The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance
Abstract
:1. Introduction
2. P450-Mediated Insecticide Resistance
2.1. Cross Resistance
2.2. Evolutionary Adaptability
3. Molecular Mechanisms of P450-Mediated Insecticide Resistance
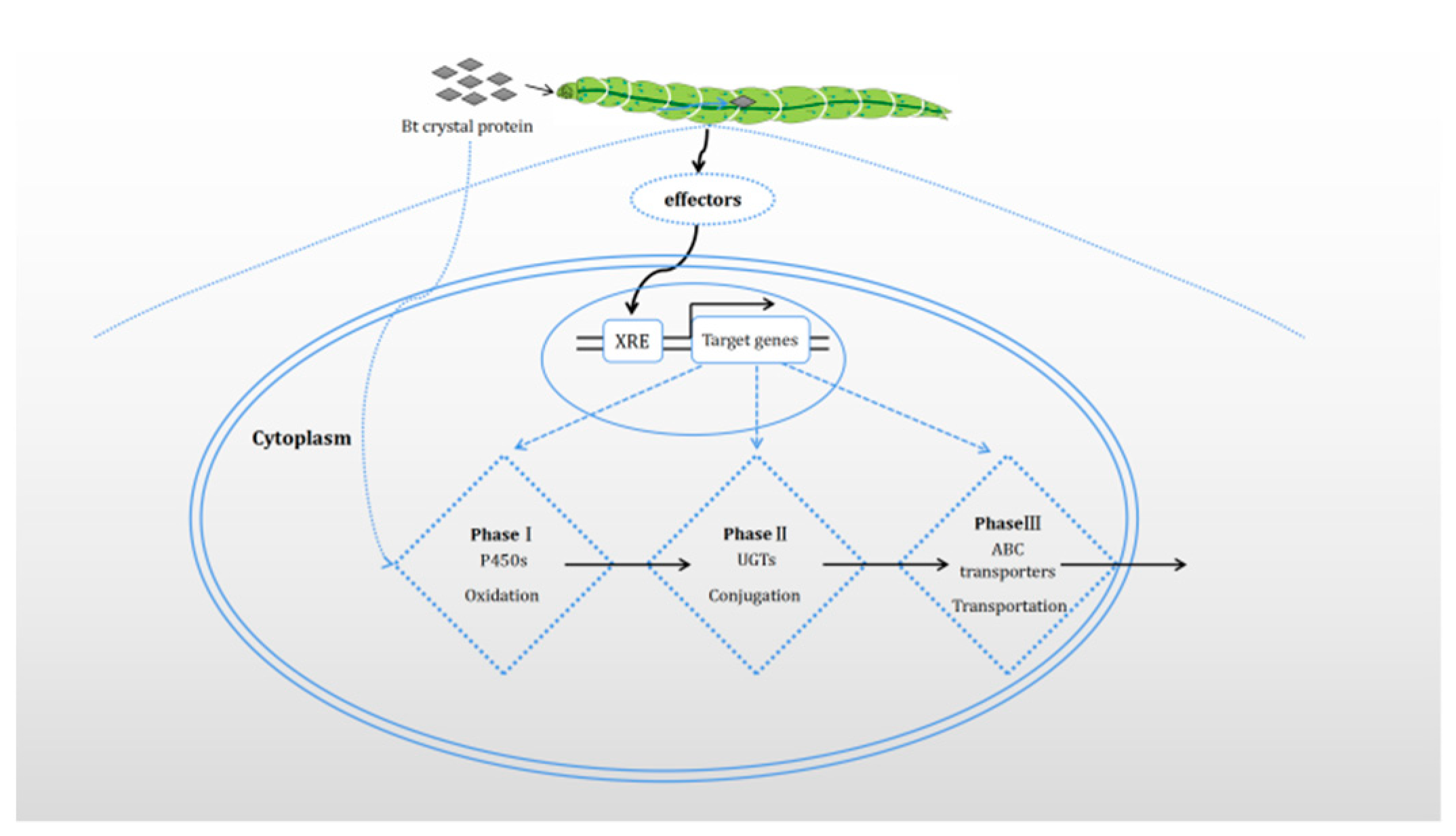
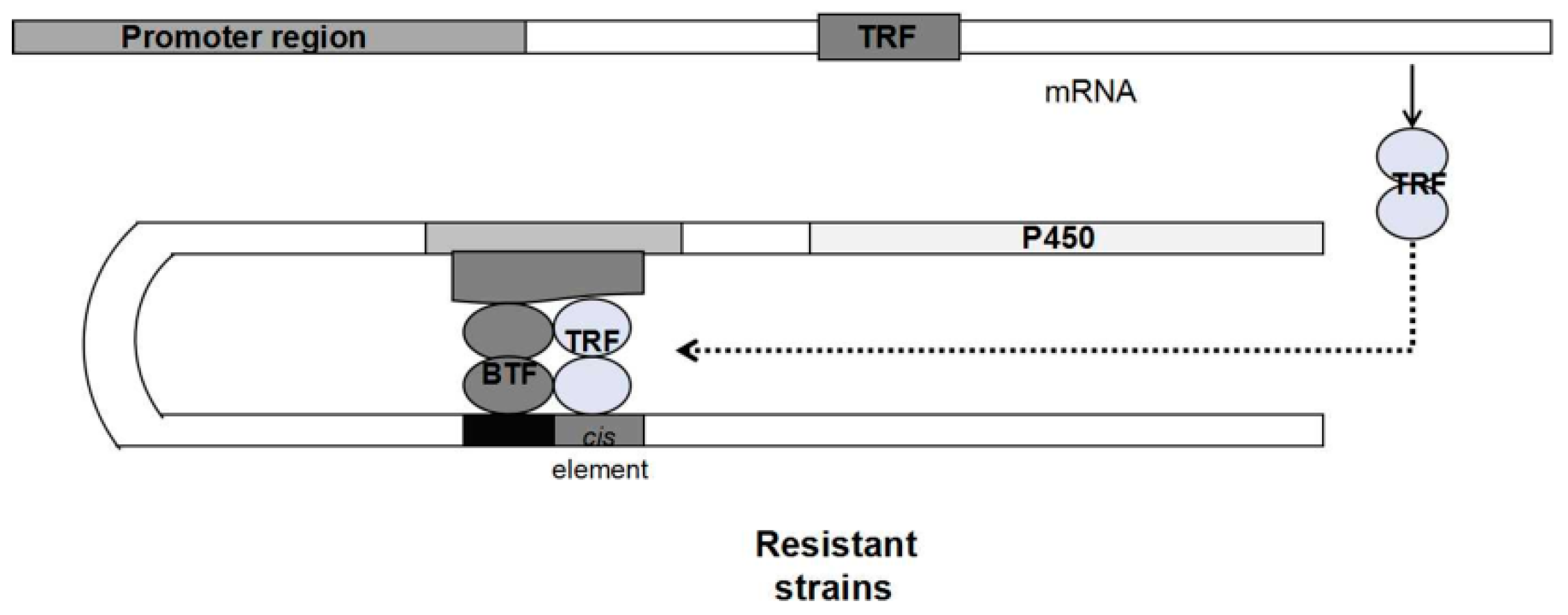
3.1. Upregulation of Enzyme Expression
3.2. Changes of Enzyme Functions
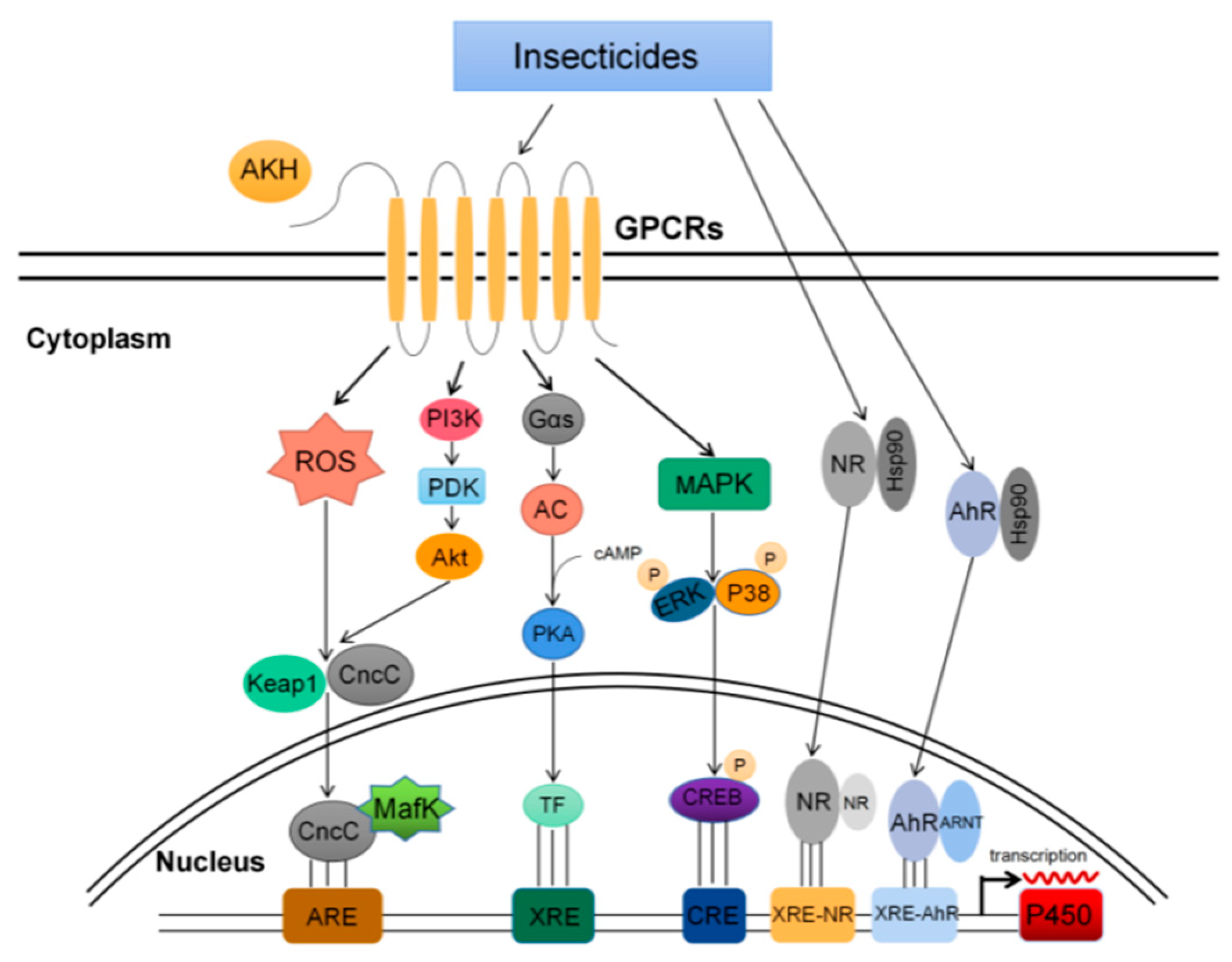
4. Effector Molecules and Signal Pathways of P450s Expression Regulation
4.1. GPCRs Pathway
4.2. MAPK Pathway
4.3. PI3K Pathway
4.4. CncC Pathway
4.5. Nuclear Receptors
5. Management of P450-Mediated Insecticide Resistance
6. Summary and Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 3003. [Google Scholar] [CrossRef] [Green Version]
- Feyereisen, R. Insect P450 Enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Iga, M.; Kataoka, H. Recent studies on insect hormone metabolic pathways mediated by cytochrome P450 enzymes. Biol. Pharm. Bull. 2012, 35, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Tang, W.; He, W.; Ma, X.; Vasseur, L.; Baxter, S.; Yang, G.; Huang, S.; Song, F.; You, M. Characterization and expression of the cytochrome P450 gene family in diamondback moth, Plutella xylostella (L.). Sci. Rep. 2015, 5, 8952. [Google Scholar] [CrossRef] [PubMed]
- Willingham, A.; Keil, T. A tissue specific cytochrome P450 required for the structure and function of Drosophila sensory organs. Mech. Dev. 2004, 121, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bassett, M.; McCarthy, J.; Waterman, M.; Sliter, T. Sequence and developmental expression of Cyp18, a member of a new cytochrome P450 family from Drosophila. Mol. Cell. Endocrinol. 1997, 131, 39–49. [Google Scholar] [CrossRef]
- Rewitz, K.; O’Connor, M.; Gilbert, L. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect. Biochem. Mol. Biol. 2007, 37, 741–753. [Google Scholar] [CrossRef]
- Kondrashov, F. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. Biol. Sci. 2012, 279, 5048–5057. [Google Scholar] [CrossRef] [Green Version]
- Simpson, A. The cytochrome P450 4 (CYP4) family. Gen. Pharmacol. 1997, 28, 351–359. [Google Scholar] [CrossRef]
- Jeschke, P. Propesticides and their use as agrochemicals. Pest Manag. Sci. 2016, 72, 210–225. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, C.; Li, M.; Sheng, C.; Liu, H.; Qiu, X. CYP9A12 and CYP9A17 in the cotton bollworm, Helicoverpa armigera: Sequence similarity, expression profile and xenobiotic response. Pest Manag. Sci. 2010, 66, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.; Lees, R.; Yunta, C.; Walker, K.; Hemmings, K.; Oladepo, F.; Hancock, P.; Weetman, D.; Paine, M.; Ismail, H. Assessing cross-resistance within the pyrethroids in terms of their interactions with key cytochrome P450 enzymes and resistance in vector populations. Parasites Vectors 2021, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- de Little, S.; Edwards, O.; van Rooyen, A.; Weeks, A.; Umina, P. Discovery of metabolic resistance to neonicotinoids in green peach aphids (Myzus persicae) in Australia. Pest Manag. Sci. 2017, 73, 1611–1617. [Google Scholar] [CrossRef]
- Fonseca-González, I.; Quiñones, M.; Lenhart, A.; Brogdon, W. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag. Sci. 2011, 67, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Wagah, M.; Korlević, P.; Clarkson, C.; Miles, A.; Lawniczak, M.; Makunin, A. Genetic variation at the Cyp6m2 putative insecticide resistance locus in Anopheles gambiae and Anopheles coluzzii. Malar. J. 2021, 20, 234. [Google Scholar] [CrossRef]
- Scott, J.G.; Wen, Z. Cytochromes P450 of insects: The tip of the iceberg. Pest Manag. Ence 2001, 57, 958–967. [Google Scholar] [CrossRef]
- Matowo, J.; Jones, C.M.; Kabula, B.; Ranson, H.; Weetman, D. Genetic basis of pyrethroid resistance in a population of Anopheles arabiensis, the primary malaria vector in Lower Moshi, north-eastern Tanzania. Parasites Vectors 2014, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Rahman, R.U.; Souza, B.; Uddin, I.; Carrara, L.; Brito, L.P.; Costa, M.M.; Mahmood, M.A.; Khan, S.; Lima, J.B.P.; Martins, A.J. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021, 11, 4555. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, A.; Xiao, W.; Zou, B.; Jiang, Y.; Xiao, J.; Deng, C.; Zhang, Y. Cytochrome P450 monooxygenase genes in the wild silkworm, Bombyx mandarina. PeerJ 2021, 9, e10818. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Wang, X.G.; Yin, Y.; Shen, L.T. Mechanisms for multiple resistances in field populations of rice stem borer, Chilo suppressalis (Lepidoptera: Crambidae) from Sichuan Province, China. Pestic. Biochem. Physiol. 2020, 171, 104720. [Google Scholar] [CrossRef]
- Liao, X.; Xu, P.F.; Gong, P.P.; Wan, H.; Li, J.H. The current susceptibilities of brown planthopper Nilaparvata lugens to triflumezopyrim and other frequently used insecticides in China. Insect Sci. 2020, 28, 115–126. [Google Scholar] [CrossRef]
- Xiao, Q.; Deng, L.; Elzaki, M.E.A.; Zhu, L.; Xu, Y.; Han, X.; Wang, C.; Han, Z.; Wu, M. The inducible CYP4C71 can metabolize imidacloprid in Laodelphax striatellus (Hemiptera: Delphacidae). J. Econ. Entomol. 2020, 113, 399–406. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Feng, X. Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bull. Entomol. Res. 2014, 104, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, J.; Zhang, J.; Che, W.; Luo, C. Characterization of flupyradifurone resistance in the whitefly Bemisia tabaci Mediterranean (Q biotype). Pest Manag. Sci. 2020, 76, 4286–4292. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Su, X.; Zhen, C.A.; Lu, L.Y.; Li, Y.S.; Ge, X.; Chen, D.M.; Pei, Z.; Shi, M.W.; Chen, X.L. Silencing of cytochrome P450 in Spodoptera frugiperda (Lepidoptera: Noctuidae) by RNA interference enhances susceptibility to chlorantraniliprole. J. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef]
- Kim, J.H.; Moreau, J.A.; Zina, J.M.; Mazgaeen, L.; Yoon, K.S.; Pittendrigh, B.R.; Clark, J.M. Identification and interaction of multiple genes resulting in DDT resistance in the 91-R strain of Drosophila melanogaster by RNAi approaches. Pestic. Biochem. Physiol. 2018, 151, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Amvongo-Adjia, N.; Wondji, M.J.; Irving, H.; Riveron, J.M.; Wondji, C.S. Pyrethroid resistance in the major malaria vector Anopheles funestus is exacerbated by overexpression and overactivity of the P450 CYP6AA1 across Africa. Genes 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.S.; Ndula, M.; Riveron, J.M.; Irving, H.; Wondji, C.S. The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 2016, 25, 3436–3452. [Google Scholar] [CrossRef] [PubMed]
- Adolfi, A.; Poulton, B.; Anthousi, A.; Macilwee, S.; Ranson, H.; Lycett, G.J. Functional genetic validation of key genes conferring insecticide resistance in the major African malaria vector, Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2019, 116, 25764–25772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edi, C.V.; Djogbénou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Kétoh, G.; Paine, M. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.N.; Stevenson, B.J.; Mueller, P.; Wilding, C.S.; Egyir-Yawson, A.; Field, S.G.; Hemingway, J.; Paine, M.; Ranson, H.; Donnelly, M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA 2012, 109, 6147–6152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, B.J.; Bibby, J.; Pignatelli, P.; Muangnoicharoen, S.; O’Neill, P.; Lian, L.Y.; Müller, P.; Nikou, D.; Steven, A.; Hemingway, J. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011, 41, 492–502. [Google Scholar] [CrossRef]
- Yunta, C.; Hemmings, K.; Stevenson, B.; Koekemoer, L.L.; Paine, M. Cross-resistance profiles of malaria mosquito P450s associated with pyrethroid resistance against WHO insecticides. Pestic. Biochem. Physiol. 2019, 161, 61–67. [Google Scholar] [CrossRef]
- Lees, R.S.; Ismail, H.M.; Logan, R.; Malone, D.; Paine, M. New insecticide screening platforms indicate that Mitochondrial Complex I inhibitors are susceptible to cross-resistance by mosquito P450s that metabolise pyrethroids. Sci. Rep. 2020, 10, 16232. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Grigoraki, L.; Morgan, J.; Tsakireli, D.; Fuseini, G.; Segura, L.; Julie, N.; Nguema, R.; Weetman, D.; Slotman, M.A. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 2018, 115, 4619–4624. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, X.; Xiang, X.; Xu, X.; Guo, Y.; Liu, Y.; Yin, Y.; Wu, Y.; Cheng, Q.; Gong, C.; et al. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Hemiptera:Delphacidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2021, 171, 104723. [Google Scholar] [CrossRef]
- Sun, X.; Gong, Y.; Shahbaz, A.; Hou, M. Mechanisms of resistance to thiamethoxam and dinotefuran compared to imidacloprid in the brown planthopper: Roles of cytochrome P450 monooxygenase and a P450 gene CYP6ER1. Pestic. Biochem. Physiol. 2018, 150, 17–26. [Google Scholar] [CrossRef]
- Jin, R.; Mao, K.; Liao, X.; Xu, P.; Li, Z.; Ali, E.; Wan, H.; Li, J. Overexpression of CYP6ER1 associated with clothianidin resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 154, 39–45. [Google Scholar] [CrossRef]
- Liao, X.; Jin, R.; Zhang, X.; Ali, E.; Mao, K.; Xu, P.; Li, J.; Wan, H. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens (Stål). Pest Manag. Sci. 2019, 75, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Gorman, K.; Slater, R.; Blande, J.; Clarke, A.; Wren, J.; McCaffery, A.; Denholm, I. Cross-resistance relationships between neonicotinoids and pymetrozine in Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Wölfel, K.; Lueke, B.; Myridakis, A.; Tsakireli, D.; Roditakis, E.; Tsagkarakou, A.; Stephanou, E.; Vontas, J. Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad spectrum catalytic efficiency. Pestic. Biochem. Physiol. 2015, 121, 3–11. [Google Scholar] [CrossRef]
- Kliot, A.; Kontsedalov, S.; Ramsey, J.; Jander, G.; Ghanim, M. Adaptation to nicotine in the facultative tobacco-feeding hemipteran Bemisia tabaci. Pest Manag. Sci. 2014, 70, 1595–1603. [Google Scholar] [CrossRef]
- Zhou, C.; Cao, Q.; Li, G.; Ma, D. Role of several cytochrome P450s in the resistance and cross-resistance against imidacloprid and acetamiprid of Bemisia tabaci (Hemiptera: Aleyrodidae) MEAM1 cryptic species in Xinjiang, China. Pestic. Biochem. Physiol. 2020, 163, 209–215. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Ning, Y.; Qiao, K.; Wang, K. Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015, 108, 1978–1985. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Li, D.; Wan, H.; You, H.; Li, J. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017, 140, 85–89. [Google Scholar] [CrossRef]
- Tabashnik, B.; Unnithan, G.; Masson, L.; Crowder, D.; Li, X.; Carrière, Y. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Natl. Acad. Sci. USA 2009, 106, 11889–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Cao, G.; Song, J.; Yin, Q.; Han, Z. Biochemical mechanisms conferring cross-resistance between tebufenozide and abamectin in Plutella xylostella. Pestic. Biochem. Physiol. 2008, 91, 175–179. [Google Scholar] [CrossRef]
- Yin, Q.; Qian, L.; Song, P.; Jian, T.; Han, Z. Molecular mechanisms conferring asymmetrical cross-resistance between tebufenozide and abamectin in Plutella xylostella. J. Asia-Pac. Entomol. 2019, 22, 189–193. [Google Scholar] [CrossRef]
- Mulamba, C.; Irving, H.; Riveron, J.; Mukwaya, L.; Birungi, J.; Wondji, C. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: A potential challenge for malaria vector control in Uganda. Parasites Vectors 2014, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Riveron, J.; Ibrahim, S.; Mulamba, C.; Djouaka, R.; Irving, H.; Wondji, M.; Ishak, I.; Wondji, C. Genome-Wide transcription and functional analyses reveal heterogeneous molecular mechanisms driving pyrethroids resistance in the major malaria vector Anopheles funestus across Africa. G3 Genes 2017, 7, 1819–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weedall, G.; Mugenzi, L.; Menze, B.; Tchouakui, M.; Ibrahim, S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.; Tchoupo, M.; Djouaka, R.; et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, 7386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; Sears, C.; Sun, H.; Mertz, R.; Kasai, S.; Scott, J. CYP-mediated resistance and cross-resistance to pyrethroids and organophosphates in Aedes aegypti in the presence and absence of kdr. Pestic. Biochem. Physiol. 2019, 160, 119–126. [Google Scholar] [CrossRef]
- Moyes, C.; Vontas, J.; Martins, A.; Ng, L.; Koou, S.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Reid, W.; Thornton, A.; Pridgeon, J.; Becnel, J.; Tang, F.; Estep, A.; Clark, G.; Allan, S.; Liu, N. Transcriptional analysis of four family 4 P450s in a Puerto Rico strain of Aedes aegypti (Diptera: Culicidae) compared with an Orlando strain and their possible functional roles in permethrin resistance. J. Med. Entomol. 2014, 51, 605–615. [Google Scholar] [CrossRef]
- Stevenson, B.J.; Patricia, P.; Dimitra, N.; Paine, M.; Kent, C. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: Developing new tools to combat insecticide resistance. PLoS Negl. Trop. Dis. 2012, 6, e1595. [Google Scholar] [CrossRef] [Green Version]
- Festucci-Buselli, R.; Carvalho-Dias, A.; de Oliveira-Andrade, M.; Caixeta-Nunes, C.; Li, H.; Stuart, J.; Muir, W.; Scharf, M.; Pittendrigh, B. Expression of Cyp6g1 and Cyp12d1 in DDT resistant and susceptible strains of Drosophila melanogaster. Insect Mol. Biol. 2005, 14, 69–77. [Google Scholar] [CrossRef]
- Gellatly, K.; Yoon, K.; Doherty, J.; Sun, W.; Pittendrigh, B.; Clark, J. RNAi validation of resistance genes and their interactions in the highly DDT-resistant 91-R strain of Drosophila melanogaster. Pestic. Biochem. Physiol. 2015, 121, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Sun, W.; McDonnell, C.; Li-Byarlay, H.; Steele, L.; Wu, J.; Xie, J.; Muir, W.; Pittendrigh, B. Genome-wide analysis of genes associated with moderate and high DDT resistance in Drosophila melanogaster. Pest Manag. Sci. 2013, 69, 930–937. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, N.; Zhang, J.; Zhang, Y.; Liu, Z. Induction of P450 genes in Nilaparvata lugens and Sogatella furcifera by two neonicotinoid insecticides. Insect Sci. 2018, 25, 401–408. [Google Scholar] [CrossRef]
- Bao, H.; Gao, H.; Zhang, Y.; Fan, D.; Fang, J.; Liu, Z. The roles of CYP6AY1 and CYP6ER1 in imidacloprid resistance in the brown planthopper: Expression levels and detoxification efficiency. Pestic. Biochem. Physiol. 2016, 129, 70–74. [Google Scholar] [CrossRef]
- Elzaki, M.; Miah, M.; Peng, Y.; Zhang, H.; Jiang, L.; Wu, M.; Han, Z. Deltamethrin is metabolized by CYP6FU1, a cytochrome P450 associated with pyrethroid resistance, in Laodelphax striatellus. Pest Manag. Sci. 2018, 74, 1265–1271. [Google Scholar] [CrossRef]
- Miah, M.; Elzaki, M.; Husna, A.; Han, Z. An overexpressed cytochrome P450 CYP439A1v3 confers deltamethrin resistance in Laodelphax striatellus Fallén (Hemiptera: Delphacidae). Arch. Insect Biochem. Physiol. 2019, 100, e21525. [Google Scholar] [CrossRef]
- Brun-Barale, A.; Héma, O.; Martin, T.; Suraporn, S.; Audant, P.; Sezutsu, H.; Feyereisen, R. Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera. Pest Manag. Sci. 2010, 66, 900–909. [Google Scholar] [CrossRef]
- Tao, X.; Xue, X.; Huang, Y.; Chen, X.; Mao, Y. Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 2012, 21, 4371–4385. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, D.; Qin, J.; Zhao, W.; Qiu, L. Over-expression of multiple cytochrome P450 genes in fenvalerate-resistant field strains of Helicoverpa armigera from north of China. Pestic. Biochem. Physiol. 2016, 132, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Wu, S.; Yue, L.; Wu, Y. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera. J. Econ. Entomol. 2006, 99, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, H.; Liu, Z.; Wu, S.; Yang, Y.; Feyereisen, R.; Heckel, D.; Wu, Y. Phylogenetic and functional characterization of ten P450 genes from the CYP6AE subfamily of Helicoverpa armigera involved in xenobiotic metabolism. Insect Biochem. Mol. Biol. 2018, 93, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Harrop, T.; Sztal, T.; Lumb, C.; Good, R.; Daborn, P.; Batterham, P.; Chung, H. Evolutionary changes in gene expression, coding sequence and copy-number at the Cyp6g1 locus contribute to resistance to multiple insecticides in Drosophila. PLoS ONE 2014, 9, e84879. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Liu, N. Differential expression of CYP6A5 and CYP6A5v2 in pyrethroid-resistant house flies, Musca domestica. Arch. Insect Biochem. Physiol. 2008, 67, 107–119. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.; Miles, A.; Harding, N.; Clarkson, C.; Lawniczak, M.; Kwiatkowski, D.; Weetman, D.; Donnelly, M. Whole-genome sequencing reveals high complexity of copy number variation at insecticide resistance loci in malaria mosquitoes. Genome Res. 2019, 29, 1250–1261. [Google Scholar] [CrossRef] [Green Version]
- Weetman, D.; Wilding, C.; Neafsey, D.; Müller, P.; Ochomo, E.; Isaacs, A.; Steen, K.; Rippon, E.; Morgan, J.; Mawejje, H.; et al. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae. Sci. Rep. 2018, 8, 2920. [Google Scholar] [CrossRef] [PubMed]
- Faucon, F.; Dusfour, I.; Gaude, T.; Navratil, V.; Boyer, F.; Chandre, F.; Sirisopa, P.; Thanispong, K.; Juntarajumnong, W.; Poupardin, R.; et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 2015, 25, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.; Corbel, V.; Sutherland, I.; Hertz, J.; et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef] [Green Version]
- Bariami, V.; Jones, C.; Poupardin, R.; Vontas, J.; Ranson, H. Gene amplification, ABC transporters and cytochrome P450s: Unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1692. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Bogwitz, M.; McCart, C.; Andrianopoulos, A.; Ffrench-Constant, R.; Batterham, P.; Daborn, P. Cis-regulatory elements in the Accord retrotransposon result in tissue-specific expression of the Drosophila melanogaster insecticide resistance gene Cyp6g1. Genetics 2007, 175, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Garud, N.; Messer, P.; Buzbas, E.; Petrov, D. Recent selective sweeps in North American Drosophila melanogaster show signatures of soft sweeps. PLoS Genet. 2015, 11, e1005004. [Google Scholar] [CrossRef] [Green Version]
- McCart, C.; Ffrench-Constant, R. Dissecting the insecticide-resistance- associated cytochrome P450 gene Cyp6g1. Pest Manag. Sci. 2008, 64, 639–645. [Google Scholar] [CrossRef]
- Morra, R.; Kuruganti, S.; Lam, V.; Lucchesi, J.; Ganguly, R. Functional analysis of the cis-acting elements responsible for the induction of the Cyp6a8 and Cyp6g1 genes of Drosophila melanogaster by DDT, phenobarbital and caffeine. Insect Mol. Biol. 2010, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.; Lam, G.; Thummel, C. Constitutive activation of the Nrf2/Keap1 pathway in insecticide-resistant strains of Drosophila. Insect Biochem. Mol. Biol. 2013, 43, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Liu, Y.; Li, M.; Zhu, S.; Li, X.; Pittendrigh, B.; Qiu, X. Nrf2/Maf-binding-site-containing functional Cyp6a2 allele is associated with DDT resistance in Drosophila melanogaster. Pest Manag. Sci. 2014, 70, 1048–1058. [Google Scholar] [CrossRef]
- Pang, R.; Li, Y.; Dong, Y.; Liang, Z.; Zhang, Y.; Zhang, W. Identification of promoter polymorphisms in the cytochrome P450 CYP6AY1 linked with insecticide resistance in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2014, 23, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Pang, R.; Dong, Y.; Sun, Z.; Ling, Y.; Zhang, W. Identification of SNPs involved in regulating a novel alternative transcript of P450 CYP6ER1 in the brown planthopper. Insect Sci. 2018, 25, 726–738. [Google Scholar] [CrossRef]
- Zimmer, C.; Garrood, W.; Singh, K.; Randall, E.; Lueke, B.; Gutbrod, O.; Matthiesen, S.; Kohler, M.; Nauen, R.; Davies, T.; et al. Neofunctionalization of duplicated P450 genes drives the evolution of insecticide resistance in the Brown Planthopper. Curr. Biol. CB 2018, 28, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, J.; Sun, H.; Wang, J.; Wu, M.; Wang, K.; Denholm, I.; Han, Z. Multiple cis-acting elements involved in up-regulation of a cytochrome P450 gene conferring resistance to deltamethrin in smal brown planthopper, Laodelphax striatellus (Fallén). Insect Biochem. Mol. Biol. 2016, 78, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wong, A.; Zhang, Y.; Ni, X.; Li, X. Common and unique cis-acting elements mediate xanthotoxin and flavone induction of the generalist P450 CYP321A1. Sci. Rep. 2014, 4, 6490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Li, D.; Luo, Y.; Qin, J.; Qiu, L. Identification of the 2-tridecanone cis-acting element in the promoter of cytochrome P450 CYP6B7 in Helicoverpa armigera. Insect Sci. 2018, 25, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, M.; Zhang, Y.; Shen, G.; Di, H.; Wang, Y.; He, L. The expression of P450 genes mediating fenpropathrin resistance is regulated by CncC and Maf in Tetranychus cinnabarinus (Boisduval). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2017, 198, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Palli, S. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; Tyagi, R.; Kasai, S.; Scott, J. CYP-mediated permethrin resistance in Aedes aegypti and evidence for trans-regulation. PLoS Negl. Trop. Dis. 2018, 12, e0006933. [Google Scholar] [CrossRef]
- Lin, G.; Scott, J. Investigations of the constitutive overexpression of CYP6D1 in the permethrin resistantLPR strain of house fly (Musca domestica). Pestic. Biochem. Physiol. 2011, 100, 130–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Shan, C.; Li, F.; Liang, P.; Smagghe, G.; Gao, X. Transcription factor FTZ-F1 and cis-acting elements mediate expression of CYP6BG1 conferring resistance to chlorantraniliprole in Plutella xylostella. Pest Manag. Sci. 2019, 75, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s--Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Yang, T.; Liu, N. Genome analysis of cytochrome P450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e29418. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Yao, J.; Li, D.; He, Y.; Zhu, Y.; Zhang, X.; Zhu, K. Cytochrome P450 genes from the aquatic midge Chironomus tentans: Atrazine-induced up-regulation of CtCYP6EX3 enhanced the toxicity of chlorpyrifos. Chemosphere 2017, 186, 68–77. [Google Scholar] [CrossRef]
- Walsh, T.; Joussen, N.; Tian, K.; McGaughran, A.; Anderson, C.; Qiu, X.; Ahn, S.; Bird, L.; Pavlidi, N.; Vontas, J.; et al. Multiple recombination events between two cytochrome P450 loci contribute to global pyrethroid resistance in Helicoverpa armigera. PLoS ONE 2018, 13, e0197760. [Google Scholar] [CrossRef] [Green Version]
- Joußen, N.; Agnolet, S.; Lorenz, S.; Schöne, S.; Ellinger, R.; Schneider, B.; Heckel, D. Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc. Natl. Acad. Sci. USA 2012, 109, 15206–15211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, A.; Joußen, N.; Lorenz, S.; Ellinger, R.; Schneider, B.; Khan, S.; Ashfaq, M.; Heckel, D. An independent occurrence of the chimeric P450 enzyme CYP337B3 of Helicoverpa armigera confers cypermethrin resistance in Pakistan. Insect Biochem. Mol. Biol. 2014, 53, 54–65. [Google Scholar] [CrossRef]
- Durigan, M.; Corrêa, A.; Pereira, R.; Leite, N.; Amado, D.; de Sousa, D.; Omoto, C. High frequency of CYP337B3 gene associated with control failures of Helicoverpa armigera with pyrethroid insecticides in Brazil. Pestic. Biochem. Physiol. 2017, 143, 73–80. [Google Scholar] [CrossRef]
- Han, Y.; Yu, W.; Zhang, W.; Yang, Y.; Walsh, T.; Oakeshott, J.; Wu, Y. Variation in P450-mediated fenvalerate resistance levels is not correlated with CYP337B3 genotype in Chinese populations of Helicoverpa armigera. Pestic. Biochem. Physiol. 2015, 121, 129–135. [Google Scholar] [CrossRef]
- Good, R.; Gramzow, L.; Battlay, P.; Sztal, T.; Batterham, P.; Robin, C. The molecular evolution of cytochrome P450 genes within and between drosophila species. Genome Biol. Evol. 2014, 6, 1118–1134. [Google Scholar] [CrossRef] [Green Version]
- Seong, K.; Coates, B.; Berenbaum, M.; Clark, J.; Pittendrigh, B. Comparative CYP-omic analysis between the DDT-susceptible and -resistant Drosophila melanogaster strains 91-C and 91-R. Pest Manag. Sci. 2018, 74, 2530–2543. [Google Scholar] [CrossRef] [Green Version]
- Amichot, M.; Tarès, S.; Brun-Barale, A.; Arthaud, L.; Bride, J.; Bergé, J. Point mutations associated with insecticide resistance in the Drosophila cytochrome P450 Cyp6a2 enable DDT metabolism. Eur. J. Biochem. 2004, 271, 1250–1257. [Google Scholar] [CrossRef]
- Ibrahim, S.; Riveron, J.; Bibby, J.; Irving, H.; Yunta, C.; Paine, M.; Wondji, C. Allelic variation of cytochrome P450s drives resistance to bednet insecticides in a major malaria vector. PLoS Genet. 2015, 11, e1005618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, H.; Riveron, J.; Ibrahim, S.; Lobo, N.; Wondji, C. Positional cloning of rp2 QTL associates the P450 genes CYP6Z1, CYP6Z3 and CYP6M7 with pyrethroid resistance in the malaria vector Anopheles funestus. Heredity 2012, 109, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, K.; Nakamura, Y.; Jouraku, A.; Akiduki, G.; Uchibori-Asano, M.; Kuwazaki, S.; Suetsugu, Y.; Daimon, T.; Yamamoto, K.; Noda, H.; et al. Genome-wide assessment and development of molecular diagnostic methods for imidacloprid-resistance in the brown planthopper, Nilaparvata lugens (Hemiptera; Delphacidae). Pest Manag. Sci. 2021, 77, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- van Noort, V.; Seebacher, J.; Bader, S.; Mohammed, S.; Vonkova, I.; Betts, M.; Kühner, S.; Kumar, R.; Maier, T.; O’Flaherty, M.; et al. Cross-talk between phosphorylation and lysine acetylation in a genome-reduced bacterium. Mol. Syst. Biol. 2012, 8, 571. [Google Scholar] [CrossRef]
- Rossignoli, G.; Phillips, R.; Astegno, A.; Menegazzi, M.; Voltattorni, C.; Bertoldi, M. Phosphorylation of pyridoxal 5’-phosphate enzymes: An intriguing and neglected topic. Amino Acids 2018, 50, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lapied, B.; Pennetier, C.; Apaire-Marchais, V.; Licznar, P.; Corbel, V. Innovative applications for insect viruses: Towards insecticide sensitization. Trends Biotechnol. 2009, 27, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliarini, D.; Dixon, J. Mitochondrial modulation: Reversible phosphorylation takes center stage? Trends Biochem. Sci. 2006, 31, 26–34. [Google Scholar] [CrossRef]
- Gai, L.; Zhu, Y.; Zhang, C.; Meng, X. Targeting canonical and non-canonical STAT signaling pathways in renal diseases. Cells 2021, 10, 1610. [Google Scholar] [CrossRef]
- Jhun, B.; Mishra, J.; Monaco, S.; Fu, D.; Jiang, W.; Sheu, S.; O-Uchi, J. The mitochondrial Ca2+ uniporter: Regulation by auxiliary subunits and signal transduction pathways. Am. J. Physiol. Cell Physiol. 2016, 311, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Morley, S.; Coldwell, M.; Clemens, M. Initiation factor modifications in the preapoptotic phase. Cell Death Differ. 2005, 12, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Vitrac, H.; MacLean, D.; Karlstaedt, A.; Taegtmeyer, H.; Jayaraman, V.; Bogdanov, M.; Dowhan, W. Dynamic lipid-dependent modulation of protein topology by post-translational phosphorylation. J. Biol. Chem. 2017, 292, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Thany, S.; Lenaers, G.; Raymond-Delpech, V.; Sattelle, D.; Lapied, B. Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2007, 28, 14–22. [Google Scholar] [CrossRef]
- Xu, T.; Yuchi, Z. Crystal structure of diamondback moth ryanodine receptor Repeat34 domain reveals insect-specific phosphorylation sites. BMC Biol. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Li, F.; Fang, Y.; Wang, H.; Chen, J.; Li, M.; Lu, Z.; Qu, J.; Li, J.; Hu, J.; et al. Effects of chlorantraniliprole exposure on detoxification enzyme activities and detoxification-related gene expression in the fat body of the silkworm, Bombyx mori. Ecotoxicol. Environ. Saf. 2019, 176, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, S.; Wu, Q.; Wang, S.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; Zhang, Y. The regulation landscape of MAPK signaling cascade for thwarting Bacillus thuringiensis infection in an insect host. PLoS Pathog. 2021, 17, e1009917. [Google Scholar] [CrossRef]
- Audsley, N.; Down, R. G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Sharan, S.; Watts, V. Genomics, GPCRs and new targets for the control of insect pests and vectors. Curr. Opin. Insect Sci. 2018, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Nowling, R.; Abrudan, J.; Shoue, D.; Abdul-Wahid, B.; Wadsworth, M.; Stayback, G.; Collins, F.; McDowell, M.; Izaguirre, J. Identification of novel arthropod vector G protein-coupled receptors. Parasites Vectors 2013, 6, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hiel, M.; Van Loy, T.; Poels, J.; Vandersmissen, H.; Verlinden, H.; Badisco, L.; Vanden Broeck, J. Neuropeptide receptors as possible targets for development of insect pest control agents. Adv. Exp. Med. Biol. 2010, 692, 211–226. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; You, C.; Zeng, X.; Gao, X. The role of G protein-coupled receptor-related genes in cytochrome P450-mediated resistance of the house fly, Musca domestica (Diptera: Muscidae), to imidacloprid. Insect Mol. Biol. 2020, 29, 92–103. [Google Scholar] [CrossRef]
- Cao, C.; Sun, L.; Du, H.; Moural, T.; Bai, H.; Liu, P.; Zhu, F. Physiological functions of a methuselah-like G protein coupled receptor in Lymantria dispar Linnaeus. Pestic. Biochem. Physiol. 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Sun, L.; Liu, P.; Zhang, C.; Du, H.; Wang, Z.; Moural, T.; Zhu, F.; Cao, C. Ocular albinism type 1 regulates deltamethrin tolerance in Lymantria dispar and Drosophila melanogaster. Front. Physiol. 2019, 10, 766. [Google Scholar] [CrossRef]
- Hu, X.; Yan, S.; Wang, W.; Yang, M.; Sun, L.; Tan, W.; Sun, J.; Qian, J.; Lei, M.; Zhang, D. Cloning and characterization of NYD-OP7, a novel deltamethrin resistance associated gene from Culex pipiens pallens. Pestic. Biochem. Physiol. 2007, 88, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Duan, B.; Xu, Y.; Ma, L.; Shen, B.; Sun, Y.; Zhu, C. NYD-OP7/PLC regulatory signaling pathway regulates deltamethrin resistance in Culex pipiens pallens (Diptera: Culicidae). Parasites Vectors 2018, 11, 419. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culex quinquefasciatus. Sci. Rep. 2014, 4, 6474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Cao, C.; Yang, T.; Zhang, L.; He, L.; Xi, Z.; Bian, G.; Liu, N. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2015, 5, 17772. [Google Scholar] [CrossRef]
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, N. The function of G-protein-coupled receptor-regulatory cascade in southern house mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, N. Role of the G-protein-coupled receptor signaling pathway in insecticide resistance. Int. J. Mol. Sci. 2019, 20, 4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, U.; Kostenko, S. Structure and function of MK5/PRAK: The loner among the mitogen-activated protein kinase-activated protein kinases. Biol. Chem. 2013, 394, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Young, S.; Lin, J.; Lin, P. Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2011, 41, 197–202. [Google Scholar] [CrossRef]
- Misra, J.; Horner, M.; Lam, G.; Thummel, C. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Cheng, Y.; Li, W.; Li, Y.; Zeng, R.; Song, Y. Activation of CncC pathway by ROS burst regulates cytochrome P450 CYP6AB12 responsible for λ-cyhalothrin tolerance in Spodoptera litura. J. Hazard. Mater. 2020, 387, 121698. [Google Scholar] [CrossRef] [PubMed]
- Plavšin, I.; Stašková, T.; Šerý, M.; Smýkal, V.; Hackenberger, B.; Kodrík, D. Hormonal enhancement of insecticide efficacy in Tribolium castaneum: Oxidative stress and metabolic aspects. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2015, 170, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, Y.; Li, Y.; Li, W.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic hormone regulates cytochrome P450-mediated imidacloprid resistance in the brown planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef]
- Cheatle Jarvela, A.; Pick, L. The function and evolution of nuclear receptors in insect embryonic development. Curr. Top. Dev. Biol. 2017, 125, 39–70. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Kozaki, T.; Scott, J. Hormone receptor-like in 96 and Broad-Complex modulate phenobarbital induced transcription of cytochrome P450 CYP6D1 in Drosophila S2 cells. Insect Mol. Biol. 2011, 20, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afschar, S.; Toivonen, J.; Hoffmann, J.; Tain, L.; Wieser, D.; Finlayson, A.; Driege, Y.; Alic, N.; Emran, S.; Stinn, J.; et al. Nuclear hormone receptor DHR96 mediates the resistance to xenobiotics but not the increased lifespan of insulin-mutant Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Li, Y.; Cheng, Y.; Li, W.; Song, Y.; Zeng, R.; Sun, Z. Activation of the NR2E nuclear receptor HR83 leads to metabolic detoxification-mediated chlorpyrifos resistance in Nilaparvata lugens. Pestic. Biochem. Physiol. 2021, 173, 104800. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Li, W.; Song, Y.; Zeng, R.; Lu, K. Inhibition of hepatocyte nuclear factor 4 confers imidacloprid resistance in Nilaparvata lugens via the activation of cytochrome P450 and UDP-glycosyltransferase genes. Chemosphere 2021, 263, 128269. [Google Scholar] [CrossRef]
- Hu, J.; Liu, P.; Hu, L.; Lu, W.; Xu, Z.; He, L. P8 nuclear receptor responds to acaricides exposure and regulates transcription of P450 enzyme in the two-spotted spider mite, Tetranychus urticae. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2019, 224, 108561. [Google Scholar] [CrossRef]
- Tsuji, N.; Fukuda, K.; Nagata, Y.; Okada, H.; Haga, A.; Hatakeyama, S.; Yoshida, S.; Okamoto, T.; Hosaka, M.; Sekine, K.; et al. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio 2014, 4, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Beischlag, T.; Luis Morales, J.; Hollingshead, B.; Perdew, G. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.; McDonnell, C.; Berenbaum, M.; Schuler, M. Regulation of an insect cytochrome P450 monooxygenase gene (CYP6B1) by aryl hydrocarbon and xanthotoxin response cascades. Gene 2005, 358, 39–52. [Google Scholar] [CrossRef]
- McDonnell, C.; Brown, R.; Berenbaum, M.; Schuler, M. Conserved regulatory elements in the promoters of two allelochemical-inducible cytochrome P450 genes differentially regulate transcription. Insect Biochem. Mol. Biol. 2004, 34, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.; Niamsup, H.; Berenbaum, M.; Schuler, M. Transcriptional response elements in the promoter of CYP6B1, an insect P450 gene regulated by plant chemicals. Biochim. Biophys. Acta 2003, 1619, 269–282. [Google Scholar] [CrossRef]
- Peng, T.; Chen, X.; Pan, Y.; Zheng, Z.; Wei, X.; Xi, J.; Zhang, J.; Gao, X.; Shang, Q. Transcription factor aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator is involved in regulation of the xenobiotic tolerance-related cytochrome P450 CYP6DA2 in Aphis gossypii Glover. Insect Mol. Biol. 2017, 26, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, T.; Xu, P.; Zeng, X.; Shang, Q. Transcription factors AhR/ARNT regulate the expression of CYP6CY3 and CYP6CY4 switch conferring nicotine adaptation. Int. J. Mol. Sci. 2019, 20, 4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, D.; Smith, H.; Bennett, J.; Torrance, P.; Huffman, E.; Sparks, A.; Gruver, C.; Dunn, T.; Champagne, D. Regional survey of diamondback moth (Lepidoptera: Plutellidae) response to maximum dosages of insecticides in Georgia and Florida. J. Econ. Entomol. 2020, 113, 2458–2464. [Google Scholar] [CrossRef]
- Attique, M.; Khaliq, A.; Sayyed, A.H. Could resistance to insecticides in Plutella xylostella (Lep., Plutellidae) be overcome by insecticide mixtures? J. Appl. Entomol. 2006, 130, 122–127. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 inhibitors and insecticides: Challenges and opportunities. Pest Manag. Sci. 2015, 71, 793–800. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Gu, L.; Knipple, D.C. Recent advances in RNA interference research in insects: Implications for future insect pest management strategies. Crop Prot. 2013, 45, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, H.; Miao, X. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci. 2013, 20, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kogel, K. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Kumar, P.; Pandit, S.; Baldwin, I. Tobacco rattle virus vector: A rapid and transient means of silencing manduca sexta genes by plant mediated RNA interference. PLoS ONE 2012, 7, e31347. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Xue, X.; Tao, X.; Yang, C.; Wang, L.; Chen, X. Cysteine protease enhances plant-mediated bollworm RNA interference. Plant Mol. Biol. 2013, 83, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, X.; Ma, J.; Zhao, J. Silencing of cytochrome P450 CYP6B6 gene of cotton bollworm (Helicoverpa armigera) by RNAi. Bull. Entomol. Res. 2013, 103, 584–591. [Google Scholar] [CrossRef]
- Scott, J.; Michel, K.; Bartholomay, L.; Siegfried, B.; Hunter, W.; Smagghe, G.; Zhu, K.; Douglas, A. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Kim, Y.; Soumaila Issa, M.; Cooper, A.; Zhu, K. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic. Biochem. Physiol. 2015, 120, 109–117. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Nayak, B.; Xiong, L.; Xie, C.; Dong, Y.; You, M.; Yuchi, Z.; You, S. The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance. Agriculture 2022, 12, 53. https://doi.org/10.3390/agriculture12010053
Ye M, Nayak B, Xiong L, Xie C, Dong Y, You M, Yuchi Z, You S. The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance. Agriculture. 2022; 12(1):53. https://doi.org/10.3390/agriculture12010053
Chicago/Turabian StyleYe, Min, Bidhan Nayak, Lei Xiong, Chao Xie, Yi Dong, Minsheng You, Zhiguang Yuchi, and Shijun You. 2022. "The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance" Agriculture 12, no. 1: 53. https://doi.org/10.3390/agriculture12010053
APA StyleYe, M., Nayak, B., Xiong, L., Xie, C., Dong, Y., You, M., Yuchi, Z., & You, S. (2022). The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance. Agriculture, 12(1), 53. https://doi.org/10.3390/agriculture12010053






