Influence of Temperature and Screw Pressing on the Quality of Cassava Leaf Fractions
Abstract
:1. Introduction
2. Materials and Methods
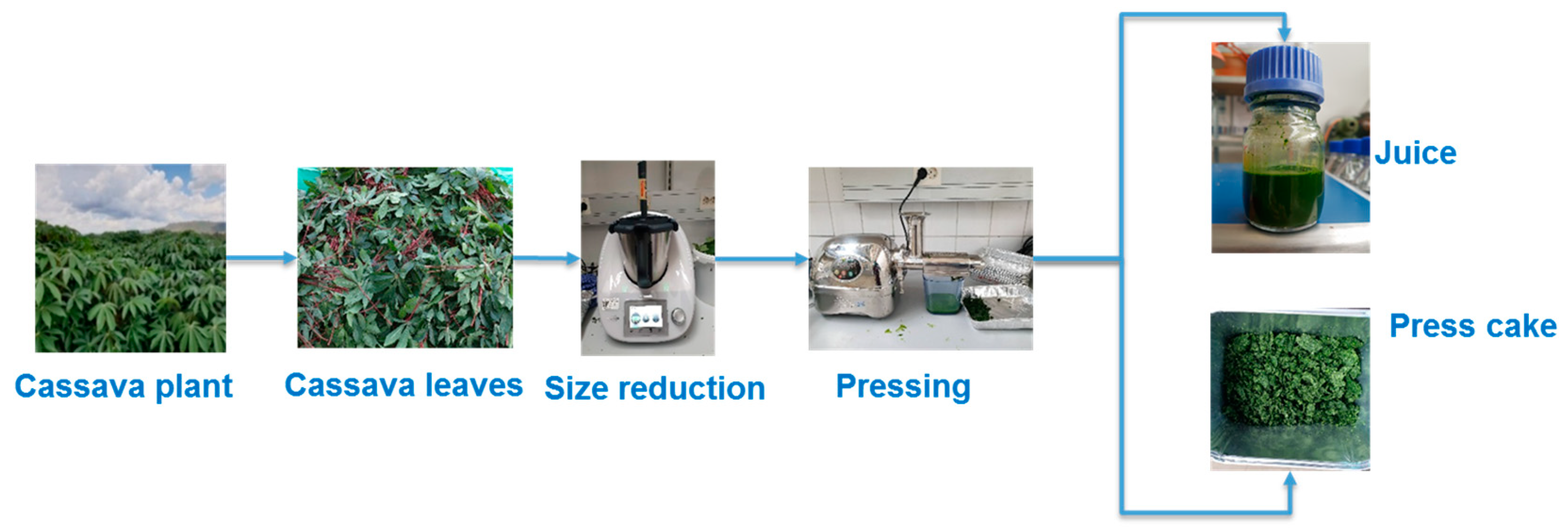
2.1. Plant Material
2.2. Treatments
2.3. Sample Analysis
2.3.1. Antinutritional Factors
2.3.2. Micronutrients
2.3.3. Moisture, Ash, and Crude Protein Content
2.3.4. Acid Detergent Fiber, Acid Detergent Lignin, and Neutral Detergent Fiber
2.3.5. Protein Fractioning
2.4. Statistical Analysis
3. Results and Discussion
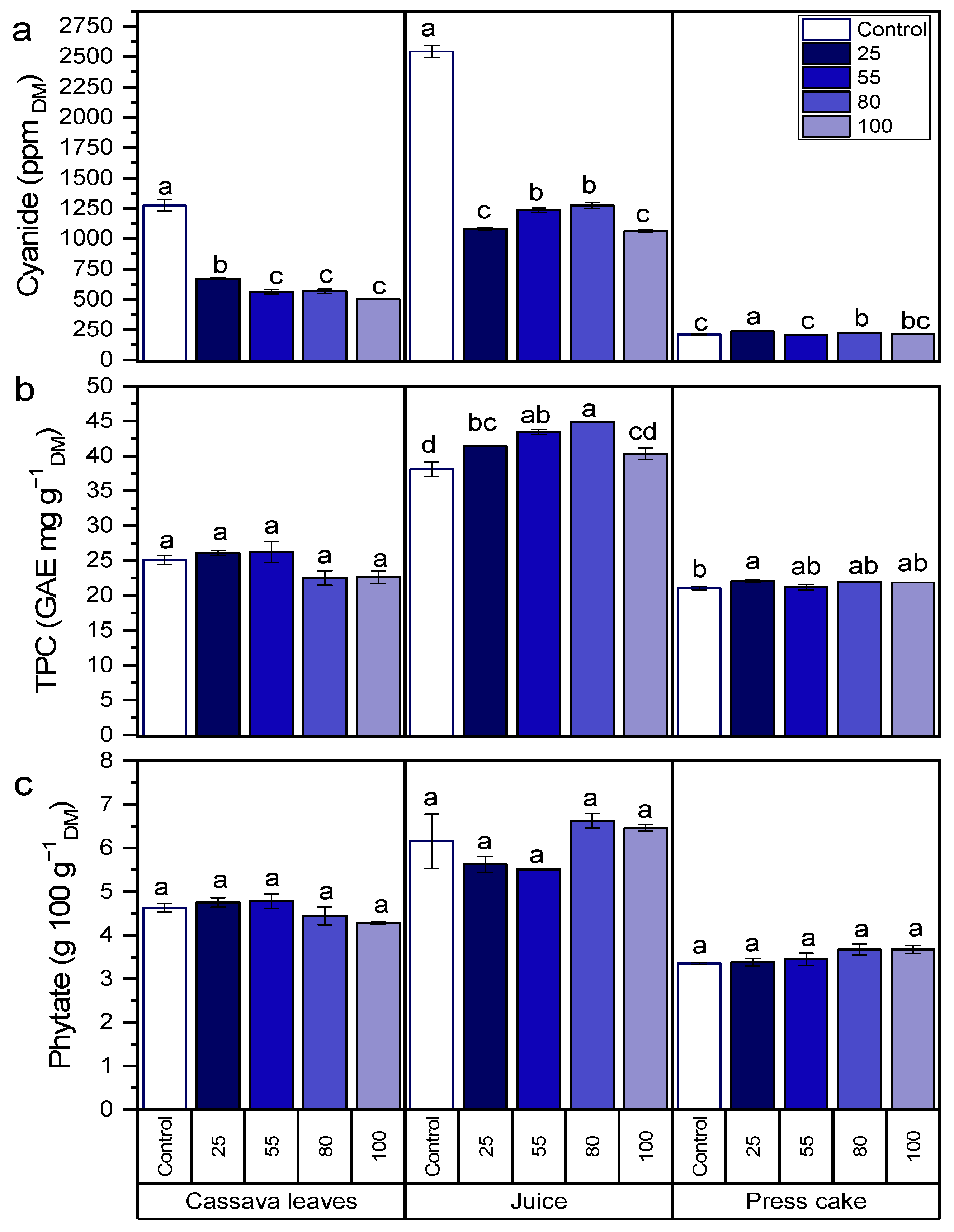
3.1. Antinutrients
3.2. Micronutrients (Vitamin C, Beta-Carotene, Lutein, Chlorophyll a, and Chlorophyll b)
3.3. Macronutrient (Ash, Crude Protein, Acid Detergent Fiber, Acid Detergent Lignin, and Neutral Detergent Fiber)
3.4. Protein Fractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Z.; Zhang, Y.; Xiao, Y.; Liu, F.; Wang, M.; Zhu, X.; Liu, P.; Sun, Q.; Wang, W.; Peng, M.; et al. Transcriptome response of cassava leaves under natural shade. Sci. Rep. 2016, 6, 31673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, A.; Fikre, A.; Sturm, B.; Hensel, O. Post-harvest management and associated food losses and by-products of cassava in southern Ethiopia. Food Secur. 2018, 10, 419–435. [Google Scholar] [CrossRef]
- Daba, M. Nutritional and Hydrogen Cyanide Compositions and Consumers Preference in Cassava Varieties Grown in East Hararghe Zone of Oromia, Ethiopia. Int. J. Sci. Technol. Res. 2019, 8, 6. [Google Scholar]
- Latif, S.; Müller, J. Potential of cassava leaves in human nutrition: A review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Jamila, S.S.; Bujangb, A. Nutrient and antinutrient composition of different variety of cassava (Manihot esculenta Crantz) leaves. J. Teknol. 2016, 78, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Chaiareekitwat, S.; Latif, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Amawan, S.; Müller, J. Protein composition, chlorophyll, carotenoids, and cyanide content of cassava leaves (Manihot esculenta Crantz) as influenced by cultivar, plant age, and leaf position. Food Chem. 2022, 372, 131173. [Google Scholar] [CrossRef]
- Ogbuji, C.A.; David-Chukwu, N.P. Phytochemical, Antinutrient and Mineral Compositions of Leaf Extracts of Some Cassava Varieties. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 4. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Denton, I.C. Mild method for removal of cyanogens from cassava leaves with retention of vitamins and protein. Food Chem. 2014, 158, 417–420. [Google Scholar] [CrossRef]
- Popoola, J.O.; Egwari, L.O.; Bilewu, Y.; Omonigbehin, E.; Ogunlana, O.O.; Daramola, F. Proximate analysis and SDS-PAGE protein profiling of cassava leaves: Utilization as leafy vegetable in Nigeria. MOJ Ecol. Environ. Sci. 2019, 4, 5. [Google Scholar] [CrossRef]
- Latif, S.; Zimmermann, S.; Barati, Z. Detoxification of Cassava Leaves by Thermal, Sodium Bicarbonate, Enzymatic, and Ultrasonic Treatments. J. Food Sci. 2019, 84, 1986–1991. [Google Scholar] [CrossRef]
- Poonsri, T.; Jafarzadeh, S.; Ariffin, F.; Abidin, S.Z.; Barati, Z.; Latif, S.; Müller, J. Improving nutrition, physicochemical and antioxidant properties of rice noodles with fiber and protein-rich fractions derived from cassava leaves. J. Food Nutr. Res. 2019, 7, 325–332. [Google Scholar] [CrossRef]
- Hawashi, M.; Aparamarta, H.; Widjaja, T.; Gunawan, S. Optimization of Solid State Fermentation Conditions for Cyanide Content Reduction in Cassava Leaves using Response Surface Methodology. Int. J. Technol. 2019, 10, 291–319. [Google Scholar] [CrossRef] [Green Version]
- Leguizamón, A.J.; Rompato, K.M.; Hoyos, R.E.; Audisio, M.C. Nutritional evaluation of three varieties of cassava leaves (Manihot esculenta Crantz) grown in Formosa, Argentina. J. Food Compos. Anal. 2021, 101, 103986. [Google Scholar] [CrossRef]
- Diasolua Ngudi, D.; Kuo, Y.-H.; Lambein, F. Amino acid profiles and protein quality of cooked cassava leaves or ‘saka-saka’. J. Sci. Food Agric. 2003, 83, 529–534. [Google Scholar] [CrossRef]
- Hidayat, A.; Zuraida, N.; Hanarida, I. The Cyanogenic Potential of Roots and Leaves of Ninety Nine Cassava Cultivars. Indones. J. Agric. Sci. 2002, 3, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Colas, D.; Doumeng, C.; Pontalier, P.Y.; Rigal, L. Green crop fractionation by twin-screw extrusion: Influence of the screw profile on alfalfa (Medicago sativa) dehydration and protein extraction. Chem. Eng. Process. Process Intensif. 2013, 72, 1–9. [Google Scholar] [CrossRef]
- Ayele, H.H.; Latif, S.; Bruins, M.E.; Müller, J. Partitioning of Proteins and Anti-Nutrients in Cassava (Manihot esculenta Crantz) Leaf Processing Fractions after Mechanical Extraction and Ultrafiltration. Foods 2021, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Tamayo Tenorio, A.; Gieteling, J.; de Jong, G.A.H.; Boom, R.M.; van der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilization. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Romuli, S.; Barati, Z.; Müller, J. CFD assisted investigation of mechanical juice extraction from cassava leaves and characterization of the products. Food Sci. Nutr. 2020, 8, 3089–3098. [Google Scholar] [CrossRef] [Green Version]
- Hawashi, M.; Sitania, C.; Caesy, C.; Aparamarta, H.W.; Widjaja, T.; Gunawan, S. Kinetic data of extraction of cyanide during the soaking process of cassava leaves. Data Brief. 2019, 25, 104279. [Google Scholar] [CrossRef]
- Ravindran, V. Cassava leaves as animal feed: Potential and limitations. J. Sci. Food Agric. 1993, 61, 141–150. [Google Scholar] [CrossRef]
- Rizki, I.L.N.; Yulianto, M.E.; Hartati, I.; Paramita, V.; Abidin, Z.; Nisa’, Q.A.y.K.; Waspada, I. Optimization of simultaneous enzymatic inactivation and extraction of linamarin from cassava leaf by UV-assisted photobioextraction. AIP Conf. Proc. 2018, 2026, 020016. [Google Scholar] [CrossRef]
- EL, G.; TPR, S.; DS, F.; MM, M. Cassava derivatives in the preparation of unconventional gluten-free snacks. Int. Food Res. J. 2019, 26, 801–809. [Google Scholar]
- Bradbury, M.G.; Egan, S.V.; Bradbury, J.H. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. J. Sci. Food Agric. 1999, 79, 593–601. [Google Scholar] [CrossRef]
- Egan, S.V.; Yeoh, H.H.; Bradbury, J.H. Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. J. Sci. Food Agric. 1998, 76, 39–48. [Google Scholar] [CrossRef]
- Yeoh, H.-H.; Bradbury, J.H.; Egan, S.V. A simple and rapid method for isolating cassava leaf linamarase suitable for cassava cyanide determination. J. Sci. Food Agric. 1997, 75, 258–262. [Google Scholar] [CrossRef]
- Latta, M.; Eskin, M. A simple and rapid colorimetric method for phytate determination. J. Agric. Food Chem. 1980, 28, 1313–1315. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.-S.; Kang, I.-K.; Choung, M.-G. Metabolic association of lipophilic pigments in the organs of soybean sprouts. Food Sci. Biotechnol. 2015, 24, 859–865. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Ravindran, V.; Kornegay, E.; Rajaguru, A. Influence of processing methods and storage time on the cyanide potential of cassava leaf meal. Anim. Feed Sci. Technol. 1987, 17, 227–234. [Google Scholar] [CrossRef]
- Joint, F.; WHO Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Padmaja, G. Cyanide detoxification in cassava for food and feed uses. Crit. Rev. Food Sci. Nutr. 1995, 35, 299–339. [Google Scholar] [CrossRef]
- Castellanos, R.; Altamirano, S.; Moretti, R. Nutritional characteristics of cassava (Manihot esculenta Crantz) leaf protein concentrates obtained by ultrafiltration and acidic thermocoagulation. Plant Foods Hum. Nutr. 1994, 45, 357–363. [Google Scholar] [CrossRef]
- Raja, K.S.; Taip, F.S.; Azmi, M.M.Z.; Shishir, M.R.I. Effect of pre-treatment and different drying methods on the physicochemical properties of Carica papaya L. leaf powder. J. Saudi Soc. Agric. Sci. 2019, 18, 150–156. [Google Scholar] [CrossRef]
- Ismanto, S.D. The Effect of Drying Temperature to Chemical Components of Surian Herbal Tea Leaves (Toona sureni, (Blume) Merr.). Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 229–238. [Google Scholar]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Processing Techniques to Reduce Toxicity and Antinutrients of Cassava for Use as a Staple Food. Compr. Rev. Food Sci. Food Saf. 2009, 8, 17–27. [Google Scholar] [CrossRef]
- Santos, M.A.I.; Fraguas, R.M.; Braga, M.A.; Marques, T.R.; Duarte, M.H.; dos Santos, C.M.; Freire, J.M.; Correcirc, A.D. Antioxidants and chlorophyll in cassava leaves at three plant ages. Afr. J. Agric. Res. 2013, 8, 3724–3730. [Google Scholar]
- Karri, V.R.; Nalluri, N. Cassava: Meeting the global protein need. Plant Sci. Today 2016, 3, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Mou, B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Lin, X.-M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Achir, N.; Randrianatoandro, V.A.; Bohuon, P.; Laffargue, A.; Avallone, S. Kinetic study of β-carotene and lutein degradation in oils during heat treatment. Eur. J. Lipid Sci. Technol. 2010, 112, 349–361. [Google Scholar] [CrossRef]
- Sánchez, C.; Baranda, A.B.; Martínez de Marañón, I. The effect of High Pressure and High Temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef]
- Oresegun, A.; Fagbenro, O.A.; Ilona, P.; Bernard, E.; Yildiz, F. Nutritional and anti-nutritional composition of cassava leaf protein concentrate from six cassava varieties for use in aqua feed. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Salvador, E.; Steenkamp, V.; McCrindle, C.M.E. Production, consumption and nutritional value of cassava (Manihot esculenta, Crantz) in Mozambique: An overview. J. Agric. Biotechnol. Sustain. Dev. 2014, 6, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Ujong, A.; Baridia, D. Effect of Processing on the Chemical and Anti-Nutritional Properties of Cassava Leaves (Sweet and Bitter Varieties). ARC J. Nutr. Growth 2020, 6, 6–12. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Lübeck, M. Production of leaf protein concentrates in green biorefineries as alternative feed for monogastric animals. Anim. Feed Sci. Technol. 2020, 268, 114605. [Google Scholar] [CrossRef]
- Tenorio, A.T. Sugar Beet Leaves for Functional Ingredients. Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2017. [Google Scholar]
- Achidi, A.U.; Ajayi, O.A.; Maziya-Dixon, B.; Bokanga, M. The Effect of Processing on the Nutrient Content of Cassava (Manihot esculenta Crantz) Leaves. J. Food Process. Preserv. 2008, 32, 486–502. [Google Scholar] [CrossRef]
- Lyimo, M.; Nyagwegwe, S.; Mnkeni, A. Investigations on the effect of traditional food processing, preservation and storage methods on vegetable nutrients: A case study in Tanzania. Plant Foods Hum. Nutr. 1991, 41, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Paengkoum, P.; Thongpea, S.; Paengkoum, S. Utilization of concentrate supplements containing varying levels of cassava leaf pellet by growing goats fed a basal diet of pangola hay. Indian J. Anim. Res. 2017, 51, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Jayanegara, A.; Dewi, S.; Laylli, N.; Laconi, E.; Nahrowi, N.; Ridla, M. Determination of cell wall protein from selected feedstuffs and its relationship with ruminal protein digestibility in vitro. Media Peternak. 2016, 39, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Schwab, C.G.; Tylutki, T.; Ordway, R.; Sheaffer, C.; Stern, M.D. Characterization of proteins in feeds. J. Dairy Sci. 2003, 86, E88–E103. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Haque, M.; Hossain, S. Non-protein nitrogen (NPN) test protocol for raw materials of feed. Ijppr. Hum. 2016, 6, 129–140. [Google Scholar]
- Stanton, T.L. Urea and NPN for Cattle and Sheep. Ph.D. Dissertation, Colorado State University. Libraries, Fort Collins, CO, USA, 1981. [Google Scholar]
- Das, L.K.; Kundu, S.S.; Kumar, D.; Datt, C. Fractionation of carbohydrate and protein content of some forage feeds of ruminants for nutritive evaluation. Vet. World 2015, 8, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Tham, H.T.; Man, N.V.; Preston, T.R. Estimates of protein fractions of various heat-treated feeds in ruminant production. Livest. Res. Rural Dev. 2008, 20. Available online: http://www.lrrd.org/lrrd20/supplement/tham2.htm (accessed on 25 March 2021).
- Hakl, J.; Fuksa, P.; Konečná, J.; Šantrůček, J. Differences in the crude protein fractions of lucerne leaves and stems under different stand structures. Grass Forage Sci. 2016, 71, 413–423. [Google Scholar] [CrossRef]

| Fraction | Temperature (°C) | DM | Ash | CP | ADF | ADL | NDF |
|---|---|---|---|---|---|---|---|
| (%) | (g 100 g−1DM) | (g 100 g−1DM) | (g 100 g−1DM) | (g 100 g−1DM) | (g 100 g−1DM) | ||
| Leaves | Control | 29.1 ± 0.1 a | 9.7 ± 0.1 b | 31.0 ± 0.5 a | 23.8 ± 0.4 a | 7.5 ± 0.1 a | 25.7 ± 0.2 a |
| 25 | 27.7 ± 0.2 b | 9.6 ± 0.1 b | 30.5 ± 0.4 a b | 22.6 ± 0.9 a | 7.0 ± 0.1 a | 23.0 ± 0.5 a | |
| 55 | 27.9 ± 0.1 b | 9.9 ± 0.0 b | 29.9 ± 0.3 a b c | 21.8 ± 1.0 a | 7.0 ± 0.1 a | 23.7 ± 0.5 a | |
| 80 | 28.3 ± 0.4 a b | 10.4 ± 0.2 a | 29.4 ± 0.3 b c | 24.3 ± 0.3 a | 7.6 ± 0.3 a | 25.1 ± 0.6 a | |
| 100 | 28.4 ± 0.4 a b | 10.4 ± 0.0 a | 28.9 ± 0.2 c | 22.4 ± 0.6 a | 7.6 ± 0.1 a | 25.2 ± 1.3 a | |
| Juice | Control | 13.6 ± 0.0 a | 12.4 ± 0.1 b | 27.7 ± 0.2 b | 3.2 ± 0.2 a b | 1.1 ± 0.0 a | 3.8 ± 0.3 c |
| 25 | 13.1 ± 0.3 a | 12.4 ± 0.3 b | 29.6 ± 0.7 a | 3.1 ± 0.1 a b | 1.1 ± 0.0 a | 5.2 ± 0.9 b c | |
| 55 | 12.9 ± 0.1 a | 12.7 ± 0.3 a b | 26.6 ± 0.5 b c | 2.9 ± 0.1 b | 0.9 ± 0.0 b | 8.6 ± 1.9 a b | |
| 80 | 13.1 ± 0.3 a | 13.1 ± 0.0 a b | 25.8 ± 1.1 c | 3.1 ± 0.0 a b | 1.1 ± 0.0 a | 11.5 ± 0.6 a | |
| 100 | 13.6 ± 0.1 a | 13.3 ± 0.0 a | 27.8 ± 0.0 b | 3.5 ± 0.1 a | 1.1 ± 0.0 a | 12.2 ± 0.0 a | |
| Press cake | Control | 52.5 ± 0.1 b | 8.6 ± 0.3 a | 31.0 ± 0.4 a | 24.9 ± 0.4 a b | 10.4 ± 0.6 a | 28.7 ± 0.3 a b |
| 25 | 54.8 ± 0.2 a | 8.5 ± 0.0 a | 31.2 ± 0.0 a | 26.6 ± 0.2 a | 9.0 ± 0.1 a | 31.9 ± 1.0 a | |
| 55 | 56.1 ± 0.8 a | 8.6 ± 0.1 a | 31.2 ± 0.2 a | 25.1 ± 1.0 a b | 9.5 ± 0.0 a | 29.2 ± 1.1 a b | |
| 80 | 55.1 ± 0.3 a | 9.1 ± 0.1 a | 31.0 ± 0.1 a | 23.9 ± 0.1 b | 9.7 ± 0.3 a | 29.4 ± 0.9 a b | |
| 100 | 54.5 ± 0.6 a | 9.1 ± 0.1 a | 29.5 ± 0.1 b | 25.1 ± 0.6 a b | 9.4 ± 0.4 a | 28.2 ± 0.5 b |
| Fraction | CP (g 100 g−1DM) | SP (g 100 g−1DM) | NPN (g 100 g−1DM) | NDICP (g 100 g−1DM) | ADICP (g 100 g−1DM) | A (g 100 g−1 CP) | B1 (g 100 g−1 CP) | B2 (g 100 g−1 CP) | B3 (g 100 g−1 CP) | C (g 100 g−1 CP) |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | 31.0 ± 0.5 a | 8.9 ± 0.3 a | 7.4 ± 0.1 a | 2.1 ± 0.0 a | 1.3 ± 0.0 a | 24.0 ± 0.2 a | 4.7 ± 0.8 a | 64.6 ± 0.6 b | 2.5 ± 0.2 b | 0.7 ± 0.0 a |
| Juice | 27.7 ± 0.2 b | 9.9 ± 0.5 a | 8.3 ± 0.6 a | 0.3 ± 0.0 b | 0.1 ± 0.0 b | 30.0 ± 2.5 a | 5.8 ± 0.5 a | 63.3 ± 2.4 b | 0.6 ± 0.0 c | 0.1 ± 0.0 b |
| Press cake | 31.0 ± 0.4 a | 2.3 ± 0.1 b | 1.5 ± 0.1 b | 2.6 ± 0.3 a | 1.3 ± 0.1 a | 5.0 ± 0.4 b | 2.4 ± 0.1 b | 84.2 ± 1.3 a | 4.4 ± 0.6 a | 0.7 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayele, H.H.; Latif, S.; Müller, J. Influence of Temperature and Screw Pressing on the Quality of Cassava Leaf Fractions. Agriculture 2022, 12, 42. https://doi.org/10.3390/agriculture12010042
Ayele HH, Latif S, Müller J. Influence of Temperature and Screw Pressing on the Quality of Cassava Leaf Fractions. Agriculture. 2022; 12(1):42. https://doi.org/10.3390/agriculture12010042
Chicago/Turabian StyleAyele, Haimanot Hailegiorgis, Sajid Latif, and Joachim Müller. 2022. "Influence of Temperature and Screw Pressing on the Quality of Cassava Leaf Fractions" Agriculture 12, no. 1: 42. https://doi.org/10.3390/agriculture12010042







