Differential Physiological Characteristics and Fungal Composition of Alfalfa under Salt Stress in Degraded Grasslands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Conditions
2.3. Alfalfa Preparation
2.4. Analysis of Physiological Characteristics
2.5. DNA Extraction and PCR Amplification
2.6. Illumina Hiseq2500 Sequencing and Microbial Diversity Analysis
2.7. Statistical Analysis
3. Results
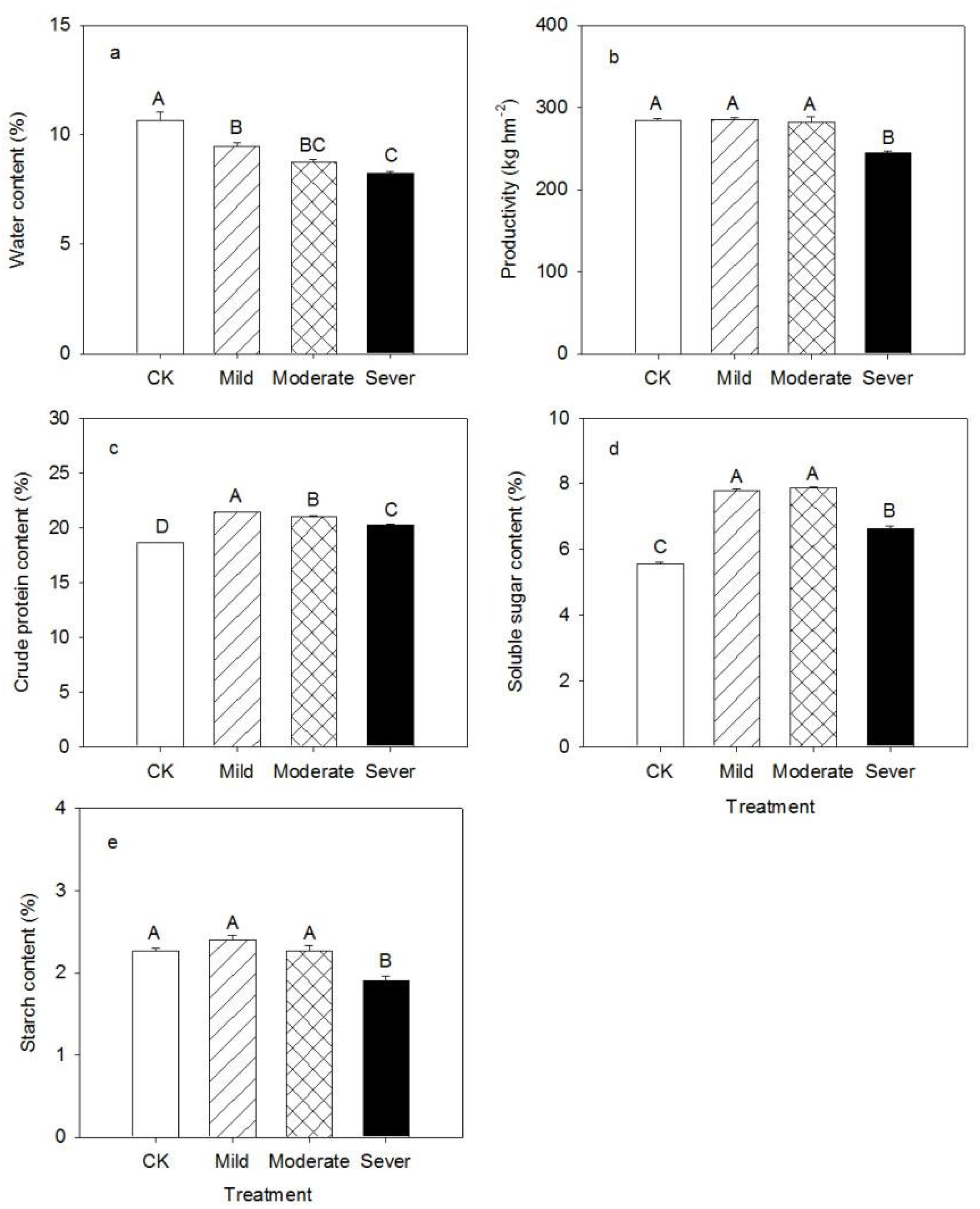
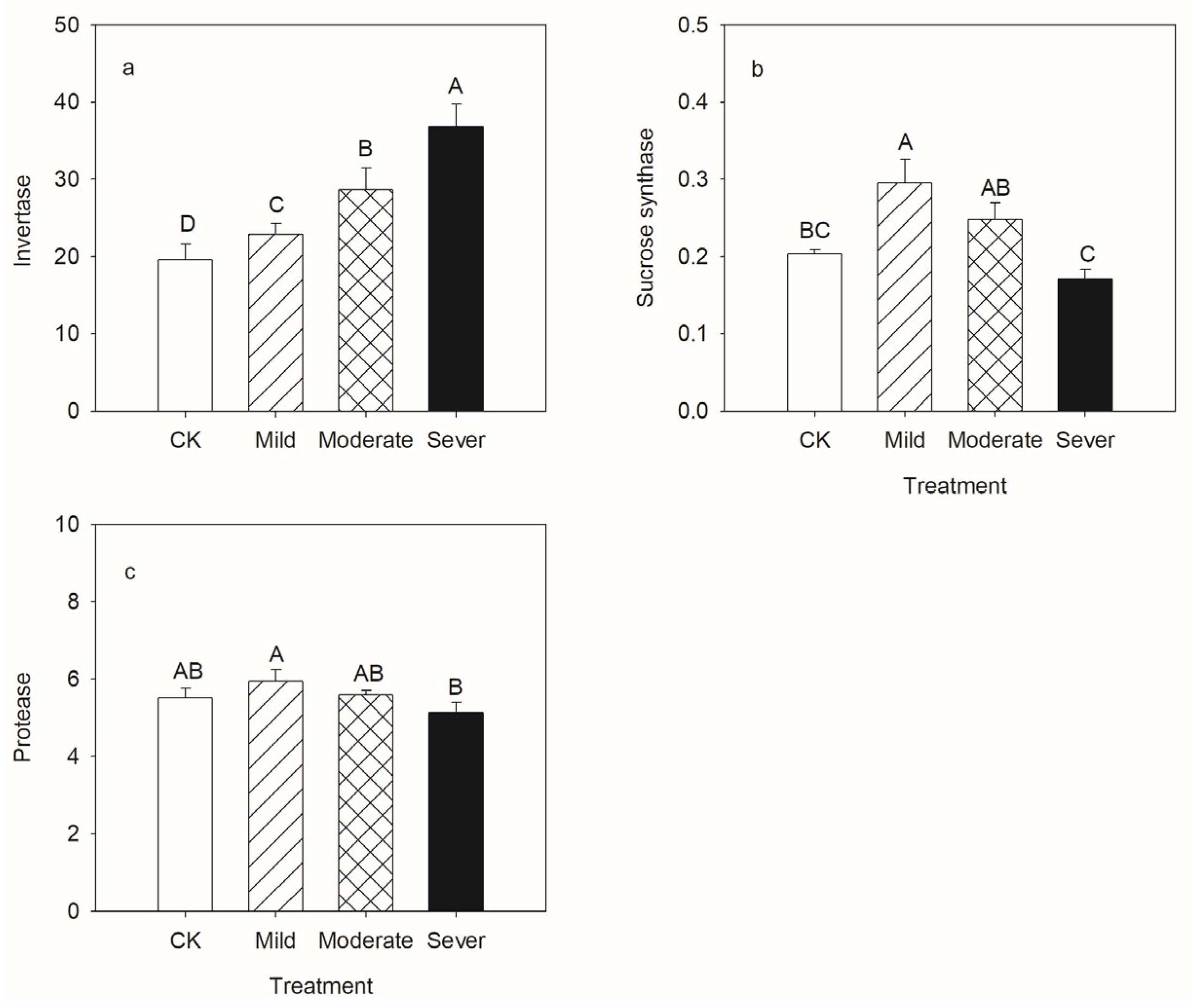
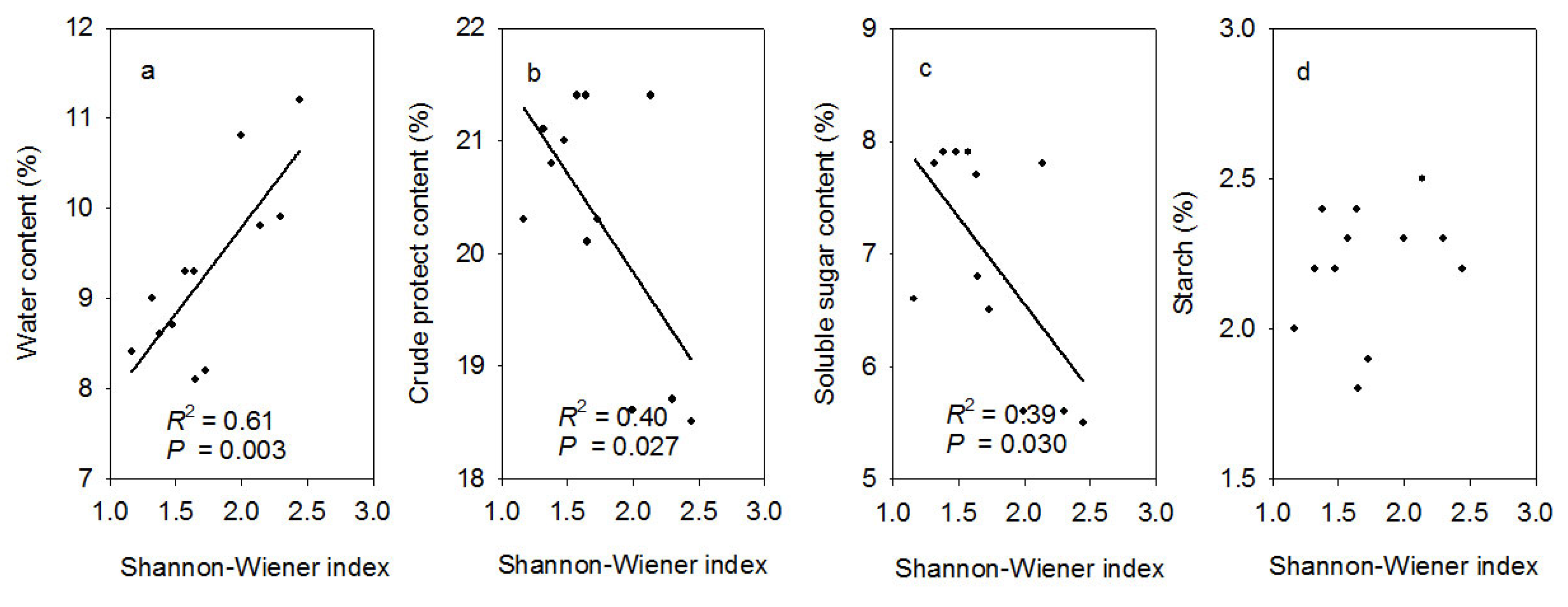
3.1. Physiological Characteristics of Alfalfa
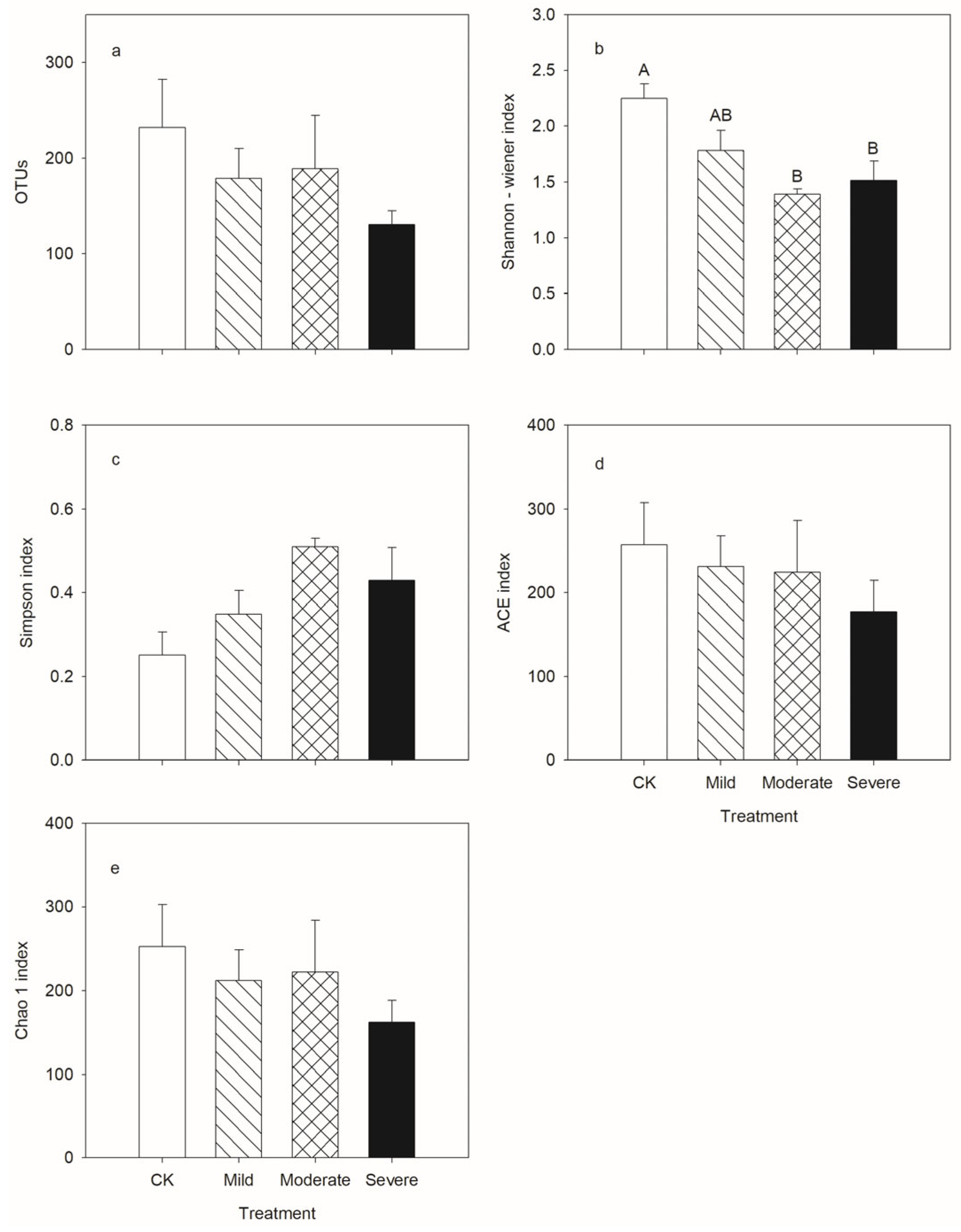
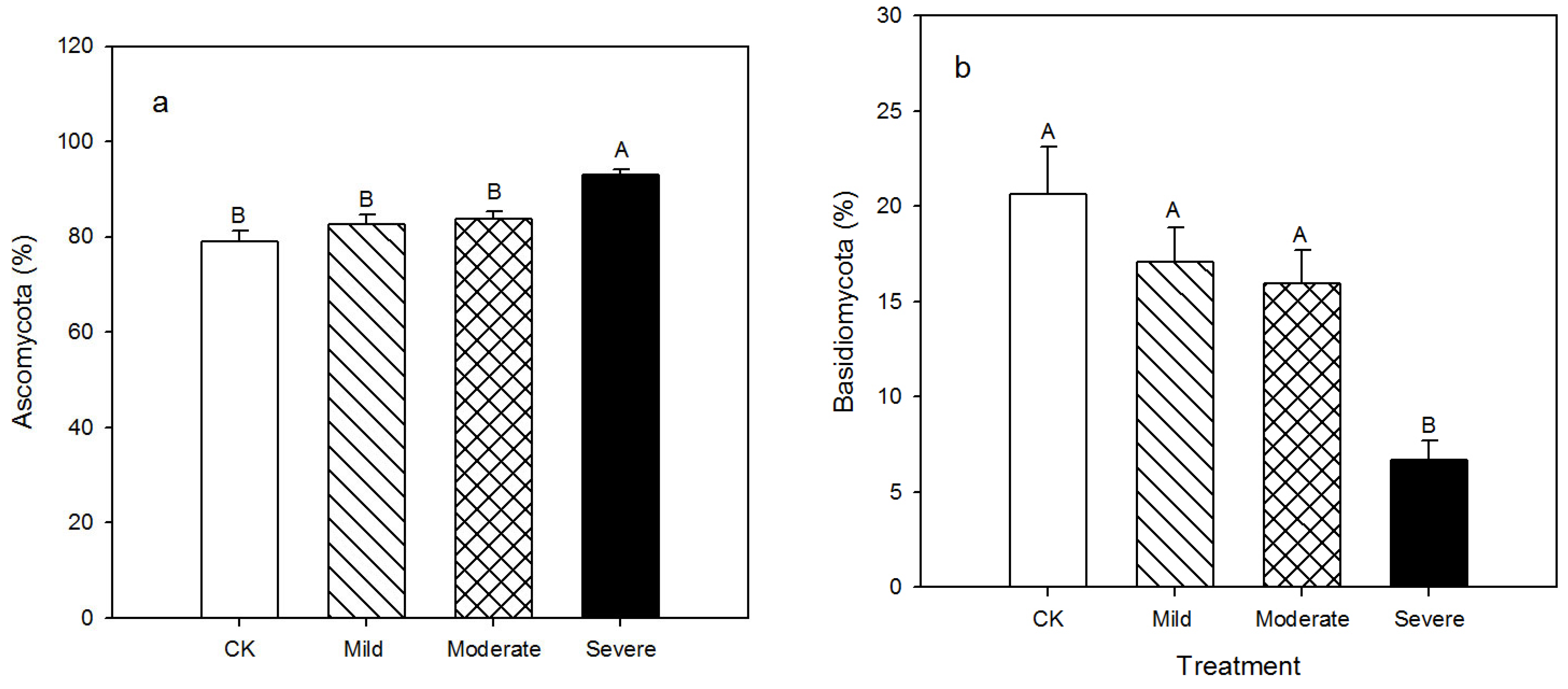
3.2. Fungal Community Diversity and Composition
3.3. Fungal Functional Groups
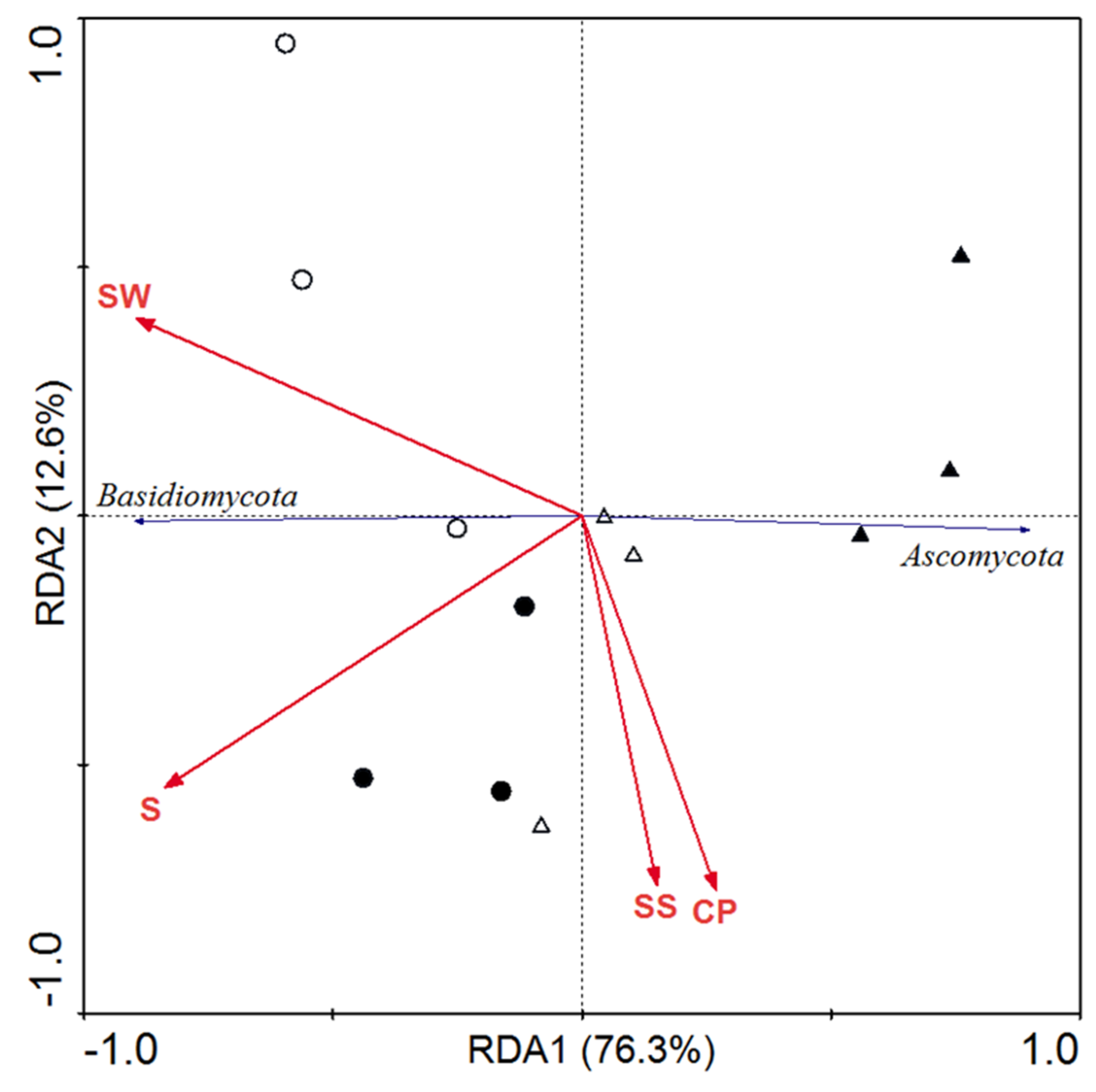
3.4. Multivariate Statistical Analyses of Plant Physiological Characteristics, Microbial Diversity, and Community Composition
4. Discussions
4.1. Physiological Characteristics of Alfalfa
4.2. Alfalfa Fungi and Response to Salt Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, C.; Tao, S.; Cui, Z.; Ying, Z. Response of Soil Properties and Microbial Communities to Increasing Salinization in the Meadow Grassland of Northeast China. Microb. Ecol. 2021, 82, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kang, S.; Ding, R.; Zhao, Q. A crude protein and fiber model of alfalfa incorporating growth age under water and salt stress. Agric. Water Manag. 2021, 255, 107037. [Google Scholar] [CrossRef]
- Lu, Q.; Ge, G.T.; Sa, D.W.; Wang, Z.; Hou, M.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ 2021, 9, e11729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Zhao, Z.; Wu, C.; Yu, B. Using soil erosion to locate nonpoint source pollution risks in coastal zones: A case study in the Yellow River Delta, China. Environ. Pollut. 2021, 283, 117117. [Google Scholar] [CrossRef]
- Gabrijel, O.; Santosha, R.; Kallakeri, K.M.; Channappa, G.; Madhyavenkatapura, S.A.; Akshay, S.S.; Brajendra, P.; Brahamdeo, K.Y.; Nirmala, B.; Farzana, R.; et al. Salt Stress in Plants and Mitigation Approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V. Arresting cytokinin signaling for salt-stress tolerance. Plant Sci. 2022, 314, 111–116. [Google Scholar] [CrossRef]
- Jia, X.M.; Wang, H.; Svetla, S.; Zhu, Y.F.; Hu, Y.; Cheng, L.; Zhao, T.; Wang, Y.X. Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Sci. Hortic. 2019, 245, 154–162. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Liu, J.; Ren, X.; Zhang, Z.; Wang, Q. Effects of microplastics on humification and fungal community during cow manure composting. Sci. Total Environ. 2022, 803, 150029. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Al-Farsi, S.M.; Nawaz, A.; Aneesur, R.; Nadaf, S.K.; Farooq, M. Effects, tolerance mechanisms and management of salt stress in lucerne (Medicago sativa). Crop Pasture Sci. 2020, 7, 411–428. [Google Scholar] [CrossRef]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021, 130, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, K.; Barbara, K. Influence of drought and salt stress on the growth of young Populus nigra ‘Italica’ plants and associated mycorrhizal fungi and non-mycorrhizal fungal endophytes. New For. 2022, 53, 679–694. [Google Scholar] [CrossRef]
- Řezáčová, V.; Řezáč, M.; Gryndler, M.; Hrelová, H.; Gryndlerová, H.; Michalová, T. Plant invasion alters community structure and decreases diversity of arbuscular mycorrhizal fungal communities. Appl. Soil Ecol. 2021, 167, 104039. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wang, S.; Wei, M.; Du, D. Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 2021, 768, 144518. [Google Scholar] [CrossRef] [PubMed]
- Mello, I.N.K.; Moreira, B.C.; Kasuya, M.C.; Arajo, J.V.; Freitas, L.G. Nematophagus fungi increasing phosphorus uptake and promoting plant growth. Biol. Control 2018, 23, 71–75. [Google Scholar] [CrossRef]
- Peterson, L.W.; Huffaker, R.C. Loss of ribulose 1,5-Diphosphate carboxylase and increase in proteolytic activity during senescence of detached primary barley leaves. Plant Physiol. 1975, 55, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Sergeeva, L.I.; Keurentjes, J.J.B.; Bentsink, L. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 2994–2999. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.Y.; Liu, E.K.; Yan, C.R.; Tian, J.; Zhang, H.H.; Zhang, Y.Q. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- Bostrom, C.; Obrien, K.; Roos, C.; Ekebom, J. Environmental variables explaining structural and functional diversity of seagrass macrofauna in an archipelago landscape. J. Exp. Mar. Biol. Ecol. 2006, 335, 52–73. [Google Scholar] [CrossRef]
- Kusvuran, A.; Bilgici, M.; Kusvuran, S.; Bilgici, M.; Kuvuran, V.; Nazli, R. The effect of different organic matters on plant growth regulation and nutritional components under salt stress in sweet sorghum [Sorghum bicolor (L.) Moench.]. Maydica 2021, 66, 1–9. [Google Scholar]
- Yang, X.; Li, Y.; Chen, H.; Huang, J.; Zhang, Y.; Qi, M.; Liu, Y.; Li, T. Photosynthetic response mechanism of soil salinity-induced cross-tolerance to subsequent drought stress in tomato plants. Plants 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, F.A.; Kazemeini, S.A.; Pirasteh-Anosheh, H.; Ghadiri, H.; Mohammad, P. Forage yield and quality as affected by salt stress in different ratios of Sorghum bicolor—Bassia indica intercropping. J. Plant Nutr. 2020, 43, 2579–2589. [Google Scholar] [CrossRef]
- Mao, X.; Li, Q.; Ren, L. Application of molybdenum fertilizer enhanced quality and production of alfalfa in northern China under non-irrigated conditions. J. Plant Nutr. 2018, 41, 1009–1019. [Google Scholar] [CrossRef]
- Lu, Q.; Zhen, W.; Sa, D.; Hou, M.; Ge, G.; Wang, Z.; Jia, Y. The potential effects on microbiota and silage fermentation of alfalfa under salt stress. Front. Microbiol. 2021, 12, 688695. [Google Scholar] [CrossRef] [PubMed]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Rupasinghe, T.; Rupasinghe, T.W.T.; Barklaet, B.J. Salt stress alters membrane lipid content and lipid biosynthesis pathways in the plasma membrane and tonoplast. Plant Physiol. 2022, 189, 805–826. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Guo, J.L.; Zhu, A.J.; Zhang, L.; Zhang, M.M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, M.; He, Y.; Gu, X. Effects of pH and nitrogen form on Nitzschia closterium growth by linking dynamic with enzyme activity. Chemosphere 2020, 249, 126154. [Google Scholar] [CrossRef]
- Gupta, A.K.; Verma, J.; Srivastava, A.; Srivastava, S.; Prasad, V. Pseudomonas aeruginosa isolate PM1 effectively controls virus infection and promotes growth in plants. Arch. Microbiol. 2022, 204, 1–18. [Google Scholar] [CrossRef]
- Kaisermann, A.; Maron, P.A.; Beaumelle, L. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 2015, 86, 158–164. [Google Scholar] [CrossRef]
- Fabiana, C.; Laura, Z.; Claudia, P.; Laura, S.; Silvano, O.; József, G. Vegetation, pH and water content as main factors for shaping fungal richness, community composition and functional guilds distribution in soils of Western Greenland. Front. Microbiol. 2019, 10, 2348. [Google Scholar] [CrossRef] [Green Version]
- Waller, F.B.; Achatz, H.; Baltruschat, J.; Fodor, K.; Becker, M. The endophytic fungus Piriformis indica reprograms barley to salt-stress tolerance, disease resistance and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]

| Index | pH | EC (mS/cm) | Total Salt (g/kg) | Organic Matter (g/kg) | Total Nitrogen (g/kg) |
|---|---|---|---|---|---|
| CK | 7.83 | 0.21 | 0.91 | 5.32 | 0.28 |
| Mild | 8.42 | 0.59 | 1.76 | 5.09 | 0.24 |
| Moderate | 8.46 | 1.35 | 2.63 | 4.89 | 0.33 |
| Severe | 8.64 | 2.30 | 3.85 | 5.29 | 0.16 |
| Index | Water Content | Crude Protein | Starch | Soluble Sugar | |
|---|---|---|---|---|---|
| Total N | R | 0.44 | −0.89 | 0.66 | 0.19 |
| p | 0.15 | <0.0001 | 0.02 | 0.55 | |
| Phylum | Family | CK | Mild | Moderate | Severe |
|---|---|---|---|---|---|
| Ascomycota | Davidiellaceae | 52.36 ± 10.74 | 59.40 ± 5.71 | 61.42 ± 10.51 | 63.07 ± 6.47 |
| Pleosporaceae | 6.27 ± 0.93 | 7.69 ± 3.47 | 7.71 ± 1.72 | 8.48 ± 0.42 | |
| Mycosphaerellaceae | 11.43 ± 8.37 | 8.67 ± 5.36 | 1.88 ± 0.82 | 9.48 ± 7.15 | |
| Pleosporales | 1.32 ± 0.77 | 0.87 ± 0.08 | 2.65 ± 2.17 | 7.31 ± 6.93 | |
| Nectriaceae | 0.77 ± 0.22 | 1.84 ± 0.62 | 6.21 ± 4.07 | 2.24 ± 1.29 | |
| Plectosphaerellaceae | 2.00 ± 1.60 | 0.52 ± 0.15 | 0.44 ± 0.10 | 0.47 ± 0.25 | |
| Xylariales | 0.54 ± 0.44 | 0.92 ± 0.74 | 0.32 ± 0.24 | 0.31 ± 0.18 | |
| Dothideomycetes | 0.16 ± 0.10 | 0.97 ± 0.54 | 0.77 ± 0.30 | 0.24 ± 0.09 | |
| Pleosporales | 0.06 ± 0.01 | 1.05 ± 1.01 | 0.38 ± 0.33 | 0.13 ± 0.12 | |
| Basidiomycota | Tremellales | 17.38 ± 1.99 | 14.54 ± 1.64 | 11.30 ± 1.28 | 5.78 ± 0.90 |
| Sporidiobolales | 1.83 ± 0.51 | 1.31 ± 1.64 | 2.58 ± 1.28 | 0.43 ± 0.19 | |
| Filobasidiaceae | 0.23 ± 0.06 | 0.56 ± 0.24 | 0.65 ± 0.31 | 0.10 ± 0.05 |
| Guild | OUTs | CK | Mild | Moderate | Sever |
|---|---|---|---|---|---|
| Animal pathogen | 58 | 7.99 ± 0.81 | 8.12 ± 0.77 | 8.61± 1.33 | 23.04 ± 6.51 |
| Dung saprotroph | 21 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 |
| Endophyte | 59 | 1.14 ± 0.29 | 3.80 ± 3.17 | 1.03 ± 0.27 | 2.88 ± 1.22 |
| Fungal parasite | 67 | 16.31 ± 2.84 | 9.92 ± 4.33 | 11.93 ± 4.54 | 25.18 ± 6.89 |
| Plant pathogen | 63 | 3.75 ± 1.07 | 14.63 ± 4.86 | 23.52 ± 7.76 | 30.53 ± 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Sa, D.; Wang, Z.; Wang, Z.; Ge, G.; Jia, Y.; Liu, T.; Sun, L. Differential Physiological Characteristics and Fungal Composition of Alfalfa under Salt Stress in Degraded Grasslands. Agriculture 2022, 12, 1636. https://doi.org/10.3390/agriculture12101636
Lu Q, Sa D, Wang Z, Wang Z, Ge G, Jia Y, Liu T, Sun L. Differential Physiological Characteristics and Fungal Composition of Alfalfa under Salt Stress in Degraded Grasslands. Agriculture. 2022; 12(10):1636. https://doi.org/10.3390/agriculture12101636
Chicago/Turabian StyleLu, Qiang, Duowen Sa, Zhen Wang, Zhijun Wang, Gentu Ge, Yushan Jia, Tingyu Liu, and Lin Sun. 2022. "Differential Physiological Characteristics and Fungal Composition of Alfalfa under Salt Stress in Degraded Grasslands" Agriculture 12, no. 10: 1636. https://doi.org/10.3390/agriculture12101636






