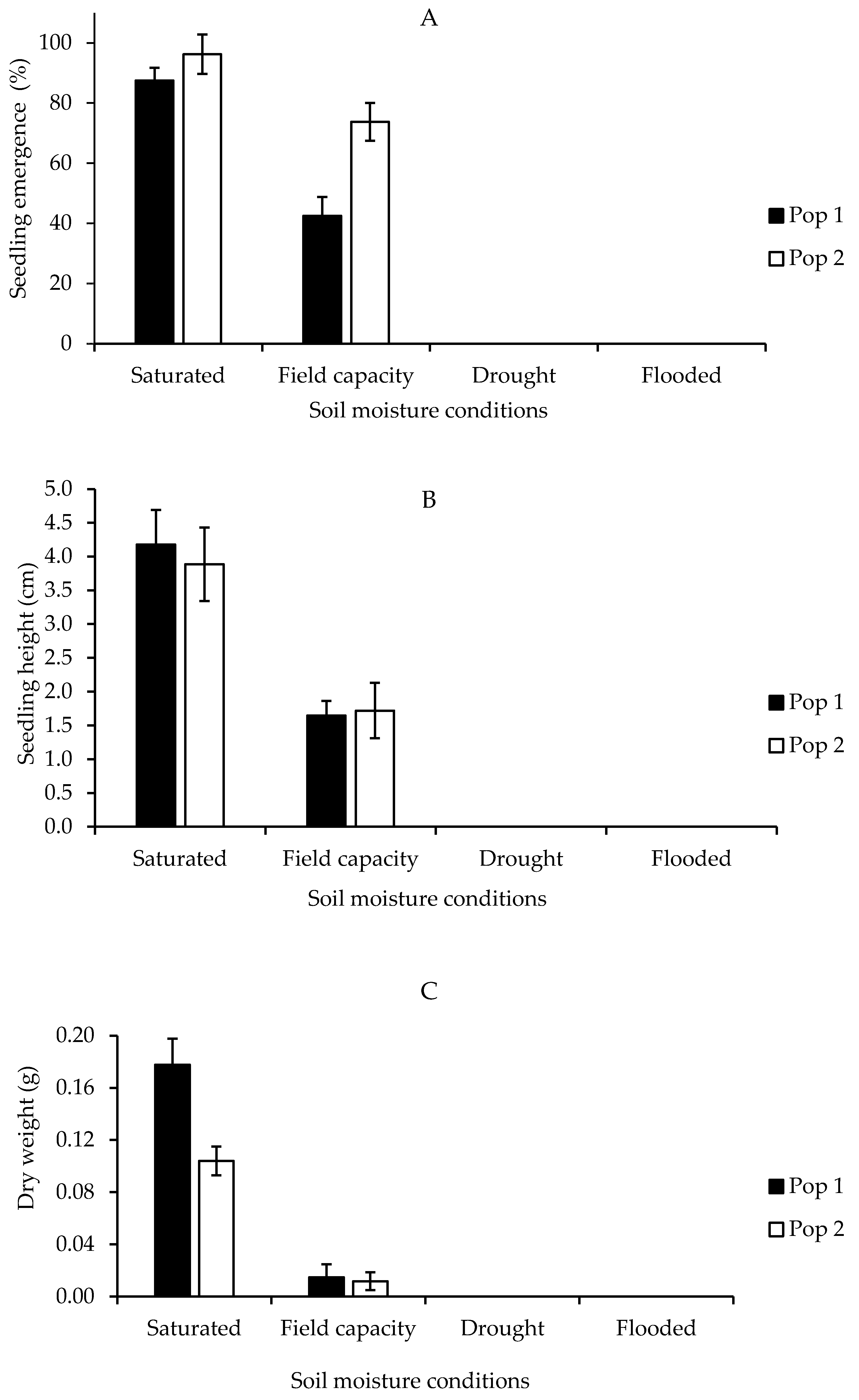
Figure 8A shows the seedling emergence capacity in each population in different soil moisture conditions: saturated, field capacity, drought, and flooded conditions. In general, there were highly significant differences in germination and growth performance including plant height, fresh and dry weight of Parthenium weed to different soil moisture conditions in both populations (p < 0.001). The emergence of Parthenium weed in Pop 1 and Pop 2 was found at its best under saturated soil condition, having 87.5% and 96.5% germination, respectively. This was then followed by field capacity (42.5% and 73.8%). Neither Pop 1 nor Pop 2 recorded any emergence in drought and flooded soils. The seedling heights of Parthenium weed in both populations 30 days after growing on different soil moisture conditions are shown in Figure 8B. The optimum plant height happened under saturated condition, followed by field capacity with 4.18 and 1.72 cm in Pop 1, while 3.89 and 1.72 cm in Pop 2, respectively. A similar pattern in dry weight was observed, where saturated soil condition yielded higher plant biomass as compared to field capacity condition (Figure 8C).
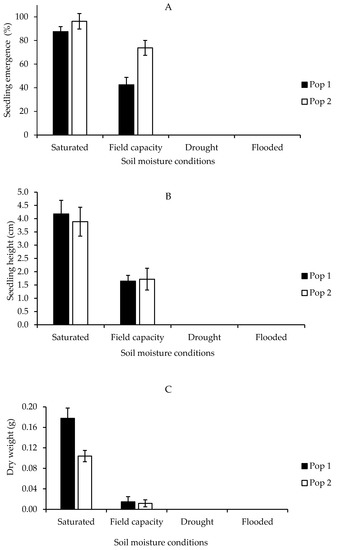
Figure 8.
Percentage of seedling emergence (A), seedling height (B), and seedling dry weight (C) of different Parthenium weed populations grown on different soil moisture conditions 30 days after sowing.
4. Discussion
4.1. Petri Dish Bioassay
Heat stress is one of the major factors exerted by the natural environment that may interrupt the plant growth process, metabolism, and productivity [30]. High temperature can become an obstacle to numerous biochemical reactions involved in plant growth and development [31]. However, some plants naturally harbor a number of adaptive, acclimation, or avoidance mechanisms to tolerate the heat stress conditions [32]. Surface temperature while burning vegetation can increase up to 550 °C for 6 min [33]. Nonetheless, this temperature can drop at a rate of 100 °C per cm under the soil surface in the first 5 cm, as described by Sanchez [34]. Seeds on soil surface might experience damage by the heat exerted during the vegetation burning but the buried seeds below 4 cm could survive the heat stress. This study suggests that Parthenium weed seeds on the soil surface or in a shallow burial depth which are exposed to the heat from vegetation burning (temperature of 100 °C and above) might be damaged, however, the deep-buried seeds might survive from the heat of 80 °C and below. The buried seed may remain in the dormant state until exposed to the light when brought onto the soil surface by tillage operations [25].
Saline condition mainly occurs in arid and semiarid regions [35,36] and it comprises approximately 40% of the earth’s area, mainly in the coastal areas [37]. It was evident that Malaysian Parthenium weed populations were capable of germinating at up to 150 mM of NaCl concentration. Nevertheless, there was no germination at 250 mM (average salt level in coastal soil in Malaysia), suggesting that it is unlikely for Parthenium weed to colonize coastal areas. In Malaysia, a non-herbicide approach of Parthenium weed control by the Department of Agriculture Malaysia using salt water is also being implemented, although the continuous use of this method is not advisable in the wake of altering soil pH into being more acidic [38]. Shoot and root lengths were severely reduced at saline water concentration of 80 mM as shoots and roots produced less than 0.4 cm length. Salt stress has been proven to affect water imbibition in seed, rate of germination, and root elongation [17]. Katembe et al. [39] suggested that the influence of NaCl on plant growth includes a combination of osmotic and specific ion effects. High concentration of NaCl reduces the seed imbibition potential by reducing the water potential and suppressing the seed germination [17]. The results on salt stress obtained in this study were similar to research conducted on Campsis radicans Seem. [26], Solanum elaeagnifolium Cav. [40], Chamaesyce maculata (L.) Small [17], and Polypogon fugax Nees ex Steud. [41], concluding that the germination in these weeds was possible until 120 mM NaCl concentration, while no germination happened when the concentration increased to 160 mM NaCl and beyond.
As expected, strong acidic condition in pH 3 is less favorable for any plant to grow, that includes Parthenium weed. However, the ability of this invasive weed species to grow in pH 4 and 5 is worrisome, making it capable of invading a wide range of soil pH. The adaptive ability of weed species across various pH levels has been reported in several weed species including Campsis radicans Seem. [26], Urena lobata L. [42], Eleusine indica (L.) Gaertn. [25], and Solanum rostratum Dunal [43]. This study suggests that pH would not be one of the factors that significantly limit the growth of Parthenium weed in various habitats in Malaysia.
Osmotic pressure treatment duplicates the soil moisture stress against germination and early growth responses of local Parthenium weed populations. The weed tolerated different levels of osmotic pressure. Germination was higher than 70% in both populations at low osmotic pressure treatments. This study demonstrates that Parthenium weed showed low sensitivity to osmotic pressure treatments. The ability of Parthenium weed to germinate and grow over a broad range of osmotic pressure might contribute to its invasiveness. Naturally, there have been reports on plant species tolerant to low osmotic pressure including Solanum sarrachoides Sendtn. [44], Bromus japonicus Thunb. [24], and Sophora davidii (Franch.) Skeels [41].
4.2. Pot Trial
The spread succession of Parthenium weed into infested countries is due to the presence of suitable soil and other favorable environmental factors [45,46]. Navie et al. [47] claimed that soil type is not a crucial limiting factor for Parthenium weed as the weed is capable of thriving in a wide range of soil types, thus aiding its vigorous infestation. It can grow on varying types of soil textures from sandy loams to clay and clay loams [4,48,49]. This is also true in our findings, where Parthenium weed seeds from both populations emerged well on many types of soil textures, although the preferable soil textures for Parthenium weed seeds’ emergence and growth were sandy loam and sandy type for both populations. Parthenium predominantly grows on course texture soil, which enhances the colonization process in new areas [15,50].
Burial depth is a recognizable factor that influences seed germination and seedling emergence in natural environment [26]. Boyd and Acker [51] also suggested that the effect of soil burial depth on seed germination relies on the type of plant species and soil physical parameters. Some weed species, such as Avena fatua (wild oat) are capable of germinating and emerging at deep burial depths up to 20 cm [52], as well as Urena lobata [42]. On the contrary, several weed species such as Rumex crispus L. [53], Eriochloa villosa (Thunb.) Kunth [54], Nicandra physalodes (L.) Gaertn. [55], Urochloa subquadripara (Trin.) R.D. Webster [56], Solanum sarrachoides Sendtn. [44], Amaranthus viridis L. [57], Hesperis matronalis L. [58], Ipomoea purpurea (L.) Roth [19], Sporobolus indicus (L.) R.Br. [59], Abutilon theophrasti Medik., Echinochloa crus-galli (L.) Beauv [60], Polypogon fugax Nees ex Steud. [41,61], and Cucumis melo L. [62], and in the present study Parthenium weed had their emergence hampered when buried deep in the soil. The optimum burial depth for Parthenium was observed on soil surface (0 cm burial depth) in both populations, suggesting that mechanical weed seed control such as deep-ploughing could be a useful method to reduce Parthenium weed emergence.
Submerging duration is one of the factors that influence the seedling emergence and distribution of many plant species [63]. Flood can be defined as a general and impermanent state of partial or complete immersion of naturally dry land regions from the overflow of tidal or inland waters, or from the unordinary and rapid accumulation of surface waters from any source. It may happen naturally by prolonged periods of substantial precipitation when natural watercourses are unable to accommodate the excess water, tsunami and via storm surge with tropical cyclone, or triggered by some other phenomena such as dam failure, topography, landslides, and tidal influences [64]. This study mimicked the real situation of seed submergence in the water (e.g., flooding, drain, ditch, and creek), to observe whether the seeds were still viable and capable of germinating after being submerged under water for a certain period. Van Eck et al. [65] suggested that flooding duration is the predominant factor influencing the distribution of plant species along the elevation gradients in river floodplains. Besides that, the time of flooding strongly affects the composition of plant species [66,67]. The seeds of Malaysia Parthenium weed populations in the current study remained viable following submergence for 30 days. This characteristic would help the dispersal of Parthenium weed via surface runoff following rainfall into water canals or drainage systems, further increasing its invasive territory in various habitats and ecosystems. Thus, it is crucial to ensure that this noxious weed species is carefully and essentially managed, especially plants that grow close to water bodies to avoid seed shattering into the water bodies.
Soil moisture is a fundamental abiotic variable that regulates the habitat suitability of one species [68]. A broad variation in soil moisture conditions in the natural environment affects the seed germination, further influencing establishment and reproductive ability in plants under both extreme soil moisture conditions, including drought and flooded conditions, and in turn leading to adaptive tolerance characters [68,69]. The deficit of water content in the soil or drought affects the physiological processes, such as transpiration, photosynthesis, and translocation of assimilates from the main source to the sinks [70]. Furthermore, low soil water content significantly decreases the concentration of shoot nutrients and increases the soil redox potential in the plant as studied by Elkheir et al. [71]. Our findings indicate that the Parthenium weed emergence was ideal in saturated (0 kPa) soil moisture and field capacity conditions. It is critical for the Parthenium weed seed to emerge under adequate soil moisture condition in the range of 0–−50 kPa as no emergence occurred in both extreme soil moisture conditions, which included drought (−70 kPa) and flooded conditions. Bavec and Mlakar [72] and Barney et al. [68] have suggested that medium moisture availability in ecosystem is particularly productive for plant growth and establishment.
5. Conclusions
The current study investigated germination and growth behavior of two Malaysian Parthenium weed populations under various biotic conditions, namely in Petri dish and pot bioassays. The Petri dish bioassay found that both populations are capable of germinating in high temperatures of up to 80 °C, moderately tolerant to salinity, and tolerate wide ranges of pH and osmotic pressure. In the pot trial, each Parthenium weed population responded to the treated abiotic factors differently, with Pop 1 having slightly higher growth capacity over Pop 2 when grown under various soil textures and burial depths. It is however noticed that mixed responses were evident in flooding durations and soil moisture conditions. An extensive pot and field trials study on growth competition of Parthenium weed with different crops is in dire need, thus one can predict the competitiveness ability of this invasive weed in the agricultural zones.
Our findings summarize that the seedling emergence and growth behavior of Parthenium weed from two distinguished ecological zones varied significantly, suggesting that both Parthenium weed populations might have originated from different locations. It is also plausible that the distinguished growth responses displayed by both populations are influenced by the original conditions of the growing area, e.g., temperature, traffic disturbance frequency, soil properties, etc., from where the populations were sampled. This information is useful as a fundamental tool to understand the growth and distribution patterns, enabling prediction of the possible infestation areas and guidance to develop a successful control method. In fact, our results have become a source of reference by the local authorities under the Department of Agriculture Malaysia to predict the potential widespread of Parthenium weed in Malaysia, essential for the future development of effective, sustainable, and promising integrated management program of this noxious weed.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture11090856/s1. Table S1: Mean squares from analysis of variance and p values of Petri dish bioassay and pot trials experiment.
Author Contributions
Conceptualization, M.S.A.-H. and M.D.; methodology, M.S.A.-H., M.D. and A.F.M.A.; validation, M.S.A.-H., M.D., U.R.S. and A.S.J.; formal analysis, A.F.M.A. and R.R.; investigation, A.F.M.A. and M.S.A.-H.; resources, M.S.A.-H., M.D., U.R.S. and A.S.J.; data curation, A.F.M.A., R.R., M.D. and M.S.A.-H.; writing—original draft preparation, A.F.M.A. and M.S.A.-H.; writing—review and editing, R.R., M.S.A.-H., M.D., U.R.S. and M.N.G.; visualization, A.F.M.A., R.R. and M.S.A.-H.; supervision, M.S.A.-H. and M.D.; project administration M.S.A.-H., M.D., U.R.S. and A.S.J.; funding acquisition, M.S.A.-H., M.D., U.R.S. and A.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Universiti Putra Malaysia (projects no. UPM/700-2/1/GP-IPS/2016/9500100; UPM/700-2/1/GP-IPB/2017/9523401; UPM/700-2/1/GP-IPB/2017/9523403) and Malaysian Agricultural Research and Development Institute (MARDI: JTI-044).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their sincere gratitude to the Research Management Centre of Universiti Putra Malaysia, under the Putra Grants (projects no. UPM/700-2/1/GP-IPS/2016/9500100; UPM/700-2/1/GP-IPB/2017/9523401; UPM/700-2/1/GP-IPB/2017/9523403) and Malaysian Agricultural Research and Development Institute (MARDI: JTI044) for providing financial support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. Hybridization between native and introduced wildlife species: Importance for conservation. Wildl. Biol. 1996, 2, 143–150. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The Population Biology of Invasive Species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Aggarwal, N.K.; Kumar, V.; Dhiman, R. Effects and Management of Parthenium hysterophorus: A Weed of Global Significance. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and Economic Costs of Nonindigenous Species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Abdulkerim-Ute, J.; Legesse, B. Parthenium hysterophorus L: Distribution, impact, and possible mitigation measures in Ethiopia. Trop. Subtrop. Agroecosys. 2016, 19, 61–72. [Google Scholar]
- Karim, S.R. Parthenium Hazards in Malaysia and Its Sustainable Management. Inaugural Lecture Presented at Faculty of Agro Based Industry Universiti Malaysia Kelantan Jeli Campus. 2015. Available online: http://umkeprints.umk.edu.my/id/eprint/4804 (accessed on 5 June 2020).
- Karim, S.M.R.; Norhafizah, M.Z.; Maszura, C.M. Incidence of Parthenium allergy on human health in Kedah, Malaysia. Int. J. Biol. Pharm. All. Sci. 2017, 6, 175–182. [Google Scholar]
- Wiesner, M.; Tessema, T.; Hoffmann, A.; Wilfried, P.; Buettner, C.; Mewis, I.; Ulrichs, C. Impact of the Pan-Tropical weed Parthenium hysterophorus L. on human health in Ethiopia. In Utilisation of Diversity in Land Use Systems: Sustainable and Organic Approaches to Meet Human Needs; Tropentag: Witzenhausen, Germany, 2007. [Google Scholar]
- Adkins, S.; Shabbir, A.; Dhileepan, K. An Introduction to the ‘Demon Plant’ Parthenium Weed. In Parthenium Weed: Biology, Ecology and Management; CABI Invasive Series 11; Adkins, S., Shabbir, A., Dhileepan, K., Eds.; CABI: Boston, MA, USA, 2019; Volume 7, pp. 1–2. ISBN 9781780645254. [Google Scholar]
- Karim, S.M.R. Malaysia Invaded: Weed It out before It Is too Late. International Parthenium News 9. 2014. Available online: https://apwss.org/documents/newsletters/parthenium/Parthenium_News_January_2014.pdf (accessed on 5 June 2020).
- National Pest Management Committee Report; Department of Agriculture, Malaysia: Putrajaya, Malaysia, 2017.
- Tanveer, A.; Khaliq, A.; Ali, H.H.; Mahajan, G.; Chauhan, B. Interference and management of parthenium: The world′s most important invasive weed. Crop. Prot. 2015, 68, 49–59. [Google Scholar] [CrossRef]
- Kohli, R.K.; Rani, D. Exhibition of allelopathy by Parthenium hysterophorus L. in agroecosystems. Trop. Ecol. 1994, 35, 295–307. [Google Scholar]
- Annapurna, C.; Singh, J.S. Variation of Parthenium hysterophorus in response to soil quality: Implications for invasiveness. Weed Res. 2003, 43, 190–198. [Google Scholar] [CrossRef]
- Forcella, F.; Arnold, R.L.B.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crop. Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Asgarpour, R.; Ghorbani, R.; Khajeh-Hosseini, M.; Mohammad, E.; Chauhan, B.S. Germination of spotted spurge (Chamaesyce maculate) seeds in response to different environmental factors. Weed Sci. 2015, 63, 502–510. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Chapter 4—physiology of dormancy and germination in relation to seed bank ecology. In Ecology of Soil Seed Banks, 1st ed.; Leck, M.A., Parker, V.E., Simpson, R.L., Eds.; Academic Press: Cambridge, MA, USA, 1989; pp. 53–65. ISBN 9780323148658. [Google Scholar]
- Singh, M.; Ramirez, A.H.M.; Sharma, S.D.; Jhala, A.J. Factors Affecting the Germination of Tall Morningglory (Ipomoea purpurea). Weed Sci. 2012, 60, 64–68. [Google Scholar] [CrossRef]
- Chauhan, B.; Gill, G.; Preston, C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006, 54, 854–860. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Ecological studies on Cyperus difformis, Cyperus iria and Fimbristylis miliacea: Three troublesome annual sedge weeds of rice. Ann. Appl. Biol. 2009, 155, 103–112. [Google Scholar] [CrossRef]
- Utilization of Tropical Foods: Fruits and Vegetables; Food and Nutrition Paper 47/7; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990.
- International Seed Testing Association. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2016. [Google Scholar]
- Li, Q.; Tan, J.; Li, W.; Yuan, G.; Du, L.; Ma, S.; Wang, J.X. Effects of Environmental Factors on Seed Germination and Emergence of Japanese Brome (Bromus japonicus). Weed Sci. 2015, 63, 641–646. [Google Scholar] [CrossRef]
- Chauhan, B.; Johnson, D.E. Germination Ecology of Goosegrass (Eleusine indica): An Important Grass Weed of Rainfed Rice. Weed Sci. 2008, 56, 699–706. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 2000, 48, 212–216. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Ahmed, H.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Rafii, Y.M.; Aslani, F.; Omar, D. Comparative phytotoxic effects of aerial and root aqueous extracts of Sida cordifolia L. on germination and seedling vigour performance of lettuce, tomato and carrot. Bangl. J. Bot. 2017, 46, 323–328. [Google Scholar]
- Brownsey, R.N.; Kyser, G.B.; DiTOMASO, J.M. Seed and Germination Biology of Dittrichia graveolents (Stinkwort). Invasive Plant Sci. Manag. 2013, 6, 371–380. [Google Scholar] [CrossRef]
- Lobell, D.; Field, C.B. Global scale climate–crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007, 2. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Cook, L. A contribution to our information on grass burning. S. Afr. J. Sci. 1939, 36, 270–282. [Google Scholar]
- Sanchez, P.A. Soil management in shifting cultivation areas. In Properties and Management of Soils in the Tropics; John Wiley and Sons: New York, NY, USA, 1976; pp. 346–412. ISBN 0471752002. [Google Scholar]
- Bernstein, L. Crop growth and salinity. In Drainage for Agriculture; Number 17 in Agronomy Series; Schilfgaarde, J.V., Ed.; American Society of Agronomy: New York, NY, USA, 1974; pp. 39–54. ISBN 9780891182115. [Google Scholar]
- Shannon, M.C. Adaptation of Plants to Salinity. Adv. Agron. 1997, 60, 75–120. [Google Scholar] [CrossRef]
- Fischer, R.A.; Turner, N.C. Plant Productivity in the Arid and Semiarid Zones. Annu. Rev. Plant Physiol. 1978, 29, 277–317. [Google Scholar] [CrossRef]
- Ahmad-Hamdani, M.S.; (Universiti Putra Malaysia, Selangor, Malaysia). Personal communication, 2019.
- Katembe, W.J.; Ungar, I.A.; Mitchell, J.P. Effect of Salinity on Germination and Seedling Growth of two Atriplexspecies (Chenopodiaceae). Ann. Bot. 1998, 82, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Stanton, R.; Wu, H.; Lemerle, D. Factors Affecting Silverleaf Nightshade (Solanum elaeagnifolium) Germination. Weed Sci. 2012, 60, 42–47. [Google Scholar] [CrossRef]
- Wang, L.; Jin, S.; Wu, L.; Zhou, X.; Liu, X.; Bai, L. Influence of environmental factors on seed germination and emergence of Asia minor bluegrass (Polypogon fugax). Weed Technol. 2016, 30, 533–538. [Google Scholar] [CrossRef]
- Wang, J.Z.; Cui, L.J.; Wang, Y.; Li, J.L. Growth, lipid peroxidation and photosynthesis in two tall fescue cultivars differing in heat tolerance. Biol. Plant. 2009, 53, 237–242. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, C.; Li, X.; Cui, H.; Huang, H.; Sui, B.; Meng, Q.; Zhang, H. Factors Affecting Buffalobur (Solanum rostratum) Seed Germination and Seedling Emergence. Weed Sci. 2009, 57, 521–525. [Google Scholar] [CrossRef]
- Zhou, J.; Deckard, E.L.; Ahrens, W.H. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005, 53, 41–45. [Google Scholar] [CrossRef]
- Navie, S.C.; McFadyen, R.E.; Panetta, F.D.; Adkins, S.W. The effect of CO2 enrichment on the growth of a C3 weed (Parthenium hysterophorus L.) and its competitive interaction with a C4 grass (Cenchrus ciliaris L.). Plant Prot. Quart. 2005, 20, 61–66. [Google Scholar]
- Karim, S.M.R. Ill Impacts of Parthenium Weed on Human Health, Livestock Production and Environment; Presented in a Seminar at West Virginia University: Morgantown, WV, USA. 2012. Available online: http://umkeprints.umk.edu.my/1059/1/Paper%201.pdf (accessed on 5 June 2020).
- Navie, S.C.; McFadyen, R.E.; Panetta, F.D.; Adkins, S.W. The Biology of Australian Weeds. 27. Parthenium hysterophorus L. Plant Prot. Quart. 1996, 11, 76–88. [Google Scholar]
- Dale, I.J. Parthenium weed in the Americas: A report on the ecology of Parthenium hysterophorus in South, Central and North America. Aust. Weeds 1981, 1, 8–14. [Google Scholar]
- Masum, S.M.; Hasanuzzaman, M.; Ali, M.H. Threats of Parthenium hysterophorus on agro-ecosystems and its management: A review. Int. J. Agric. Crop Sci. 2013, 6, 684–697. [Google Scholar]
- Bhowmik, P.C.; Sarkar, D.; Yaduraju, N.T. The Status of Parthenium Hysterophorus and its Potential Management. Ecoprint Int. J. Ecol. 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Boyd, N.; Acker, R.V. Seed germination of common weed species as affected by oxygen concentration, light, and osmotic potential. Weed Sci. 2004, 52, 589–596. [Google Scholar] [CrossRef]
- Sharma, M.P.; Born, W.V. The biology of Canadian weeds, 27: Avena fatua L. Can. J. Plant Sci. 1978, 58, 141–157. [Google Scholar] [CrossRef]
- Weaver, S.E.; Cavers, P.B. Dynamics of Seed Populations of Rumex crispus and Rumex obtusifolius (Polygonaceae) in Disturbed and Undisturbed Soil. J. Appl. Ecol. 1979, 16, 909. [Google Scholar] [CrossRef]
- Bello, I.A.; Hatterman-Valenti, H.; Owen, M.D.K. Factors affecting germination and seed production of Erichloa villosa. Weed Sci. 2000, 48, 749–754. [Google Scholar] [CrossRef]
- Watanabe, H.; Kusagaya, Y.; Saigusa, M. Environmental factors affecting germination of apple of Peru. Weed Sci. 2002, 50, 152–156. [Google Scholar] [CrossRef]
- Teuton, T.C.; Brecke, B.J.; Unruh, J.B.; Macdonald, G.E.; Miller, G.L.; Ducar, J.T. Factors affecting seed germination of tropical signalgrass (Urochloa subquadripara). Weed Sci. 2004, 52, 376–381. [Google Scholar] [CrossRef]
- Thomas, W.E.; Wicut, J.W. Influence of environmental factors on slender amaranth (Amaranthus viridis) germination. Weed Sci. 2006, 54, 316–320. [Google Scholar] [CrossRef]
- Susko, D.J.; Hussein, Y. Factors Affecting Germination and Emergence of Dame′s Rocket (Hesperis matronalis). Weed Sci. 2008, 56, 389–393. [Google Scholar] [CrossRef]
- Rana, N.; Wilder, B.J.; Sellers, B.A.; Ferrell, J.A.; Macdonald, G.E. Effects of Environmental Factors on Seed Germination and Emergence of Smutgrass (Sporobolus indicus) Varieties. Weed Sci. 2012, 60, 558–563. [Google Scholar] [CrossRef]
- Sadeghloo, A.; Asghari, J.; Ghaderi-Far, F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and Barnyardgrass (Echinochloa crus-galli). Planta Daninha 2013, 31, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, J.; Xu, H.; Dong, L. Factors Affecting Seed Germination and Seedling Emergence of Asia Minor Bluegrass (Polypogon fugax). Weed Sci. 2015, 63, 440–447. [Google Scholar] [CrossRef]
- Sohrabi, S.; Ghanbari, A.; Mohassel, M.; Gherekhloo, J.; Vidal, R. Effects of environmental factors on Cucumis melo L. subsp. agrestis var. agrestis (Naudin) Pangalo seed germination and seedling emergence. S. Afr. J. Bot. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Marañón, T. Effects of waterlogging on seed germination of three Mediterranean oak species: Ecological implications. Acta Oecol. 2009, 35, 422–428. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Flood. 2017. Available online: http://www.ga.gov.au/scientific-topics/hazards/flood (accessed on 1 December 2017).
- Van Eck, W.H.J.M.; Lenssen, J.P.M.; Van De Steeg, H.M.; Blom, C.W.P.M.; De Kroon, H. Seasonal Dependent Effects of Flooding on Plant Species Survival and Zonation: A Comparative Study of 10 Terrestrial Grassland Species. Hydrobiologia 2006, 565, 59–69. [Google Scholar] [CrossRef]
- Keddy, P.; Reznicek, A. Great Lakes Vegetation Dynamics: The Role of Fluctuating Water Levels and Buried Seeds. J. Great Lakes Res. 1986, 12, 25–36. [Google Scholar] [CrossRef]
- Warwick, N.; Brock, M.A. Plant reproduction in temporary wetlands: The effects of seasonal timing, depth, and duration of flooding. Aquat. Bot. 2003, 77, 153–167. [Google Scholar] [CrossRef]
- Barney, J.N.; Mann, J.J.; Kyser, G.B.; Blumwald, E.; Van Deynze, A.; DiTomaso, J.M. Tolerance of switchgrass to extreme soil moisture stress: Ecological implications. Plant Sci. 2009, 177, 724–732. [Google Scholar] [CrossRef]
- Porter, C.L. An analysis of variance between upland and lowland switchgrass, Panicum virgatum L., in central Oklahoma. Ecology 1966, 47, 980–992. [Google Scholar] [CrossRef]
- Bleoussi, R.T.M.; Yaou, I.; Fofana, M.; Bassole, N.H.I.; Mensah, G.A.; Kabore, N.; Tchekessi, C.K.C. Effect of different soil moisture levels at reproductive stage on rice grain quality. J. Agric. Sci. Food Technol. 2016, 2, 55–63. [Google Scholar]
- Elkheir, H.A.; Yunus, M.; Muslimin, M. Duration of soil water content between field capacity and wilting point and its effect on growth of some aerobic rice cultivars (Oryza sativa L.). Int. J. Agric. Syst. 2015, 4, 36–45. [Google Scholar]
- Bavec, F.; Mlakar, S. Effects of soil and climatic conditions on emergence of grain amaranths. Eur. J. Agron. 2002, 17, 93–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).