Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Generation of Transgenic Rice Plants
2.3. Histological Analysis of Anther Morphology
2.4. Quantitative Real–Time PCR (qRT–PCR)
2.5. Subcellular Localization of OsCCR18
2.6. Library Construction and Transcriptome Sequencing
2.7. Sequence Mapping and Enrichment Analysis
3. Results
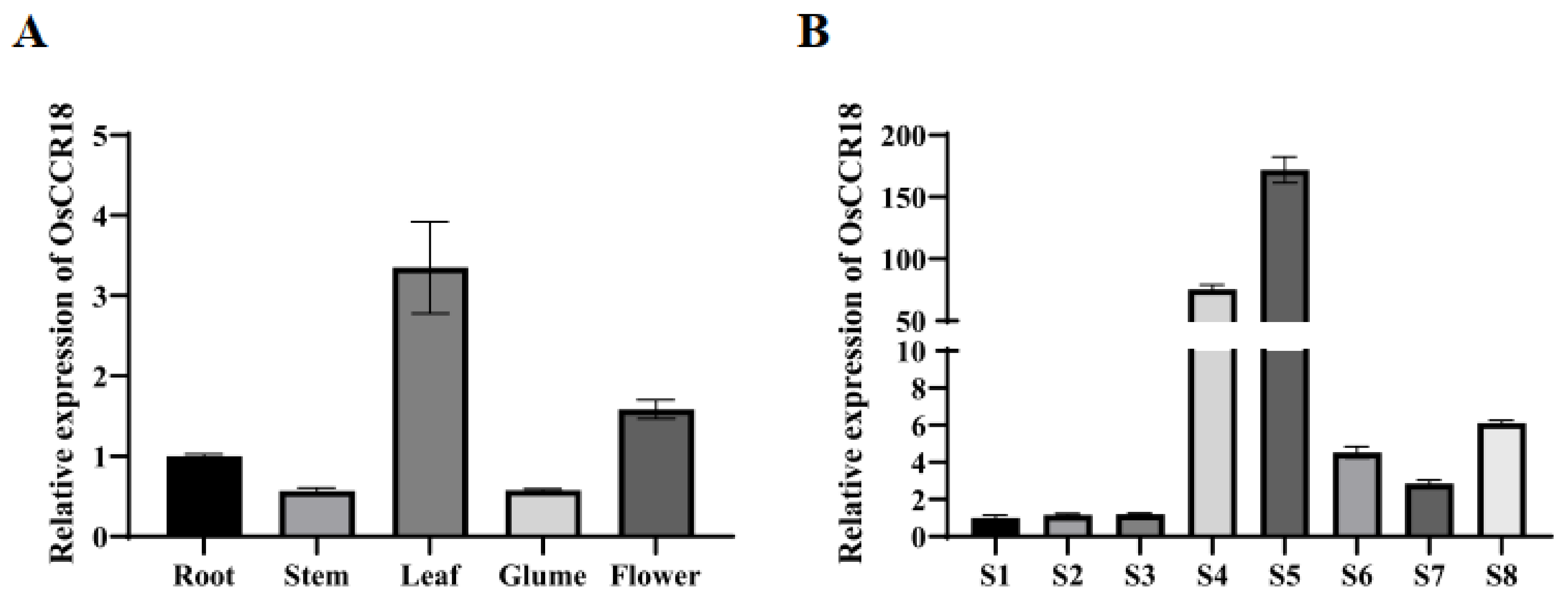
3.1. OsCCR18 Exhibits Tissue– and Developmental Stage–Specific Expression Patterns in Rice
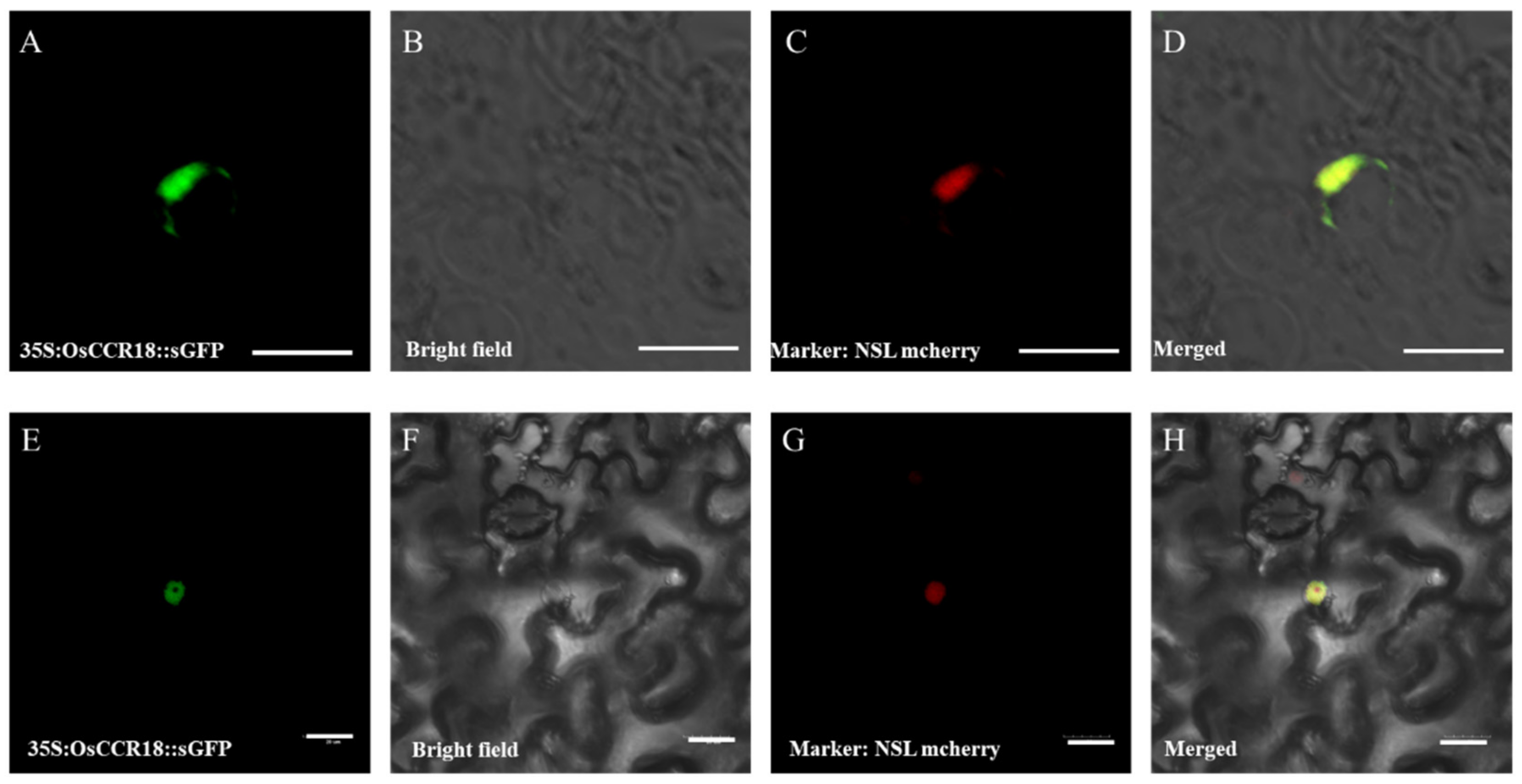
3.2. OsCCR18 Is Located in the Nucleus
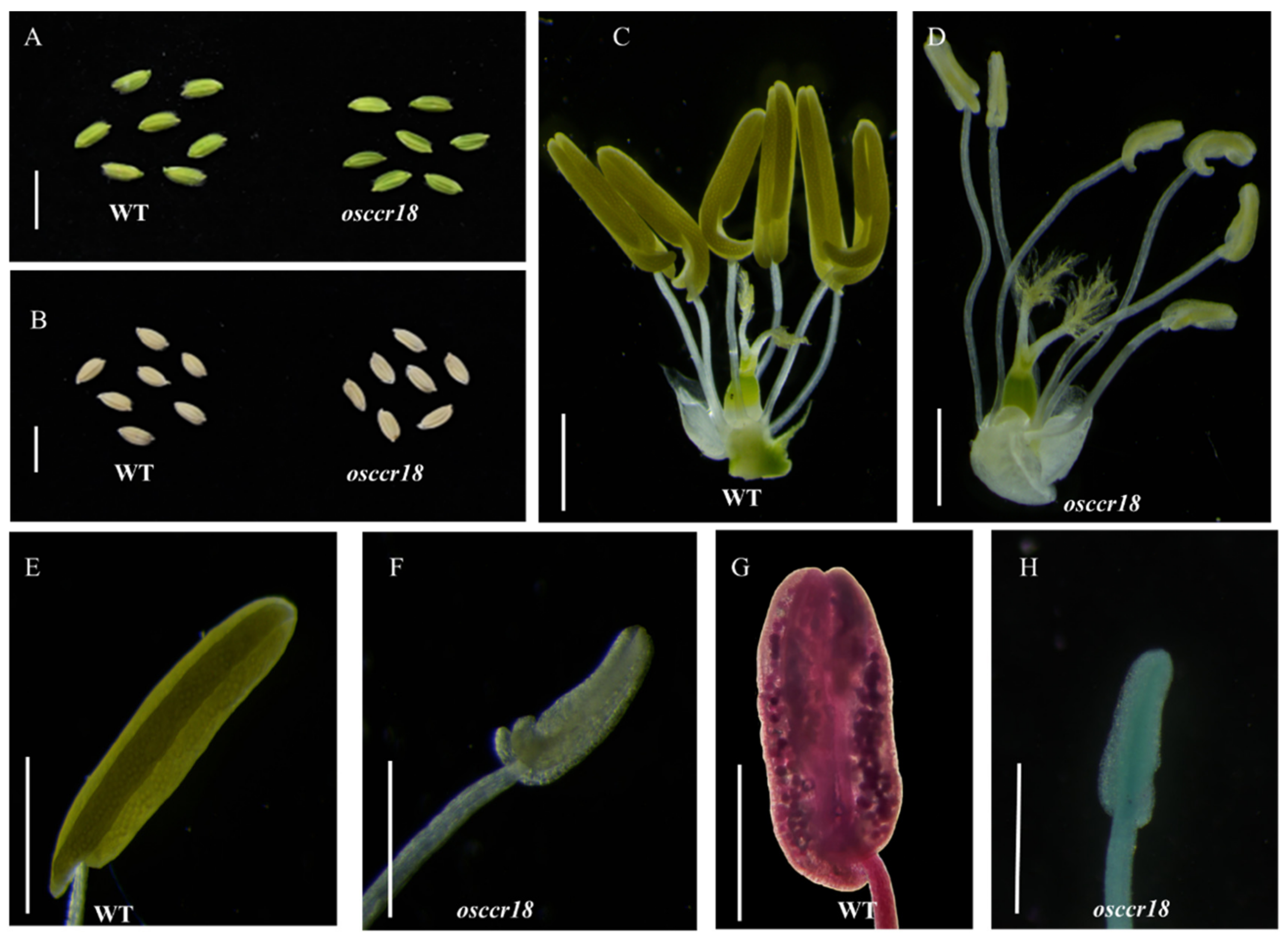
3.3. Knockout of OsCCR18 Results in Complete Male Sterility
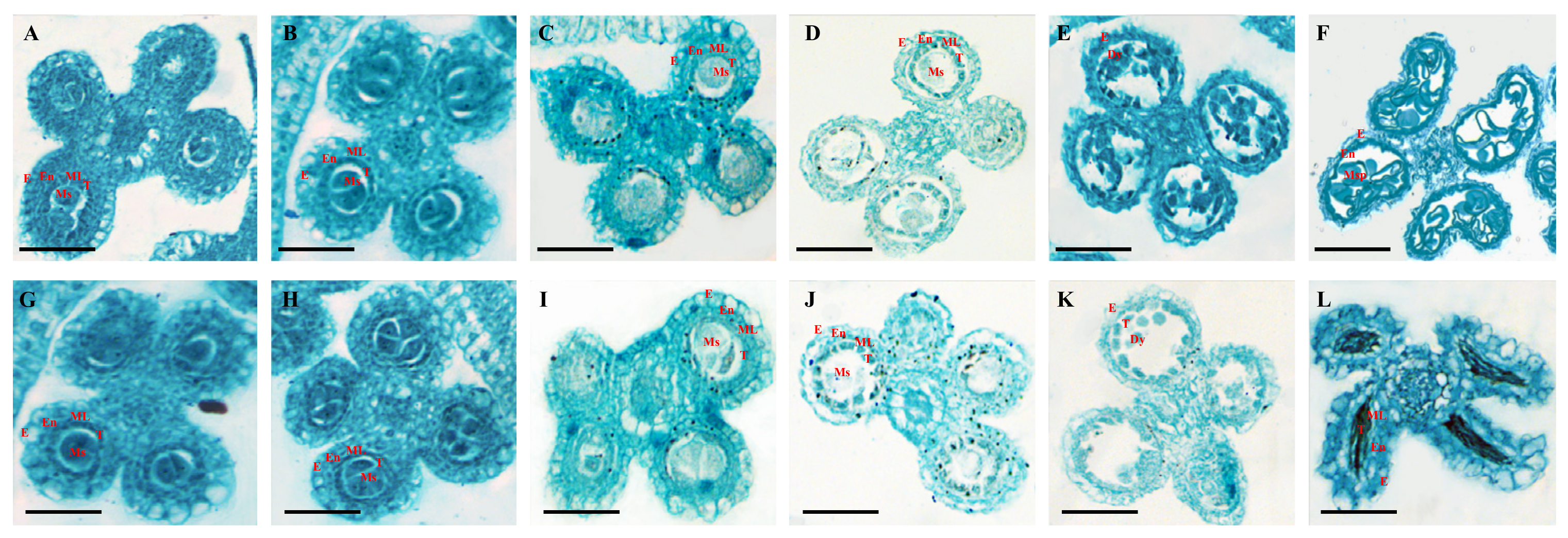
3.4. Abnormal Tapetum Degradation Contributes to the Male Sterility of the Osccr18 Mutants
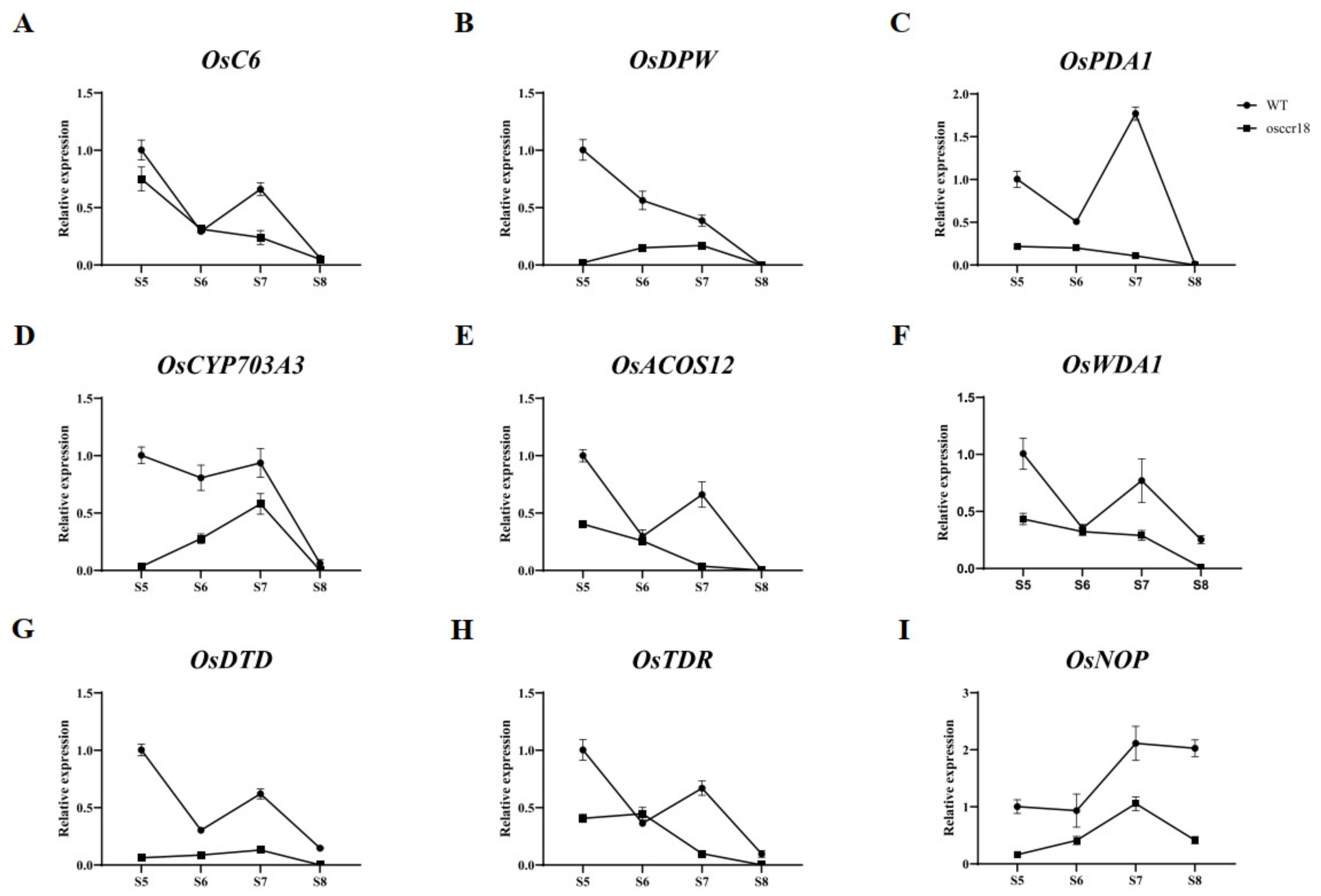
3.5. OsCCR18 Regulates the Expression of anther Development–Related Genes
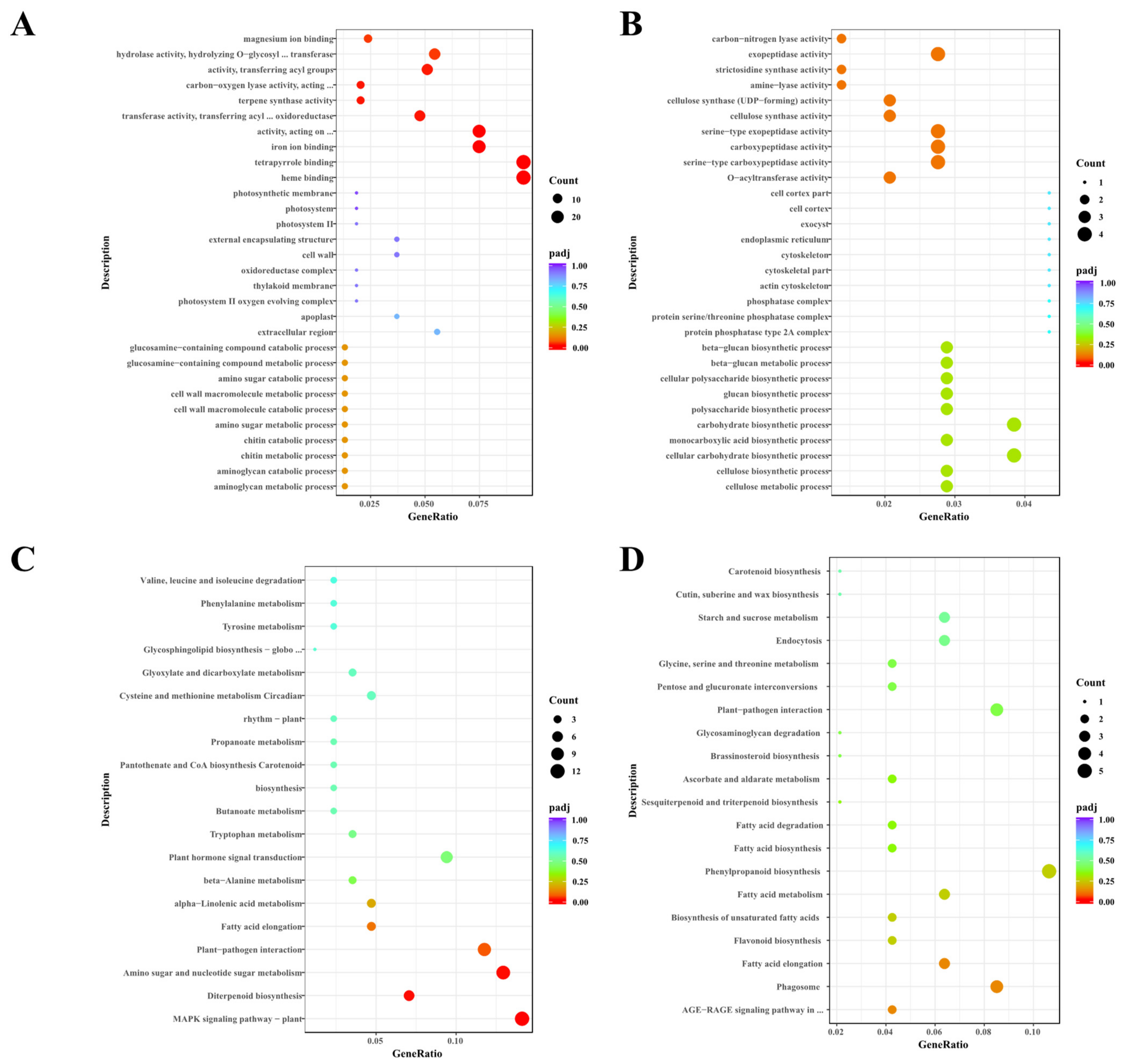
3.6. Genes Involved in Fatty Acid Metabolism Are Differentially Expressed in anther at the S9 Stage between osccr18 and WT Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Li, Z.; Liu, G.; Jiang, Y.; Maurer, H.P.; Würschum, T.; Mock, H.P.; Matros, A.; Ebmeyer, E.; Schachschneider, R.; et al. Genome-based establishment of a high-yielding heterotic pattern for hybrid wheat breeding. Proc. Natl. Acad. Sci. USA 2015, 112, 15624–15629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Fang, F.; Zhen, Y.; Liao, X. Food safety and rice production in China. Res. Agric. Mod. 2005, 26, 85–88. [Google Scholar]
- Xu, L.; Yuan, S.; Man, J. Changes in rice yield and yield stability in China during the past six decades. J. Sci. Food Agric. 2020, 100, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yu, H.; Wang, B.; Li, J. Retrospective and perspective of rice breeding in China. J. Genet. Genom. 2018, 45, 603–612. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, G.; Datta, K.; Xu, C.; He, Y.; Zhang, Q.; Khush, G.S.; Datta, S.K. Field performance of transgenic elite commercial hybrid rice expressing bacillus thuringiensis delta-endotoxin. Nat. Biotechnol. 2000, 18, 1101–1104. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, X.; Zhu, L. Cytological analysis and genetic control of rice anther development. J. Genet. Genom. 2011, 38, 379–390. [Google Scholar] [CrossRef]
- Nonomura, K.I.; Miyoshi, K.; Eiguchi, M.; Suzuki, T.; Miyao, A.; Hirochika, H.; Kurata, N. The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 2003, 15, 1728–1739. [Google Scholar] [CrossRef]
- Li, N.; Zhang, D.S.; Liu, H.S.; Yin, C.S.; Li, X.X.; Liang, W.Q.; Yuan, Z.; Xu, B.; Chu, H.W.; Wang, J.; et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 2006, 18, 2999–3014. [Google Scholar] [CrossRef]
- Huang, M.D.; Hsing, Y.I.C.; Huang, A.H.C. Transcriptomes of the anther sporophyte: Availability and uses. Plant Cell Physiol. 2011, 52, 1459–1466. [Google Scholar] [CrossRef]
- Guilford, W.J.; Schneider, D.M.; Labovitz, J.; Opella, S.J. High resolution solid state 13C NMR spectroscopy of sporopollenins from different plant taxa. Plant Physiol. 1988, 86, 134–136. [Google Scholar] [CrossRef]
- Niu, B.X.; He, F.R.; He, M.; Ren, D.; Chen, L.T.; Liu, Y.G. The ATP-binding cassette transporter OsABCG15 is required for anther development and pollen fertility in rice. J. Integr. Plant Biol. 2013, 55, 710–720. [Google Scholar] [CrossRef]
- Qin, P.; Tu, B.; Wang, Y.; Deng, L.; Quilichini, T.D.; Li, T.; Wang, H.; Ma, B.; Li, S. ABCG15 encodes an ABC transporter protein, and is essential for post-meiotic anther and pollen exine development in rice. Plant Cell Physiol. 2013, 54, 138–154. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.; Zhao, G.; Zhang, D.; Liang, W. Post-meiotic deficient anther1 (PDA1) encodes an ABC transporter required for the development of anther cuticle and pollen exine in rice. J. Plant Biol. 2013, 56, 59–68. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, W.; Yin, C.; Zong, J.; Gu, F.; Zhang, D. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol. 2010, 154, 149–162. [Google Scholar] [CrossRef]
- Yi, J.; Kim, S.R.; Lee, D.Y.; Moon, S.; Lee, Y.S.; Jung, K.H.; Hwang, I.; An, G. The rice gene DEFECTIVE TAPETUM AND MEIOCYTES 1 (DTM1) is required for early tapetum development and meiosis. Plant J. 2012, 70, 256–270. [Google Scholar] [CrossRef]
- Ji, C.; Li, H.; Chen, L.; Xie, M.; Wang, F.; Chen, Y.; Liu, Y.G. A novel rice bHLH transcription factor, DTD, acts coordinately with TDR in controlling tapetum function and pollen development. Mol. Plant 2013, 6, 1715–1718. [Google Scholar] [CrossRef]
- Yi, J.; Moon, S.; Lee, Y.S.; Zhu, L.; Liang, W.; Zhang, D.; Jung, K.H.; An, G. Defective Tapetum Cell Death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol. 2016, 170, 1611–1623. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, Y.; Tang, D.; Shi, W.; Zhang, D.; Du, G.; Zhou, Y.; Liang, G.; Li, Y.; Cheng, Z. The zinc finger protein DCM1 is required for male meiotic cytokinesis by preserving callose in rice. PLoS Genet. 2018, 14, e1007769. [Google Scholar] [CrossRef]
- Zou, T.; Xiong, P.; Zhou, F.; Zhou, D.; Chen, H.; Li, G.; Peng, K.; Zheng, K.; Han, Y.; Zhang, K.; et al. Grass-specific ABERRANT MICROSPORE DEVELOPMENT 1 is required for maintaining pollen fertility in rice. Plant J. 2022. [Google Scholar] [CrossRef]
- Jung, K.H.; Han, M.J.; Lee, D.Y.; Lee, Y.S.; Schreiber, L.; Franke, R.; Faust, A.; Yephremov, A.; Saedler, H.; Kim, Y.W.; et al. Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 2006, 18, 3015–3032. [Google Scholar] [CrossRef]
- Li, H.; Pinot, F.; Sauveplane, V.; Werck-Reichhart, D.; Diehl, P.; Schreiber, L.; Franke, R.; Zhang, P.; Chen, L.; Gao, Y.; et al. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 2010, 22, 173–190. [Google Scholar] [CrossRef]
- Xu, D.; Shi, J.; Rautengarten, C.; Yang, L.; Qian, X.; Uzair, M.; Zhu, L.; Luo, Q.; An, G.; Waßmann, F.; et al. Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol. 2017, 173, 240–255. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Sun, L.; Zhang, P.; Liu, L.; Yu, P.; Xuan, D.; Xiang, X.; Wu, W.; Cao, L.; et al. Identification of cyp703a3-3 and analysis of regulatory role of CYP703A3 in rice anther cuticle and pollen exine development. Gene 2018, 649, 63–73. [Google Scholar] [CrossRef]
- Xue, J.S.; Zhang, B.; Zhan, H.; Lv, Y.L.; Jia, X.L.; Wang, T.; Yang, N.Y.; Lou, Y.X.; Zhang, Z.B.; Hu, W.J.; et al. Phenylpropanoid derivatives are essential components of sporopollenin in vascular plants. Mol. Plant 2020, 13, 1644–1653. [Google Scholar] [CrossRef]
- Costa, M.A.; Collins, R.E.; Anterola, A.M.; Cochrane, F.C.; Davin, L.B.; Lewis, N.G. An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in Arabidopsis thaliana and limitations thereof. Phytochemistry 2003, 64, 1097–1112. [Google Scholar] [CrossRef]
- Li, L.; Cheng, X.; Lu, S.; Nakatsubo, T.; Umezawa, T.; Chiang, V.L. Clarification of cinnamoyl co-enzyme A reductase catalysis in monolignol biosynthesis of aspen. Plant Cell Physiol. 2005, 46, 1073–1082. [Google Scholar] [CrossRef]
- Kawasaki, T.; Koita, H.; Nakatsubo, T.; Hasegawa, K.; Wakabayashi, K.; Takahashi, H.; Umemura, K.; Umezawa, T.; Shimamoto, K. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ruel, K.; Berrio-Sierra, J.; Derikvand, M.M.; Pollet, B.; Thévenin, J.; Lapierre, C.; Jouanin, L.; Joseleau, J.P. Impact of CCR1 silencing on the assembly of lignified secondary walls in Arabidopsis thaliana. New Phytol. 2009, 184, 99–113. [Google Scholar] [CrossRef]
- Goujon, T.; Ferret, V.; Mila, I.; Pollet, B.; Ruel, K.; Burlat, V.; Joseleau, J.P.; Barrière, Y.; Lapierre, C.; Jouanin, L. Down-regulation of the AtCCR1 gene in Arabidopsis thaliana: Effects on phenotype, lignins and cell wall degradability. Planta 2003, 217, 218–228. [Google Scholar] [CrossRef]
- Kawasaki, T.; Henmi, K.; Ono, E.; Hatakeyama, S.; Iwano, M.; Satoh, H.; Shimamoto, K. The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 1999, 96, 10922–10926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Wang, M.; Xie, L.; Wu, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Jia, Y.; Liu, Q. The miR528-D3 module regulates plant height in rice by modulating the gibberellin and abscisic acid metabolisms. Rice 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, J.L.; Longkumer, T.; Kohli, A. Rice protoplast isolation and transfection for transient gene expression analysis. Methods Mol. Biol. 2021, 2238, 313–324. [Google Scholar] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Zhang, D.; Wilson, Z.A. Stamen specification and anther development in rice. Chin. Sci. Bull. 2009, 54, 2342–2353. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D. Biosynthesis of anther cuticle and pollen exine in rice. Plant Signal Behav. 2010, 5, 1121–1123. [Google Scholar] [CrossRef]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Park, H.L.; Bhoo, S.H.; Kwon, M.; Lee, S.W.; Cho, M.H. Biochemical and expression analyses of the rice cinnamoyl-CoA reductase gene family. Front. Plant Sci. 2017, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, E.; Hawkins, S.; Van Doorsselaere, J.; Piquemal, J.; Goffner, D.; Poeydomenge, O.; Boudet, A.M.; Grima-Pettenati, J. Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: Cloning, expression and phylogenetic relationships. Plant J. 1997, 11, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, X.; Zhang, Z.; Feng, D.; Zhang, Q.; Han, L.; Wu, J.; Lu, T. GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol. 2015, 56, 497–509. [Google Scholar] [CrossRef]
- Ischebeck, T. Lipids in pollen—They are different. Biochim. Biophys. Acta 2016, 1861, 1315–1328. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, J.; Yang, X. Role of lipid metabolism in plant pollen exine development. Subcell Biochem. 2016, 86, 315–337. [Google Scholar]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid metabolism: Critical roles in male fertility and other aspects of reproductive development in plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Z.; An, X.; Long, Y.; Xue, X.; Xie, K.; Ma, B.; Zhang, D.; Guan, Y.; Niu, C.; et al. Normal structure and function of endothecium chloroplasts maintained by ZmMs33-mediated lipid biosynthesis in tapetal cells are critical for anther development in maize. Mol. Plant 2020, 13, 1624–1643. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Liu, X.; Zhu, T.; Xie, K.; Hou, Q.; Yan, T.; Niu, C.; Zhang, S.; Yang, M.; et al. ZmFAR1 and ZmABCG26 regulated by microrna are essential for lipid metabolism in maize anther. Int. J. Mol. Sci. 2021, 22, 7916. [Google Scholar] [CrossRef]
- Zhao, J.; Long, T.; Wang, Y.; Tong, X.; Tang, J.; Li, J.; Wang, H.; Tang, L.; Li, Z.; Shu, Y.; et al. RMS2 encoding a GDSL lipase mediates lipid homeostasis in anthers to determine rice male fertility. Plant Physiol. 2020, 182, 2047–2064. [Google Scholar] [CrossRef]
- An, X.; Dong, Z.; Tian, Y.; Xie, K.; Wu, S.; Zhu, T.; Zhang, D.; Zhou, Y.; Niu, C.; Ma, B.; et al. ZmMs30 encoding a novel gdsl lipase is essential for male fertility and valuable for hybrid breeding in maize. Mol. Plant 2019, 12, 343–359. [Google Scholar] [CrossRef]
- Qu, A.; Xu, Y.; Yu, X.; Si, Q.; Xu, X.; Liu, C.; Yang, L.; Zheng, Y.; Zhang, M.; Zhang, S.; et al. Sporophytic control of anther development and male fertility by glucose-6-phosphate/phosphate translocator 1 (OsGPT1) in rice. J. Genet. Genom. 2021, 48, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, P.; Knappe, S.; Geimer, S.; Fischer, K.; Schulz, B.; Unte, U.S.; Rosso, M.G.; Ache, P.; Flügge, U.I.; Schneider, A. The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 2005, 17, 760–775. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Jiang, X.; Wen, J.; Huang, M.; Wang, Y.; Wang, M.; Dong, H.; Liu, Q. Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica). Agriculture 2022, 12, 1685. https://doi.org/10.3390/agriculture12101685
Pan X, Jiang X, Wen J, Huang M, Wang Y, Wang M, Dong H, Liu Q. Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica). Agriculture. 2022; 12(10):1685. https://doi.org/10.3390/agriculture12101685
Chicago/Turabian StylePan, Xiangjian, Xiaoyue Jiang, Junli Wen, Menghan Huang, Yanqing Wang, Mei Wang, Hui Dong, and Qingpo Liu. 2022. "Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica)" Agriculture 12, no. 10: 1685. https://doi.org/10.3390/agriculture12101685
APA StylePan, X., Jiang, X., Wen, J., Huang, M., Wang, Y., Wang, M., Dong, H., & Liu, Q. (2022). Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica). Agriculture, 12(10), 1685. https://doi.org/10.3390/agriculture12101685





