Heat Stress, Varietal Difference, and Soil Amendment Influence on Maize Grain Mineral Concentrations †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Treatments, Experimental Design, and Cultural Practices
2.3. Sample Preparation and Quantification of Minerals Concentrations through Inductively Coupled Plasma Mass Spectrometry (ICP–MS)
2.4. Data Analysis
3. Results
3.1. Analysis of Variance (ANOVA)
3.2. Heat Stress, Soil Amendment, and Varietal Effects on Maize Grain’s Macro- and Trace Minerals
3.3. The Interactive Effect of Heat Stress, Variety, and Soil Amendment on Maize Grain’s Mineral Concentrations
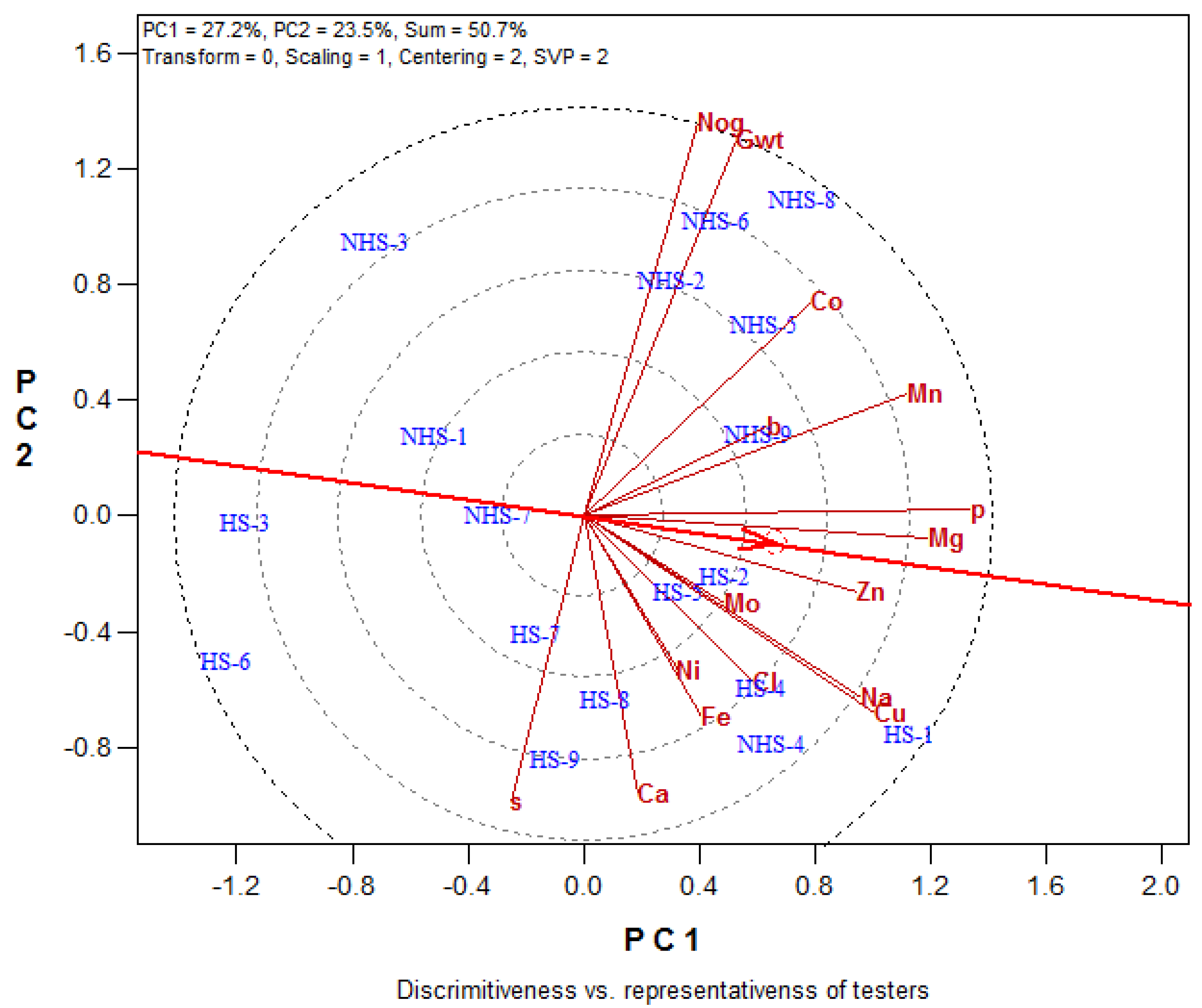
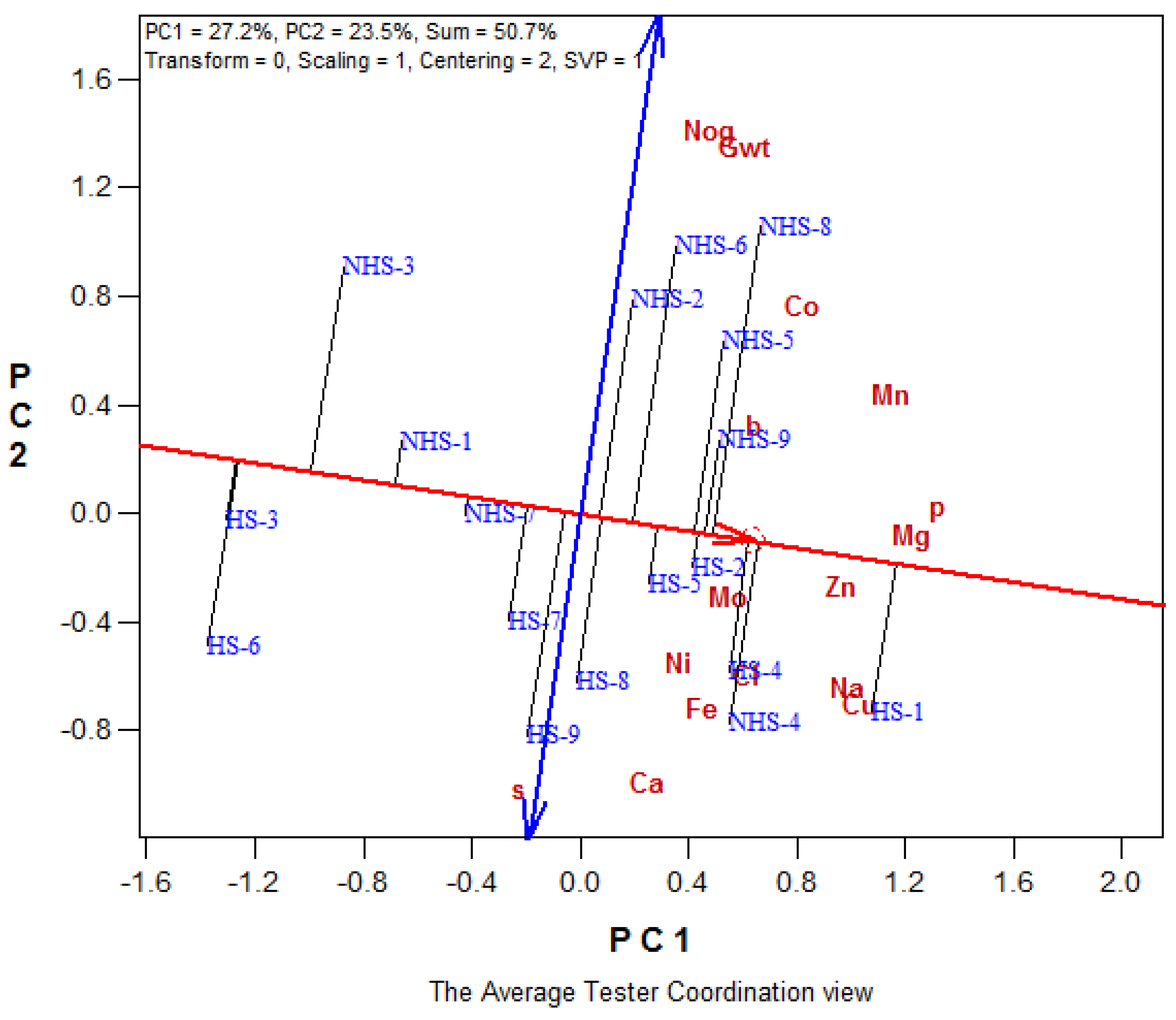
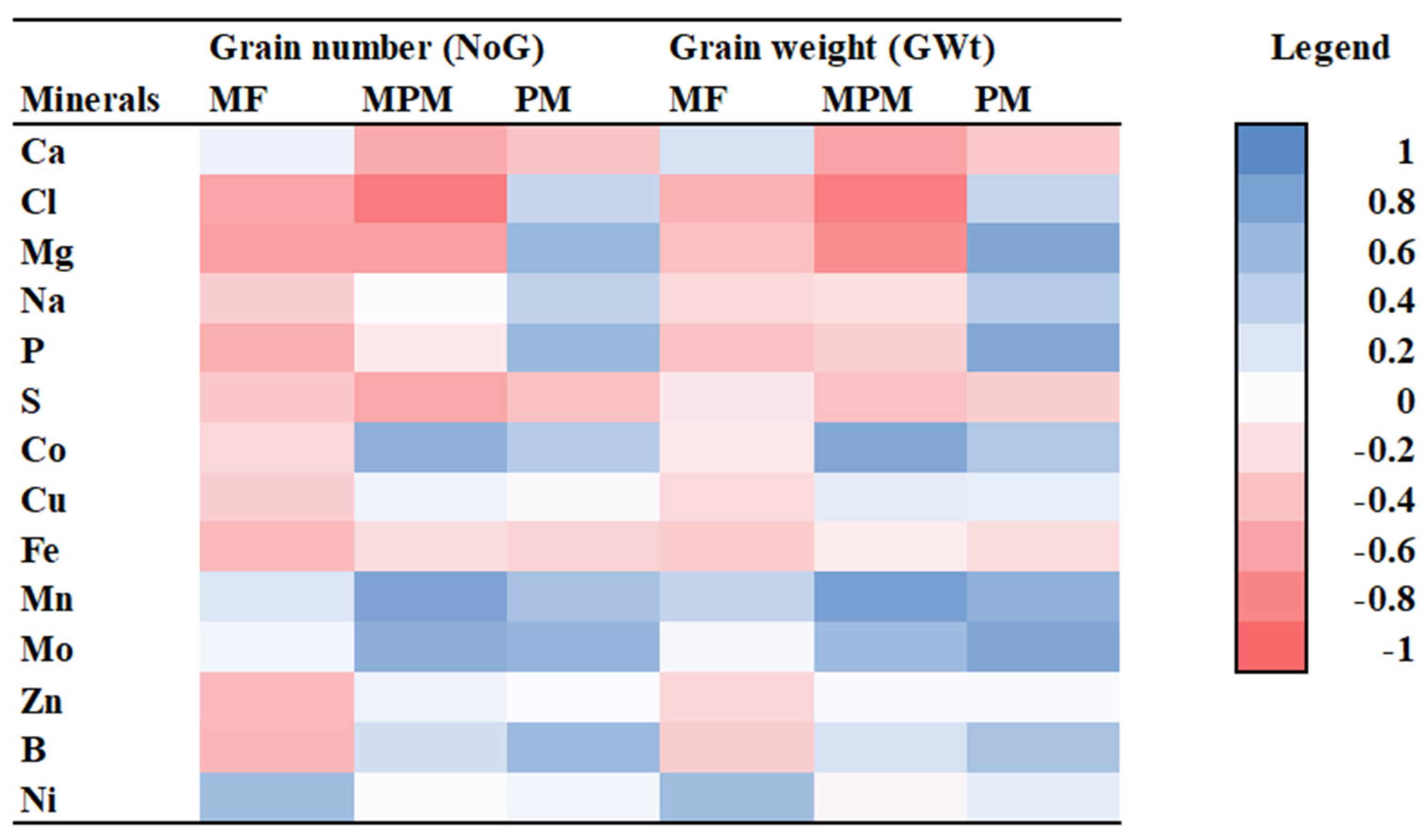
3.4. Correlation Coefficient Analysis
3.4.1. Maize Variety
3.4.2. Soil Amendment
3.4.3. Heat Stress
4. Discussion
4.1. Influence of Heat Stress on the Maize Grain Mineral Concentrations
4.2. Varietal Effect on the Maize Grain Mineral Concentrations
4.3. The Effect of Soil Amendments on Grain Mineral Concentrations
4.4. The Interactive Effect of Heat Stress, Variety, and Soil Amendment on Maize Grain’s Mineral Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- UN. The Sustainable Development Goals Report; 9789210049603; UN: New York, NY, USA, 2020; p. 66. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK, 2021; p. 41. [Google Scholar]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Heat stress effect on the grain yield of three drought-tolerant maize varieties under varying growth conditions. Plants 2021, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Lizaso, J.I.; Ruiz-Ramos, M.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.A.; Lorite, I.J.; Sánchez, D.; García, E.; Rodríguez, A. Impact of high temperatures in maize: Phenology and yield components. Field Crop. Res. 2018, 216, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef]
- Guzmán, C.; Autrique, J.E.; Mondal, S.; Singh, R.P.; Govindan, V.; Morales-Dorantes, A.; Posadas-Romano, G.; Crossa, J.; Ammar, K.; Peña, R.J. Response to drought and heat stress on wheat quality, with special emphasis on bread-making quality, in durum wheat. Field Crop. Res. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F. Effects of elevated CO2 and heat on wheat grain quality. Plants 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Goredema-Matongera, N.; Ndhlela, T.; Magorokosho, C.; Kamutando, C.N.; van Biljon, A.; Labuschagne, M. Multinutrient biofortification of maize (Zea mays L.) in Africa: Current status, opportunities and limitations. Nutrients 2021, 13, 1039. [Google Scholar] [CrossRef]
- Mallikarjuna, M.; Thirunavukkarasu, N.; Hossain, F.; Bhat, J.; Jha, S.; Rathore, A.; Agrawal, P.; Pattanayak, A.; Reddy, S.; Gularia, S.; et al. Stability performance of inductively coupled plasma mass spectrometry-phenotyped kernel minerals concentration and grain yield in maize in different agro-climatic zones. PLoS ONE 2015, 10, e0139067. [Google Scholar] [CrossRef] [Green Version]
- OECD/FAO. Cereals. In Agricultural Outlook 2020–2029; OECD/FAO: Rome, Italy, 2020; p. 17. [Google Scholar]
- Guo, S.; Chen, Y.; Chen, X.; Chen, Y.; Yang, L.; Wang, L.; Qin, Y.; Li, M.; Chen, F.; Mi, G.; et al. Grain mineral accumulation changes in Chinese maize cultivars released in different decades and the responses to nitrogen fertilizer. Front. Plant Sci. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Gu, R.; Chen, F.; Liu, B.; Wang, X.; Liu, J.; Li, P.; Pan, Q.; Pace, J.; Soomro, A.-A.; Lübberstedt, T.; et al. Comprehensive phenotypic analysis and quantitative trait locus identification for grain mineral concentration, content, and yield in maize (Zea mays L.). Theor. Appl. Genet. 2015, 128, 1777–1789. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhao, H.; Yu, C.; Tang, J.; Wu, W.; Yang, Q. Determination of the geographical origin of maize (Zea mays L.) using mineral element fingerprints. J. Sci. Food Agric. 2020, 100, 1294–1300. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yue, S.; Zhang, W.; Liu, D.; Cui, Z.; Chen, X.; Ye, Y.; Zou, C. Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS ONE 2014, 9, e93895. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.J.; Akingbola, O.O.; Ojeniyi, S.O. Effects of poultry manure on soil infiltration, organic matter contents and maize performance on two contrasting degraded alfisols in southwestern Nigeria. Int. J. Recycl. Org. Waste Agric. 2019, 8, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Noor, J.J.; Vinayan, M.T.; Umar, S.; Devi, P.; Iqbal, M.; Seetharam, K.; Zaidi, P.H. Morpho-physiological traits associated with heat stress tolerance in tropical maize (Zea mays L.) at reproductive stage. Aust. J. Crop Sci. 2019, 13, 536–545. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Tesfaye, K.; Zaidi, P.; Gbegbelegbe, S.; Boeber, C.; Getaneh, F.; Seetharam, K.; Erenstein, O.; Stirling, C. Climate change impacts and potential benefits of heat-tolerant maize in South Asia. Theor. Appl. Climatol. 2017, 130, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.; De Bang, T.; Laursen, K.; Pedas, P.; Husted, S.; Schjoerring, J. Multielement plant tissue analysis using ICP spectrometry. In Plant Mineral Nutrients; Maathuis, F.J.M., Ed.; Methods and Protocols, Methods in Molecular Biology; Springer: Totowa, NJ, USA, 2013; Volume 953, pp. 121–141. [Google Scholar]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the stress: Breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C.; Ramalho, J.C. IV. Heat stress in Triticum: Kinetics of Fe and Mn accumulation. Braz. J. Plant Physiol. 2009, 21, 153–164. [Google Scholar] [CrossRef]
- Narendra, M.; Roy, C.; Kumar, S.; Virk, P.; De, N. Effect of terminal heat stress on physiological traits, grain zinc and iron content in wheat (Triticum aestivum L.). Czech J. Genet. Plant Breed. 2021, 57, 43–50. [Google Scholar] [CrossRef]
- Enakiev, Y.I.; Bahitova, A.R.; Lapushkin, V.M. Microelements (Cu, Mo, Zn, Mn, Fe) in corn grain according to their availability in the fallow sod-podzolic soil profile. Bulg. J. Agric. Sci. 2018, 24, 285–289. [Google Scholar]
- Oikeh, S.O.; Menkir, A.; Maziya-Dixon, B.; Welch, R.; Glahn, R.P. Assessment of concentrations of iron and zinc and bioavailable iron in grains of early-maturing tropical maize varieties. J. Agric. Food Chem. 2003, 51, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Akinwale, R.O.; Adewopo, O.A. Grain iron and zinc concentrations and their relationship with selected agronomic traits in early and extra-early maize. J. Crop Improv. 2016, 30, 641–656. [Google Scholar] [CrossRef]
- Prasanna, B.; Mazumdar, S.; Chakraborti, M.; Hossain, F.; Manjaiah, K.; Agrawal, P.; Guleria, S.; Gupta, H. Genetic variability and genotype× environment interactions for kernel iron and zinc concentrations in maize (Zea mays) genotypes. Indian J. Agric. Sci. 2011, 81, 704–711. [Google Scholar]
- Jin, T.; Zhou, J.; Chen, J.; Zhu, L.; Zhao, Y.; Huang, Y. The genetic architecture of zinc and iron content in maize grains as revealed by QTL mapping and meta-analysis. Breed. Sci. 2013, 63, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, Z.X.; Kopittke, P.M.; Harper, S.M.; O’Hare, T.J.; Wang, P.; Paterson, D.J.; de Jonge, M.D.; Bell, M.J. In situ analyses of inorganic nutrient distribution in sweetcorn and maize kernels using synchrotron-based X-ray fluorescence microscopy. Ann. Bot. 2018, 123, 543–556. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.-H.; Li, S.-s.; Diao, C.-p.; Liu, L.; Hui, X.-L.; Huang, M.; Luo, L.-C.; He, G.; Cao, H.-b.; et al. Identification of high-yield and high-Zn wheat cultivars for overcoming “yield dilution” in dryland cultivation. Eur. J. Agron. 2018, 101, 57–62. [Google Scholar] [CrossRef]
- Gyori, Z. Essential mineral element status in wheat and maize grains. EC Nutr. 2018, 13, 1–3. [Google Scholar]
- Dada, O.A.; Kutu, F.R. Bioavailability and health risk assessment of potentially toxic elements in popcorn kernel from sandy loam Ferric Luvisol amended with municipal solid waste compost. Environ. Geochem. Health 2021, 43, 2389–2405. [Google Scholar] [CrossRef]
- Baiyeri, K.P.; Chukwudi, U.P.; Chizaram, C.A.; Aneke, N. Maximizing rice husk waste for Daucus carota production. Int. J. Recycl. Org. Waste Agric. 2019, 8, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Pasley, H.R.; Cairns, J.E.; Camberato, J.J.; Vyn, T.J. Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutr. Cycl. Agroecosyst. 2019, 115, 373–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukwudi, U.P.; Agbo, C.U.; Echezona, B.C.; Eze, E.I.; Kutu, F.R.; Mavengahama, S. Variability in morphological, yield and nutritional attributes of ginger (Zingiber officinale) germplasm in Nigeria. Res. Crop. 2020, 21, 634–642. [Google Scholar] [CrossRef]
- Behera, S.K.; Shukla, A.K.; Singh, M.V.; Wanjari, R.H.; Singh, P. Yield and zinc, copper, manganese and iron concentration in maize (Zea mays L.) grown on Vertisol as influenced by zinc application from various zinc fertilizers. J. Plant Nutr. 2015, 38, 1544–1557. [Google Scholar] [CrossRef]
- Imran, M.; Garbe-Schönberg, D.; Neumann, G.; Boelt, B.; Mühling, K.H. Zinc distribution and localization in primed maize seeds and its translocation during early seedling development. Environ. Exp. Bot. 2017, 143, 91–98. [Google Scholar] [CrossRef]



| Source of Variation | Ca | Cl | Mg | Na | P | S | Co | Cu | Fe | Mn | Mo | Zn | B | Ni | NoG | GWt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Env | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.061 | <0.001 | <0.001 | <0.001 | <0.001 | 0.639 | <0.001 | <0.001 | <0.001 |
| Mva | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.635 | <0.001 | 0.66 | 0.912 |
| Sam | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.519 | <0.001 | 0.021 | <0.001 |
| Env.Mva | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.029 | 0.04 |
| Env.Sam | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.026 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 |
| Mva.Sam | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Env.Mva.Sam | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Minerals | Environment | Maize Variety | Soil Amendment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS | NHS | WE3128 | WE5323 | ZM1523 | MF | MPM | PM | |||||||||
| Macro | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD |
| Ca | 175.30 | 40.2 a | 152.91 | 43.3 b | 138.38 | 15.14 c | 179.02 | 43.6 a | 174.91 | 50.5 b | 170.30 | 46.5 a | 156.50 | 14.9 c | 165.50 | 56.9 b |
| Cl | 9565. | 1477 a | 7138. | 2163 b | 7484. | 2739 c | 9628. | 1462 a | 7942. | 1681 b | 8111. | 2852 b | 9011. | 1152 a | 7933. | 2236 b |
| Mg | 520.90 | 120.9 a | 513.25 | 82.9 b | 501.55 | 110.2 c | 531.06 | 104.2 a | 518.62 | 97.3 b | 511.90 | 81.6 b | 566.10 | 65.1 a | 473.20 | 132 c |
| Na | 11.79 | 3.8 a | 10.62 | 3.1 b | 10.66 | 4.9 b | 12.60 | 2.5 a | 10.35 | 1.9 b | 13.89 | 2.7 a | 11.92 | 2.6 b | 7.80 | 1.7 c |
| P | 409.41 | 89.1 a | 401.76 | 64.9 b | 395.54 | 88.6 c | 422.08 | 80.6 a | 399.14 | 61.7 b | 389.80 | 85.5 b | 455.60 | 33.5 a | 371.40 | 77.6 c |
| S | 13.47 | 6.1 a | 7.12 | 4.9 b | 10.33 | 7.6 b | 8.61 | 6.6 c | 11.95 | 4.2 a | 13.53 | 5.8 a | 6.18 | 2.6 c | 11.19 | 7.4 b |
| Trace | ||||||||||||||||
| Co | 0.13 | 0.05 b | 0.20 | 0.12 a | 0.15 | 0.06 b | 0.15 | 0.05 ab | 0.20 | 0.15 a | 0.14 | 0.03 b | 0.23 | 0.13 a | 0.12 | 0.06 c |
| Cu | 12.78 | 5.58 a | 12.11 | 4.9 a | 9.64 | 4.8 c | 11.97 | 6.3 b | 15.74 | 1.7 a | 16.15 | 3.7 a | 13.05 | 2.5 b | 8.14 | 5.5 c |
| Fe | 136.75 | 70.2 a | 95.21 | 34.2 b | 87.85 | 40.7 c | 112.17 | 35.1 b | 147.92 | 76.6 a | 105.20 | 36.6 b | 136.90 | 51.3 a | 105.90 | 77.6 b |
| Mn | 20.20 | 5.1 b | 28.15 | 5.6 a | 23.36 | 5.9 b | 23.80 | 7.4 b | 25.38 | 6.8 a | 25.39 | 6.4 b | 26.12 | 5.6 a | 21.02 | 7.1 c |
| Mo | 21.38 | 27.6 b | 34.55 | 22.2 a | 23.58 | 29.4 b | 38.19 | 19.4 a | 22.13 | 25.5 b | 46.16 | 30.1 a | 11.79 | 16.1 c | 25.94 | 16.1 b |
| Zn | 38.80 | 13.3 a | 33.51 | 4.9 b | 40.84 | 14.4 a | 34.86 | 7.6 b | 32.77 | 5.3 c | 37.95 | 15.3 a | 37.6 | 4.8 a | 32.92 | 7.5 b |
| B | 1.63 | 0.63 a | 1.58 | 0.76 a | 1.54 | 0.65 a | 1.67 | 0.85 a | 1.60 | 0.6 a | 1.57 | 0.7 a | 1.69 | 0.5 a | 1.55 | 0.8 a |
| Ni | 3.10 | 1.4 b | 3.83 | 2.3 a | 2.53 | 1.9 c | 4.18 | 2.4 a | 3.68 | 0.7 b | 5.15 | 2.1 a | 2.99 | 0.9 b | 2.25 | 1.0 c |
| NoG | 76.0 | 66.6 b | 338.0 | 139 a | 205.0 | 183 a | 223.0 | 171 a | 194.0 | 159 a | 163.0 | 82.9 c | 256.0 | 198. a | 203.0 | 199 b |
| GWt | 18.2 | 16.8 b | 87.0 | 40 a | 50.9 | 44 a | 53.9 | 46 a | 53.0 | 49 a | 34.5 | 14.9 b | 69.2 | 52.7 a | 54.1 | 55 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwudi, U.P.; Mavengahama, S.; Kutu, F.R.; Motsei, L.E. Heat Stress, Varietal Difference, and Soil Amendment Influence on Maize Grain Mineral Concentrations. Agriculture 2022, 12, 1633. https://doi.org/10.3390/agriculture12101633
Chukwudi UP, Mavengahama S, Kutu FR, Motsei LE. Heat Stress, Varietal Difference, and Soil Amendment Influence on Maize Grain Mineral Concentrations. Agriculture. 2022; 12(10):1633. https://doi.org/10.3390/agriculture12101633
Chicago/Turabian StyleChukwudi, Uchechukwu Paschal, Sydney Mavengahama, Funso Raphael Kutu, and Lebogang Ezra Motsei. 2022. "Heat Stress, Varietal Difference, and Soil Amendment Influence on Maize Grain Mineral Concentrations" Agriculture 12, no. 10: 1633. https://doi.org/10.3390/agriculture12101633





