Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions, Plant Material, and Trial Design
2.2. Pedoclimatic Conditions
2.3. Cultivar + Environmental Combinations
2.4. Bud Fruitfulness and Vegetative Behaviour
2.5. Vegetative Behaviour and Vine Water Status Measurements
2.6. Crop Yield and Berry Characteristics
2.7. Statistical Analyses
3. Results
3.1. Climatic Conditions
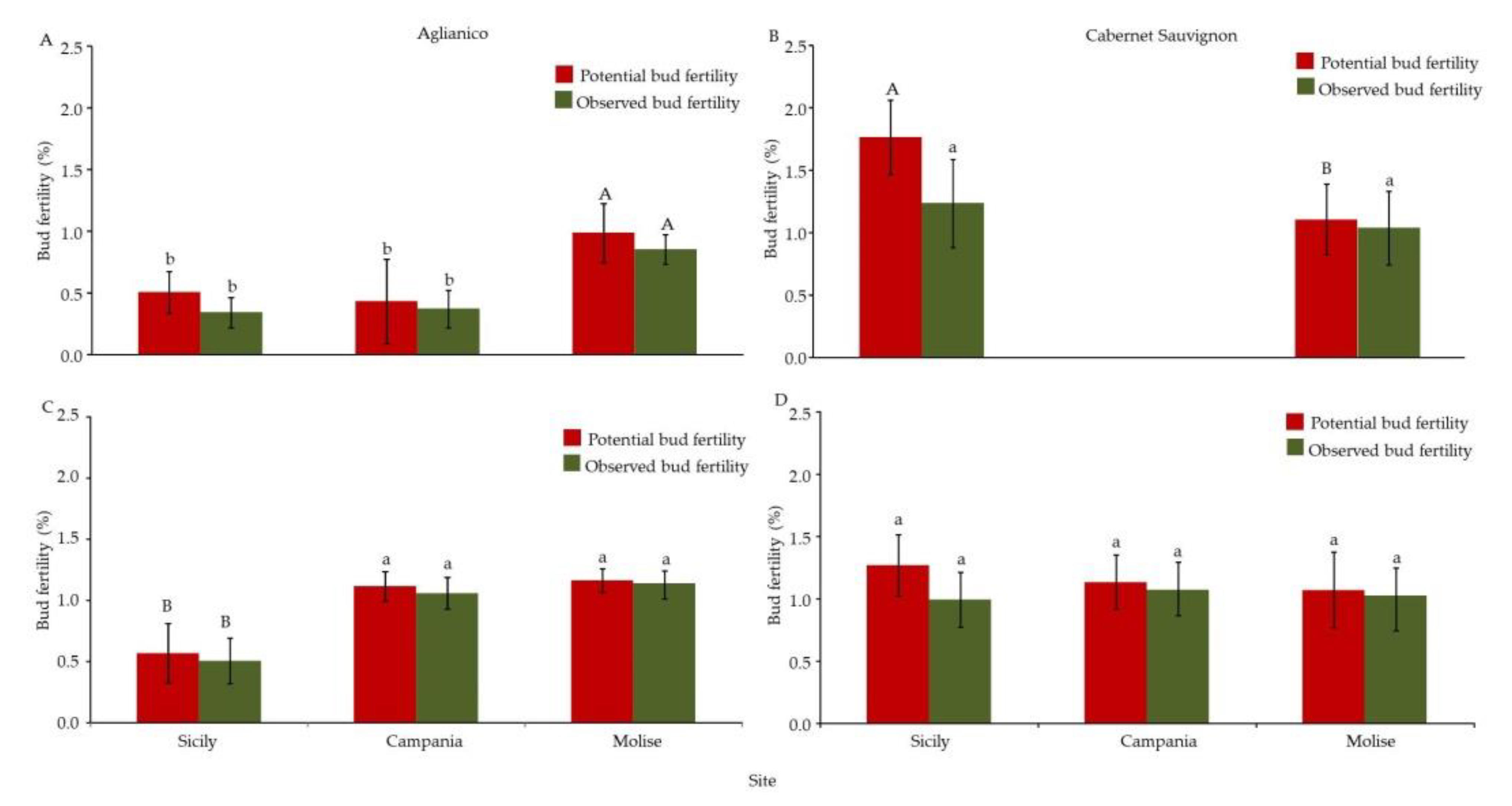
3.2. Bud Fruitfulness
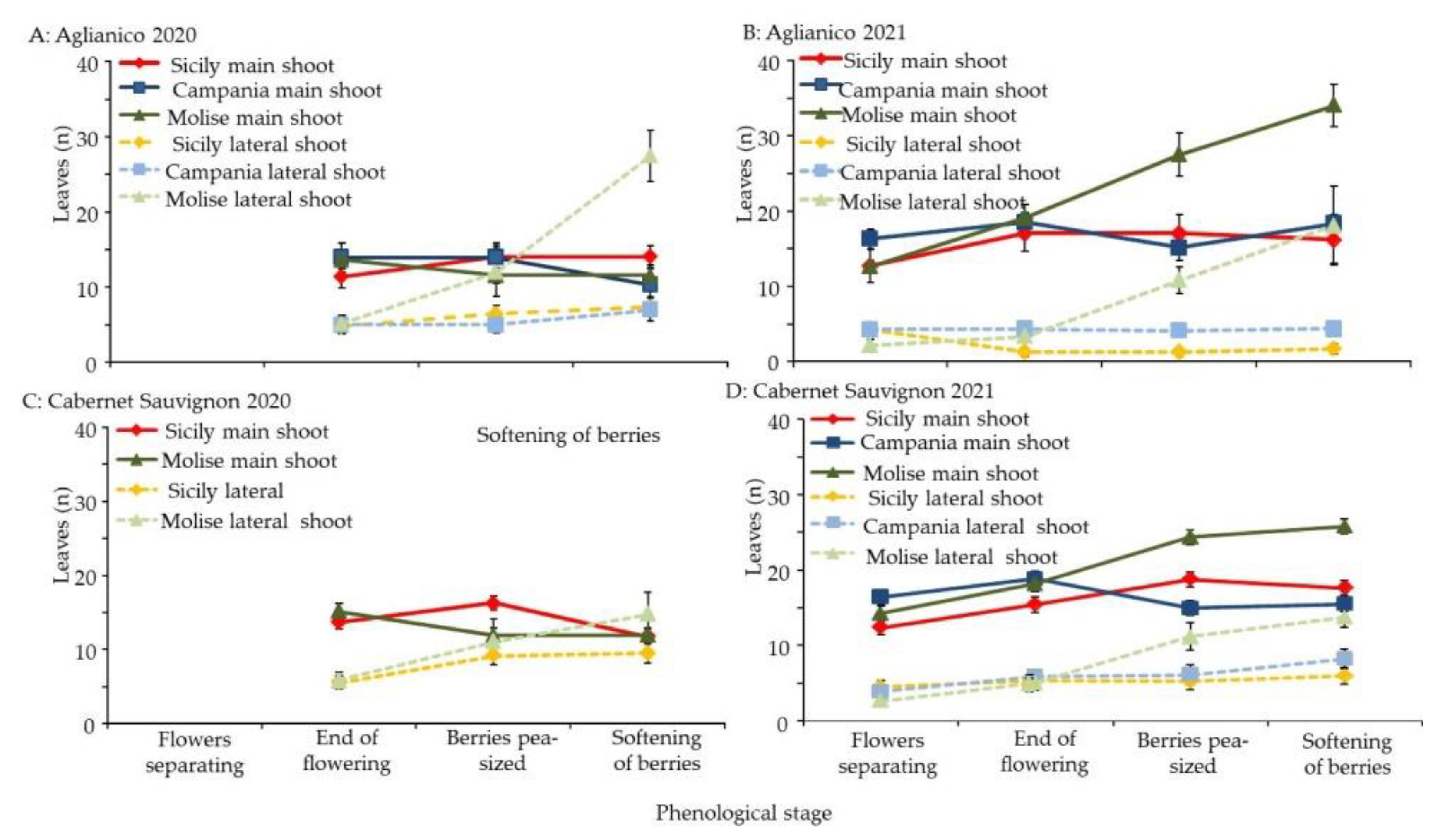
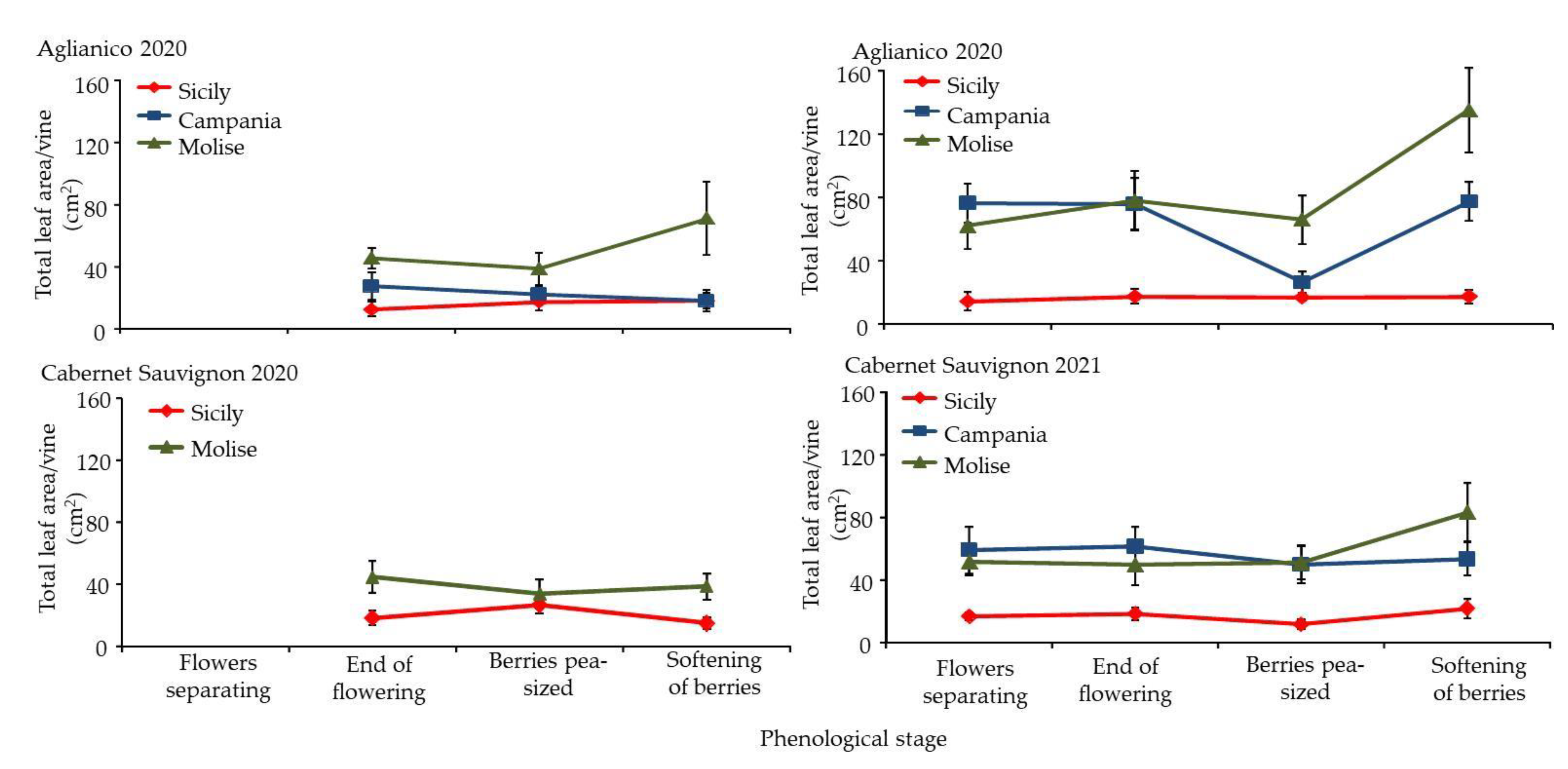
3.3. Morphological Response
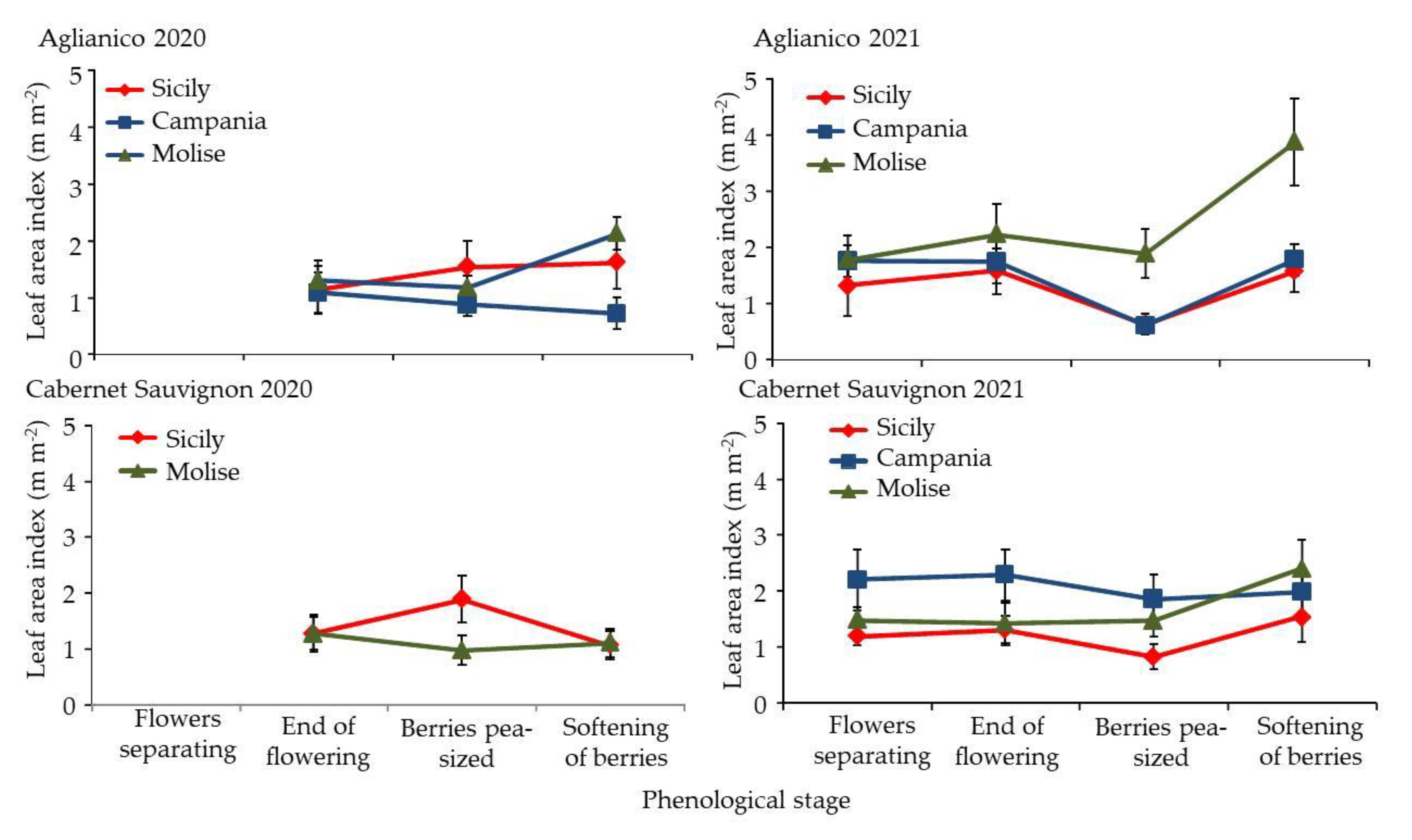
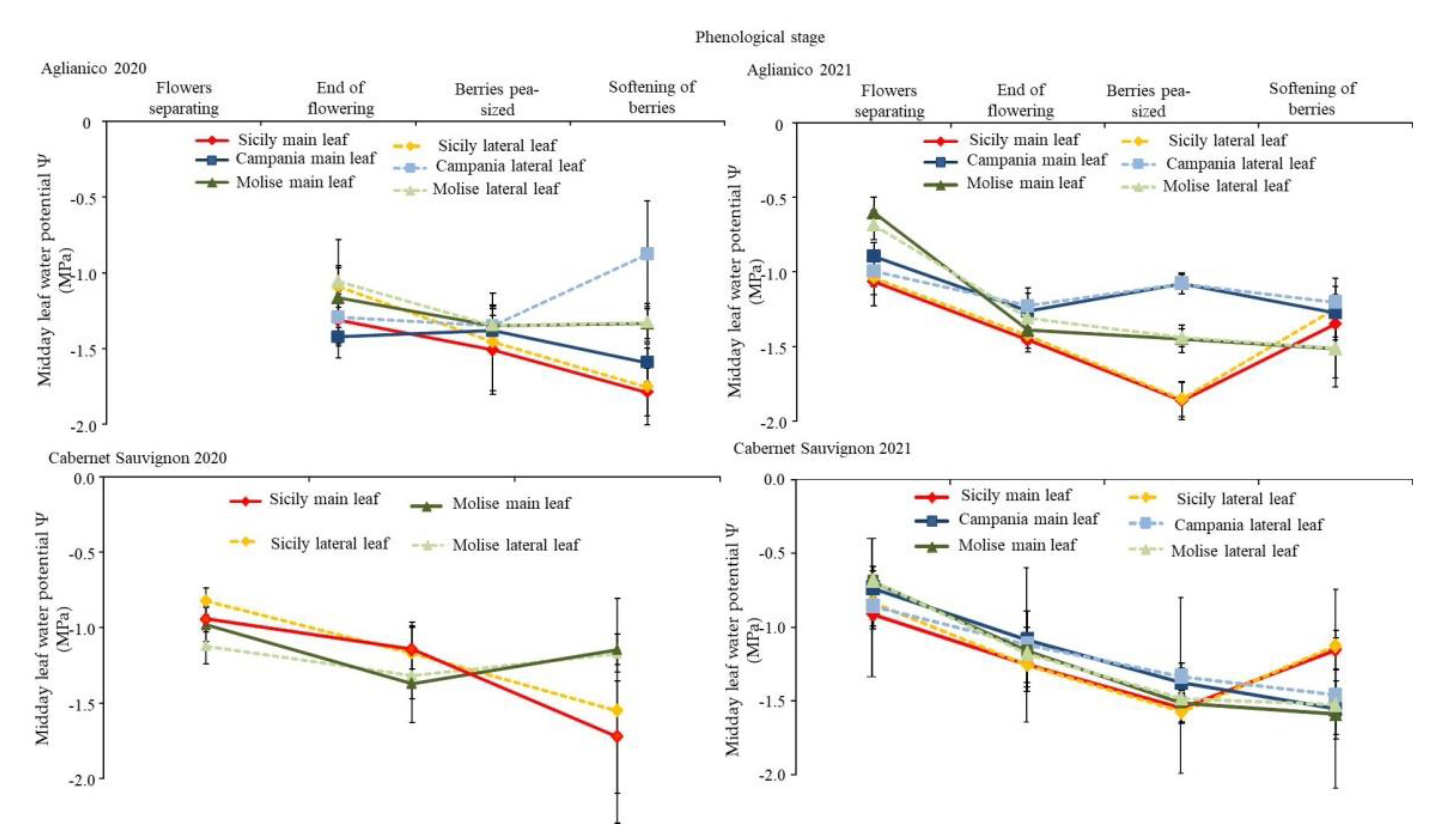
3.4. Vines Physiological Behaviour
3.5. Crop Yield and Characteristics
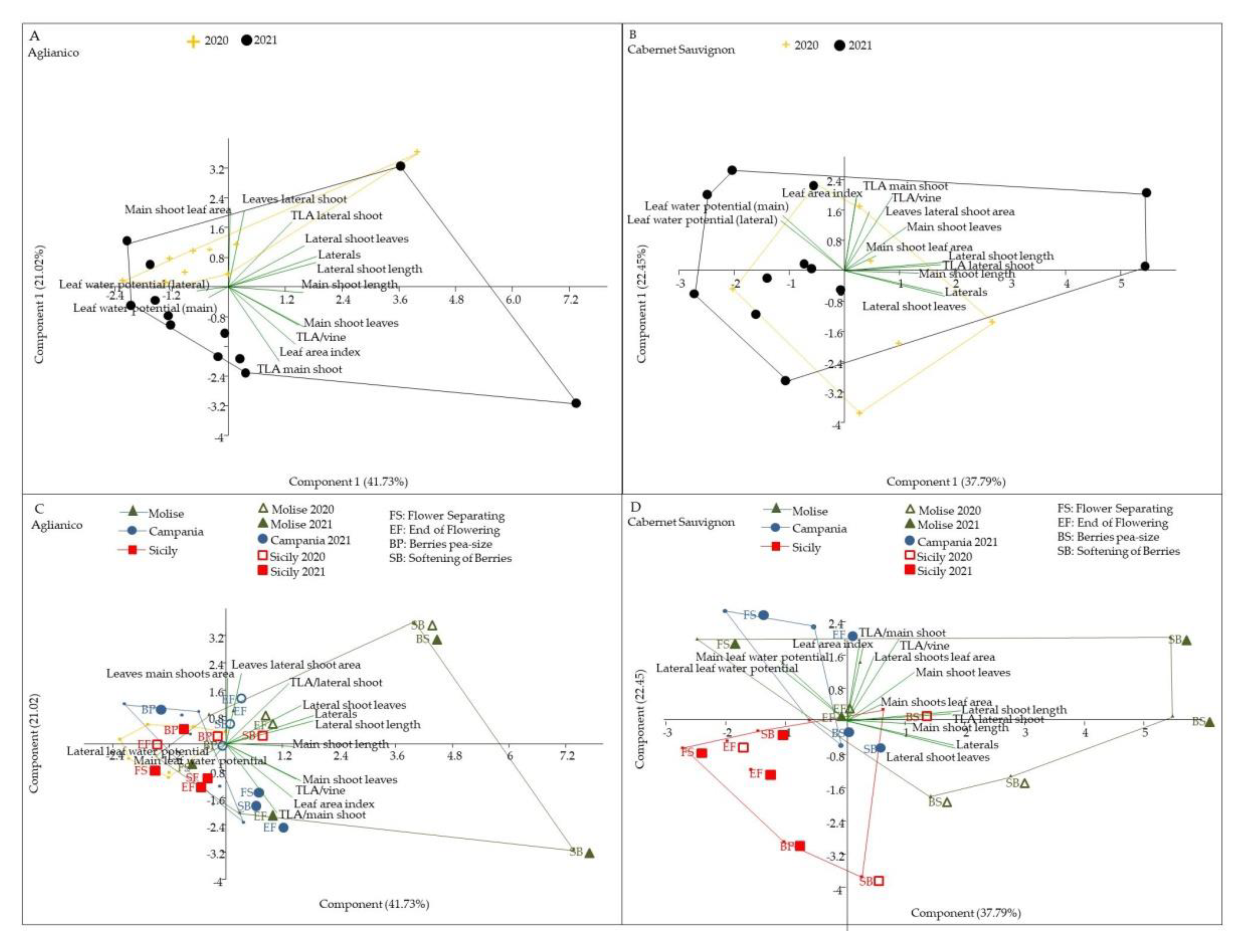
3.6. Vine Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, G.V.; Webb, L.B. Climate change, viticulture, and wine: Challenges and opportunities. J. Wine Res. 2010, 21, 103–106. [Google Scholar] [CrossRef]
- Bonfante, A.; Brillante, L. Terroir analysis and its complexity. OENO One 2022, 56, 375–388. [Google Scholar] [CrossRef]
- Grishkevich, V.; Yanai, I. The genomic determinants of genotype × environment interactions in gene expression. Trends Genet. 2013, 29, 479–487. [Google Scholar] [CrossRef]
- El-Soda, M.; Malosetti, M.; Zwaan, B.J.; Koornneef, M.; Aarts, M.G.M. Genotype × environment interaction QTL mapping in plants: Lessons from Arabidopsis. Trends Plant Sci. 2014, 19, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Des Marais, D.L.; Hernandez, K.M.; Juenger, T.E. Genotype-by-Environment Interaction and Plasticity: Exploring Genomic Responses of Plants to the Abiotic Environment. Ann. Rev. Ecol. Evol. Syst. 2013, 44, 5–29. [Google Scholar] [CrossRef] [Green Version]
- Dal Santo, S.; Tornielli, G.B.; Zenoni, S.; Fasoli, M.; Farina, L.; Anesi, A.; Guzzo, F.; Delledonne, M.; Pezzotti, M. The plasticity of the grapevine berry transcriptome. Genome Biol. 2013, 14, r54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 17. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F. Plantphenotypic plasticity in a changing climate. Trends Plant Sci. 2020, 15, 684–692. [Google Scholar] [CrossRef]
- Ciaccia, C.; Testani, E.; Fiore, A.; Iocola, I.; Di Pierro, M.; Mele, G.; Ferlito, F.; Cutuli, M.; Montemurro, F.; Farina, R.; et al. Organic Agroforestry Long-Term Field Experiment Designing Trough Actors’ Knowledge towards Food System Sustainability. Sustainability 2021, 13, 5532. [Google Scholar] [CrossRef]
- Ferlito, F.; Di Guardo, M.; Allegra, M.; Nicolosi, E.; Continella, A.; La Malfa, S.; Gentile, A.; Distefano, G. Assessment of Chilling Requirement and Threshold Temperature of a Low Chill Pear (Pyrus communis L.) Germplasm in the Mediterranean Area. Horticulturae 2021, 7, 45. [Google Scholar] [CrossRef]
- Nicolosi, E.; Ferlito, F.; Allegra, M.; Cicala, A.; Trovato, F.; La Malfa, S. Influences of aspect and tillage on two winegrape cultivars on Mount Etna. N. Z. J. Crop Hortic. Sci. 2016, 44, 83–102. [Google Scholar] [CrossRef] [Green Version]
- Lo Cicero, L.; Puglisi, I.; Nicolosi, E.; Gentile, A.; Ferlito, F.; Continella, A.; Lo Piero, A.R. Anthocyanin levels and expression analysis of biosynthesis-related genes during ripening of sicilian and international grape berries subjected to leaf removal and water deficit. J. Agric. Sci. 2016, 18, 1333–1344. [Google Scholar]
- Bonfante, A.; Alfieri, S.M.; Albrizio, R.; Basile, A.; De Mascellis, R.; Gambuti, A.; Giorio, P.; Langella, G.; Manna, P.; Monaco, E.; et al. Evaluation of the effects of future climate change on grape quality through a physically based model application: A case study for the Aglianico grapevine in Campania region, Italy. Agric. Syst. 2017, 152, 100–109. [Google Scholar] [CrossRef]
- Nicolosi, E.; Continella, A.; Gentile, A.; Cicala, A.; Ferlito, F. Influence of early leaf removal on autochthonous and international grapevines in Sicily. Sci. Hortic. 2012, 146, 1–6. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C.; Richards, C.L. Phenotypic plasticity and interactions among plants. Ecology 2003, 84, 1115–1128. [Google Scholar] [CrossRef] [Green Version]
- Bennici, S.; Las Casas, G.; Distefano, G.; Di Guardo, M.; Continella, A.; Ferlito, F.; Gentile, A.; La Malfa, S. Elucidating the contribution of wild related species on autochthonous pear germplasm: A case study from Mount Etna. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ferlito, F.; Nicolosi, E.; La Malfa, S.; Cicala, A.; Gentile, A. First characterisation of minor and neglected Vitis vinifera L. cultivars from mount Etna. Hortic. Sci. 2018, 45, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Ferlito, F.; Allegra, M.; Torrisi, B.; Pappalardo, H.; Gentile, A.; La Malfa, S.; Continella, A.; Stagno, F.; Nicolosi, E. Early defoliation effect on water status, fruit yield and must quality of ‘Nerello mascalese’ grapes. Sci. Agric. 2020, 77, 1–10. [Google Scholar] [CrossRef]
- Saitta, D.; Vanella, D.; Ramírez-Cuesta, J.M.; Longo-Minnolo, G.; Ferlito, F.; Consoli, S. Comparison of Orange Orchard Evapotranspiration by Eddy Covariance, Sap Flow, and FAO-56 Methods under Different Irrigation Strategies. J. Irrig. Drain. 2020, 146, 05020002. [Google Scholar] [CrossRef]
- Saitta, D.; Consoli, S.; Ferlito, F.; Torrisi, B.; Allegra, M.; Longo-Minnolo, G.; Ramírez-Cuesta, J.M.; Vanella, D. Adaptation of citrus orchards to deficit irrigation strategies. Agric. Water Manag. 2021, 247, 106734. [Google Scholar] [CrossRef]
- Vanella, D.; Ferlito, F.; Torrisi, B.; Giuffrida, A.; Pappapardo, S.; Saitta, D.; Longo-Minnolo, G.; Consoli, S. Long-term monitoring of deficit irrigation regimes on citrus orchards in Sicily. Chem. Eng. 2022, 52, 1193. [Google Scholar] [CrossRef]
- Castellarin, S.; Matthews, M.A.; Gaspero, G.D.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C. Terroir: The effect of the physical environment on vine growth, grape ripening and wine sensory attributes Managing Wine Quality. Vitic. Wine Qual. 2010, 1, 273–315. [Google Scholar]
- Van Leeuwen, C.; Trégoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? OENO One 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Tisseyre, B.; Ojeda, H.; Guillaume, S. Spatial extrapolation of the vine (Vitis vinifera L.) water status: A first step towards a spatial prediction model. Irrig. Sci. 2010, 28, 143–155. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Interactive effects of deficit irrigation and shoot and cluster thinning on grapevine cv. Tempranillo. Water relations, vine performance and berry and wine composition. Irrig. Sci. 2011, 29, 443–454. [Google Scholar] [CrossRef]
- Bonfante, A.; Agrillo, A.; Albrizio, R.; Basile, A.; Buonomo, R.; De Mascellis, R.; Gambuti, A.; Giorio, P.; Guida, G.; Langella, G.; et al. Functional homogeneous zones (fHZs) in viticultural zoning procedure: An Italian case study on Aglianico vine. Soil 2015, 1, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Brillante, L.; Martínez-Luscher, J.; Yu, R.; Oberholster, A.; Kurtural, S.K. Assessing Spatial Variability of Grape Skin Flavonoids at the Vineyard Scale Based on Plant Water Status Mapping. J. Agric. Food Chem. 2017, 65, 5255–5265. [Google Scholar] [CrossRef]
- Ferlito, F.; Nicolosi, E.; Gentile, A.; Lo Piero, A.R.; Squadrito, M.; Continella, A. Response of four grapevines to water stress and canopy management in an arid environment. Vitis 2014, 53, 73–80. [Google Scholar]
- Ferlito, F.; Distefano, G.; Gentile, A.; Allegra, M.; Lakso, A.N.; Nicolosi, E. Scion–rootstock interactions influence the growth and behaviour of the grapevine root system in a heavy clay soil. Aust. J. Grape Wine Res. 2020, 26, 68–78. [Google Scholar] [CrossRef]
- Las Casas, G.; Ciaccia, C.; Iovino, V.; Ferlito, F.; Torrisi, B.; Lodolini, E.M.; Giuffrida, A.; Catania, R.; Nicolosi, E.; Bella, S. Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants 2022, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Ciaccia, C.; La Torre, A.; Ferlito, F.; Testani, E.; Battaglia, V.; Salvati, L.; Roccuzzo, G. Agroecological Practices and Agro biodiversity: A Case Study on Organic Orange in Southern Italy. Agronomy 2019, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, E.; Iovino, V.; Distefano, G.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Palliotti, A.; Las Casas, G.; Ferlito, F. Mid-Term Effects of Conservative Soil Management and Fruit-Zone Early Leaf Removal Treatments on the Performance of Nerello Mascalese (Vitis vinifera L.) Grapes on Mount Etna (Southern Italy). Agronomy 2021, 11, 1070. [Google Scholar] [CrossRef]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet–Sauvignon) under contrasting water status: Leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Gòmez del Campo, M.; Ruiz, C.; Baeza, P.; Lissarrague, J.R. Drought adaptation strategies of four grapevine cultivars (Vitis vinifera L.): Modification of the properties of the leaf area. J. Int. Sci. Vigne Vin 2003, 37, 131–143. [Google Scholar]
- Toumi, I.; M’Sehli, W.; Bourgou, S.; Jallouli, N.; Bensalem-Fnayou, A.; Ghorbel, A.; Mliki, A. Response of ungrafted and grafted grapevine cultivars and rootstocks (Vitis spp.) to water stress. J. Int. Sci. Vigne Vin 2007, 41, 85–93. [Google Scholar]
- Lovisolo, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Ollat, N.; Tandonnet, J.; Bordenave, L.; Decroocq, S.; Geny, L.; Gaudillere, J.; Fouquet, R.; Barrieu, F.; Hamdi, S. Vigour conferred by rootstock: Hypotheses and direction for research. Bulletin de l’OIV 2003, 76, 581–595. [Google Scholar]
- Rebolledo, M.; Dingkuhn, M.; Courtois, B.; Gibon, Y.; Clement-Vidal, A.; Cruz, D.F.; Duitama, J.; Lorieux, M.; Luquet, D. Phenotypic and genetic dissection of component traits for early vigour in rice using plant growth modelling, sugar content analyses and association mapping. J. Exp. Bot. 2015, 66, 5555–5566. [Google Scholar] [CrossRef] [Green Version]
- Endeshaw, S.T.; Lodolini, E.M.; Neri, D. Effects of olive shoot residues on shoot and root growth of potted olive plantlets. Sci. Hortic. 2015, 182, 31–40. [Google Scholar] [CrossRef]
- Gerzon, E.; Biton, I.; Yaniv, Y.; Zemach, H.; Netzer, Y.; Schwartz, A.; Fait, A.; Ben-Ari, G. Grapevine Anatomy as a Possible Determinant of Isohydric or Anisohydric Behavior. Am. J. Enol. Vitic. 2015, 66, 340–347. [Google Scholar] [CrossRef]
- Hirose, T.; Werger, M.J.A. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 1987, 72, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Guilpard, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Rives, M. Vigour, pruning, cropping in the grapevine (Vitis vinifera L.). Agronomie 2000, 20, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Pallas, B.; Louarn, G.; Cristophe, A.; Lebon, E.; Lecoeur, J. Influence of intra-shoot trophic competition on shoot development in two grapevines cultivars (Vitis vinifera). Physiol. Plant. 2008, 134, 49–63. [Google Scholar] [CrossRef]
- Pallas, B.; Christophe, A.; Lecoeur, J. Are the common assimilate pool and trophic relationships appropriate for dealing with the observed plasticity of grapevine development? Ann. Bot. 2010, 105, 233–247. [Google Scholar] [CrossRef]
- Wilkie, D.J.; Sedgley, M.; Olesen, T. Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008, 59, 3215–3228. [Google Scholar] [CrossRef]
- Srinivasan, C.; Mullins, M.G. Physiology of flowering in the grapevine—A review. Am. J. Enol. Vitic. 1981, 132, 47–63. [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants, BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry Quedlinburg (Germany): Quedlinburg, Germany, 2001.
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research, R package version 2021; Northwestern University: Evanston, IL, USA, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 22 May 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Amerine, M.A.; Winkler, R.A.J. Composition and quality of musts and wines of California grapes. Hilgardia 1944, 15, 493–673. [Google Scholar] [CrossRef] [Green Version]
- Bonfante, A.; Monaco, E.; Langella, G.; Mercogliano, P.; Bucchignani, E.; Manna, P.; Terribile, F. A dynamic viticultural zoning to explore the resilience of terroir concept under climate change. Sci. Total Environ. 2018, 624, 294–308. [Google Scholar] [CrossRef]
- Turri, S. Fenologia e climatologia applicata alla Vitis vinifera: Appunti metodologici. Atti Acc. Ital. Vite Vino 1987, 40, 191. [Google Scholar]
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuno, M.F.; Rodrigues, C.M.; Lopes, M.L.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Hulme, P.E. Phenotypic plasticity and plant invasions: Is it all Jack? Funct. Ecol. 2008, 22, 3–7. [Google Scholar] [CrossRef]
- Matthews, M.; Anderson, M. Reproductive development in grape (Vitis vinifera L.): Responses to seasonal water deficits. Am. J. Enol. Vitic. 1989, 40, 52–60. [Google Scholar]
- Vasconcelos, M.; Greven, M.; Winefield, C.; Trought, M.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar]
- Moriana, A.; Fereres, E. Plant indicators for scheduling irrigation of young olive trees. Irrig. Sci. 2002, 21, 83–90. [Google Scholar]
- Smart, D.R.; Schwass, E.; Lakso, A.; Morano, L. Grapevine Rooting Patterns: A Comprehensive Analysis and a Review. Am. J. Enol. Vitic. 2006, 57, 89–104. [Google Scholar]
- Pellegrino, A.; Lebon, E.; Simmoneau, T.; Wery, J. Towards a simple indicator of water stress in grapevine (Vitis vinifera L.) based on the differential sensitivities of vegetative growth components. Aust. J. Grape Wine Res. 2005, 11, 306–315. [Google Scholar] [CrossRef]
- Clingeleffer, P.R. Plant management research: Status and what it can offer to address challenges and limitations. Aust. J. Grape Wine Res. 2010, 16, 25–32. [Google Scholar] [CrossRef]
- Dry, P. Canopy management for fruitfulness. Aust. J. Grape Wine Res. 2020, 6, 109–115. [Google Scholar] [CrossRef]
- Barbagallo, M.G.; Guidoni, S.; Hunter, J.J. Berry size and qualitative characteristics of Vitis vinifera L. cv Syrah. S. Afr. J. Enol. Vitic. 2011, 32, 129–136. [Google Scholar]
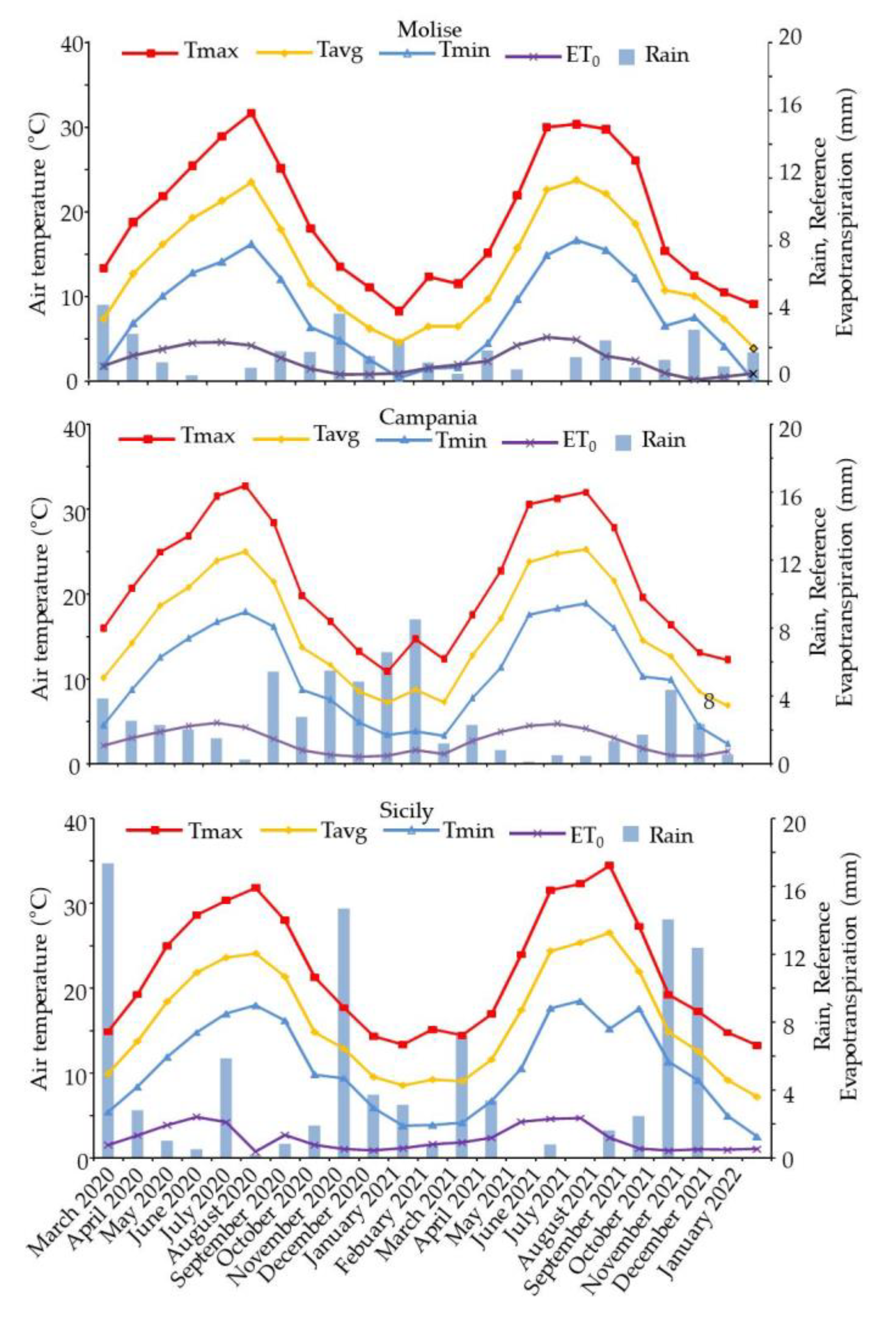

| Site | Latitude | Longitude | Elevation [m] | Chill Hours (Hours < +7 °C) | Growing Degree Days | Soil Moisture [%] | Soil Temperature |
|---|---|---|---|---|---|---|---|
| [°C] | |||||||
| MOL (San Biase, CB) | 41°72′ N | 14°57′ E | 600 | 1560 | 1650 | 55 | 12.8 |
| CAM (Galluccio, CE) | 41°33′ N | 13° 89′ E | 125 | 840 | 2112 | 41 | 17.8 |
| SIC (Zafferana Etnea, CT) | 37°41′ N | 15° 7′ E | 720 | 580 | 2045 | 15 | 16.5 |
| Parameters | Experimental Site | ||
|---|---|---|---|
| MOL (San Biase, CB) | CAM (Galluccio, CE) | SIC (Zafferana Etnea, CT) | |
| pH | 8.3 | 7.1 | 7.0 |
| EC (dS/m) | 0.135 | 0.047 | 0.034 |
| Sand (coarse) (g/kg) | 56.4 | 172.5 | 439.9 |
| Sand (fine) (g/kg) | 234.2 | 297.7 | 478.9 |
| Silt (g/kg) | 195.0 | 215.9 | 68.4 |
| Clay (g/kg) | 514.2 | 313.9 | 12.7 |
| Organic carbon (g/kg) | 10.8 | 3.7 | 25.0 |
| Organic matter (g/kg) | 18.6 | 6.4 | 43.2 |
| Nitrogen (g/kg) | 1.3 | 0.4 | 2.3 |
| C/N ratio | 7.6 | 7.0 | 10.7 |
| Assimilable phosphorus (P2O5) (mg/kg) | 25.9 | 42.5 | 23.9 |
| CSC (meq/100 g) | 31.0 | 17.4 | 17.3 |
| Exchangeable Ca (meq/100 g) | 25.7 | 3.7 | 9.8 |
| Exchangeable Mg (meq/100 g) | 3.4 | 1.8 | 0.7 |
| Exchangeable Na (meq/100 g) | 0.3 | 0.1 | 0.1 |
| Exchangeable K (meq/100 g) | 1.4 | 1.5 | 0.6 |
| Exchangeable Fe (mg/kg) | 14.8 | 49.0 | 61.5 |
| Exchangeable Cu (mg/kg) | 17.0 | 4.5 | 4.5 |
| Exchangeable Zn (mg/kg) | 2.6 | 5.9 | 0.9 |
| Exchangeable Mn (mg/kg) | 6.9 | 56.6 | 0.5 |
| Cultivar | Site | Main Shoots (n) | Bourillon and Crown Shoots (n) | Main Bunches (n) | Bourillon and Crown Inflorescences (n) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| AGL | SIC | 2.53 ± 1.19 C | 3.33 ± 1.23 C | 1.53 ± 1.30 c | 2.40 ±0.74 B | 1.20 ± 0.86 C | 1.73 ± 0.59 C | 0.13 ± 0.09 c | 0.40 ±0.04 C |
| CAM | 14.27 ± 1.91 a | 18.47 ± 1.64 B | 2.93 ± 1.28 b | 5.93 ± 1.39 a | 5.93 ± 4.32 B | 20.52 ± 2.61 B | 1.27 ± 1.10 b | 3.80 ± 1.37 B | |
| MOL | 12.53 ± 1.51 b | 22.93 ± 1.53 A | 4.73 ± 1.28 a | 6.53 ± 1.19 a | 12.13 ± 2.13 A | 26.40 ± 1.30 A | 2.20 ± 0.94 a | 12.07 ± 1.67 A | |
| CAB | SIC | 5.87 ± 1.51 B | 6.67 ± 0.90 B | 3.13 ± 1.41 B | 2.33 ± 0.90 C | 10.13 ± 2.17 B | 8.33 ± 1.45 C | 3.67 ± 1.82 a | 1.13 ± 0.92 C |
| CAM | 19.27 ± 1.67 a | 10.80 ± 1.42 a | 21.60 ± 3.54 a | 5.40 ± 1.40 A | |||||
| MOL | 12.27 ± 2.22 A | 18.60 ± 2.67 a | 5.53 ± 1.81 A | 9.07 ± 2.02 b | 13.07 ± 1.79 A | 19.13 ± 2.64 b | 3.00 ± 1.41 a | 3.13 ± 0.74 B | |
| Cultivar | Site | Blind buds of main nodes (n) | Main buds (n) | Latent bud shoots (n) | Latent bud bunches (n) | ||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| AGL | SIC | 1.00 ± 0.53 B | 0.33 ± 0.04 b | 3.53 ± 1.25 c | 3.67± 1.29 C | 2.67 ± 0.98 a | 0.53 ± 0.02 B | 0.40 ± 0.83 a | 0.13 ± 0.06 a |
| CAM | 2.47 ± 0.83 a | 1.00± 0.76 a | 16.76 ± 2.19 a | 19.47 ± 1.77 B | 2.13 ± 0.92 a | 5.73 ± 1.28 a | 0.80 ± 0.09 a | 0.33 ± 0.09 a | |
| MOL | 2.13 ± 0.99 a | 0.33 ± 0.05 b | 14.67 ± 2.16 b | 23.27 ± 1.49 A | 2.00 ± 0.76 a | 5.60 ± 0.99 a | 0.80 ± 0.68 a | 0.47 ± 0.09 a | |
| CAB | SIC | 2.67 ± 1.59 a | 1.93 ± 0.96 a | 8.53 ± 1.92 B | 8.60± 1.40 B | 9.20 ± 3.12 A | 5.27 ± 1.16 a | 2.80 ± 1.27 a | 1.00 ± 0.76 a |
| CAM | 1.13 ± 0.92 b | 20.40 ± 1.99 a | 5.87 ± 1.41 a | 0.73 ± 0.09 ab | |||||
| MOL | 0.93 ± 0.22 b | 0.73 ± 0.09 b | 13.20 ± 2.60 A | 19.33 ± 2.82 a | 4.73 ± 1.53 B | 0.60 ± 0.03 B | 0.67 ± 0.28 b | 0.20 ± 0.08 b | |
| Parameters | AGL | |||||
|---|---|---|---|---|---|---|
| MOL | CAM | SIC | ||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| Yield (t/ha) | 19.53 ± 5.39 A | 26.16 ± 3.76 a | 4.86 ± 1.63 B | 14.34 ± 5.11 b | 2.01 ± 0.89 B | 4.09 ± 0.87 b |
| Bunch weight (g) | 558.60 ± 161.5 A | 345.80 ± 42.08 n.s. | 348 ± 56.35 b | 298.20 ± 104.28 n.s. | 190 ± 15.41 B | 315.40 ± 85.23 n.s. |
| Bunch length (cm) | 16.80 ± 0.84 n.s. | 19.40 ± 1.82 n.s. | 16.80 ± 2.49 n.s. | 19.20 ± 1.10 n.s. | 16 ± 1.87 n.s. | 18.20 ± 1.30 n.s. |
| Berry number | 147.88 ± 88.59 n.s. | 126.50 ± 13.10 n.s. | 129.96 ± 25.11 n.s. | 129.80 ± 51.69 n.s. | 79.80 ± 7.66 n.s. | 106.6 ± 24.47 n.s. |
| Berry weight (g) | 2.64 ± 0.22 a | 2.59 ± 0.22 b | 2.58 ± 0.17 a | 2.19 ± 0.27 a | 2.15 ± 0.03 b | 2.38 ± 0.19 ab |
| Rachis weight (g) | 16.80 ± 1.64 a | 18.80 ± 2.77 a | 14.60 ± 2.07 ab | 17.40 ± 1.14 ab | 18.46 ± 1.50 b | 14.44 ± 1.24 b |
| CAB | ||||||
| MOL | CAM | SIC | ||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| Yield (t/ha) | 13.91 ± 2.74 A | 24.61 ± 4.47 A | - | 18.36 ± 8.56 b | 17.15 ± 2.97 B | 19.50 ±4.58 B |
| Bunch weight (g) | 352.60 ± 74.82 a | 444.6 ± 64.92 n.s. | - | 318.80 ± 102.27 n.s. | 258.40 ± 25.38 b | 346.60 ± 56.68 n.s. |
| Bunch length (cm) | 20.60 ± 2.07 b | 18.60 ± 1.95 n.s. | - | 19.20 ± 1.10 n.s. | 17.60 ± 1.52 a | 19.00 ± 1.87 n.s. |
| Berry number | 169.20 ± 35.97 a | 154.00 ± 15.07 n.s. | - | 161.40 ± 69.09 n.s. | 125.80 ± 19.23 b | 238.85 ± 74.35 n.s. |
| Berry weight (g) | 1.97 ± 0.17 n.s. | 2.75 ± 0.18 A | - | 1.89 ± 0.22 b | 1.90 ± 0.12 n.s. | 1.50 ± 0.23 c |
| Rachis weight (g) | 18.60 ±1.82 b | 19.00 ± 2.35 n.s. | - | 20.60 ±3.05 n.s. | 21.20± 1.64 a | 17.40 ±1.82 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolosi, E.; Sicilia, A.; Ferlito, F.; Bonfante, A.; Monaco, E.; Lo Piero, A.R. Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions. Agriculture 2022, 12, 1660. https://doi.org/10.3390/agriculture12101660
Nicolosi E, Sicilia A, Ferlito F, Bonfante A, Monaco E, Lo Piero AR. Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions. Agriculture. 2022; 12(10):1660. https://doi.org/10.3390/agriculture12101660
Chicago/Turabian StyleNicolosi, Elisabetta, Angelo Sicilia, Filippo Ferlito, Antonello Bonfante, Eugenia Monaco, and Angela Roberta Lo Piero. 2022. "Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions" Agriculture 12, no. 10: 1660. https://doi.org/10.3390/agriculture12101660
APA StyleNicolosi, E., Sicilia, A., Ferlito, F., Bonfante, A., Monaco, E., & Lo Piero, A. R. (2022). Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions. Agriculture, 12(10), 1660. https://doi.org/10.3390/agriculture12101660










