Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koptsik, G.N. Modern approaches to remediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 707–722. [Google Scholar] [CrossRef]
- Lu, Y.; Nakicenovic, N.; Visbeck, M.; Stevance, A.S. Policy: Five priorities for the UN sustainable development goals. Nature 2015, 520, 432–433. [Google Scholar] [CrossRef] [Green Version]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Hatano, R. Soil organic carbon and soil erodibility response to various land-use changes in northern Thailand. Catena 2022, 219, 106595. [Google Scholar] [CrossRef]
- Lal, R.; Miller, F.P.; Logan, T.J. Are intensive agricultural practices environmentally and ethically sound? J. Agric. Ethics 1988, 1, 193–210. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Kumar, S.; Bauddh, K.; Dwivedi, N.; Shukla, P.; Singh, D.P.; Barman, S.C. Toxicity assessment and accumulation of metals in radish irrigated with battery manufacturing industry effluent. Int. J. Veg. Sci. 2015, 00, 1–13. [Google Scholar] [CrossRef]
- Nevedrov, N.; Protsenko, E. Technologies for optimization of ecosystem services and functions of soils under anthropogenic impact in urban areas. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 177, p. 012009. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Gong, Y.; Chen, G.; Chen, L.; Tian, X. Highly concentrated amino-modified biochars using a plasma: Evolution of surface composition and porosity for heavy metal capture. Carbon 2020, 168, 515–527. [Google Scholar] [CrossRef]
- Yanez Espinosa, L.; Briones Gallardo, R.; Flores, J.; Alvarez del Castillo, E. Effect of heavy metals on seed germination and seedling development of Nama aff. stenophylla collected on the slope of a mine tailing dump. Int. J. Phytoremediation 2020, 22, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Tomei, M.C.; Daugulis, A.J. Ex situ bioremediation of contaminated soils: An overview of conventional and innovative technologies. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2107–2139. [Google Scholar] [CrossRef]
- Qin, G.; Gong, D.; Fan, M.Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, F.; Ravnskov, S.; Rubæk, G.H.; Sun, Z.; Andersen, M.N. Impact of wood biochar and its interactions with mycorrhizal fungi, phosphorus fertilization and irrigation strategies on potato growth. J. Agron. Crop Sci. 2017, 203, 131–145. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Dudnikova, T.; Barbashev, A.; Mazarji, M.; Chernikova, N.; Lobzenko, I.; Deryabkina, I.; Kizilkaya, R. Influence of carbon-containing and mineral sorbents on the toxicity of soil contaminated with benzo [a] pyrene during phytotesting. Environ. Geochem. Health 2022, 44, 179–193. [Google Scholar] [CrossRef]
- Karam, D.S.; Nagabovanalli, P.; Rajoo, K.S.; Ishak, C.F.; Abdu, A.; Rosli, Z.; Muharam, F.M.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Li, Y.; Ding, X.; Guo, Y.; Rong, C.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. A new method of comprehensive utilization of rice husk. J. Hazard. Mater. 2011, 186, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource utilization of rice husk biomass: Preparation of MgO flake-modified biochar for simultaneous removal of heavy metals from aqueous solution and polluted soil. Environ. Pollut. 2022, 310, 119869. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Alias, A.; Talib, N.; Rashid, Z.; Ghani, W.A.W.A.K. Characteristics of rice husk biochar blended with coal fly ash for potential sorption material. Malays. J. Anal. Sci. 2018, 22, 326–332. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Burachevskaya, M.; Minkina, T.; Zamulina, I.; Fedorenko, A.; Kalinichenko, V.; Lobzenko, I.; Sushkova, S. Effect of biochar on the lead mobility in Haplic Chernozem. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 578, p. 012012. [Google Scholar] [CrossRef]
- McClements, D.J. The future of food colloids: Next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Lu, T.; Wang, Y.; Huang, H.; Chen, Y. Influence of pyrolysis temperature and holding time on properties of biochar derived from medicinal herb (radix isatidis) residue and its effect on soil CO2 emission. J. Anal. Appl. Pyrolysis 2014, 110, 277–284. [Google Scholar] [CrossRef]
- Kirti, N.; Tekade, S.P.; Tagade, A.; Sawarkar, A.N. Pyrolysis of pigeon pea (Cajanus cajan) stalk: Kinetics and thermodynamic analysis of degradation stages via isoconversional and master plot methods. Bioresour. Technol. 2022, 347, 126440. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Cheng, L.; Andserson, B.; Zhao, X.; Wang, D.; Ding, A. Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 2018, 63, 156–173. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Y.; Qiu, Y.; Che, L.; Xue, C. Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Sci. Total Environ. 2019, 646, 1281–1289. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.S.; Tang, C.S.; Gu, K.; Shi, B. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2019, 9, 135–148. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Gu, J.; Yao, J.; Duran, R.; Sunahara, G.; Zhou, X. Alteration of mixture toxicity in nonferrous metal mine tailings treated by biochar. J. Environ. Manag. 2020, 265, 110511. [Google Scholar] [CrossRef]
- Alvarez-Rogel, J.; Gomez, M.D.T.; Conesa, H.M.; Parraga-Aguado, I.; Gonzalez-Alcaraz, M.N. Biochar from sewage sludge and pruning trees reduced porewater Cd, Pb and Zn concentrations in acidic, but not basic, mine soils under hydric conditions. J. Environ. Manag. 2018, 223, 554–565. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Liao, P.; Xiong, Q.; Deng, X.; Gao, H.; Zhang, H. Effects of biochar on the dynamic immobilization of Cd and Cu and rice accumulation in soils with different acidity levels. J. Clean. Prod. 2022, 372, 133730. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chang, C.Y.; Wang, S.Y.; Chang, C.F.; Chien, S.F.; Sun, H.F. Utilization of agricultural waste corn cob for the preparation of carbon adsorbent. J. Environ. Sci. Health Part B 2001, 36, 677–686. [Google Scholar] [CrossRef]
- Shehrawat, P.S.; Sindhu, N. Agricultural waste utilization for healthy environment and sustainable lifestyle. Ann. Agric. Biol. Res. 2015, 20, 110–114. [Google Scholar]
- Kalus, K.; Koziel, J.A.; Opaliński, S. A review of biochar properties and their utilization in crop agriculture and livestock production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef]
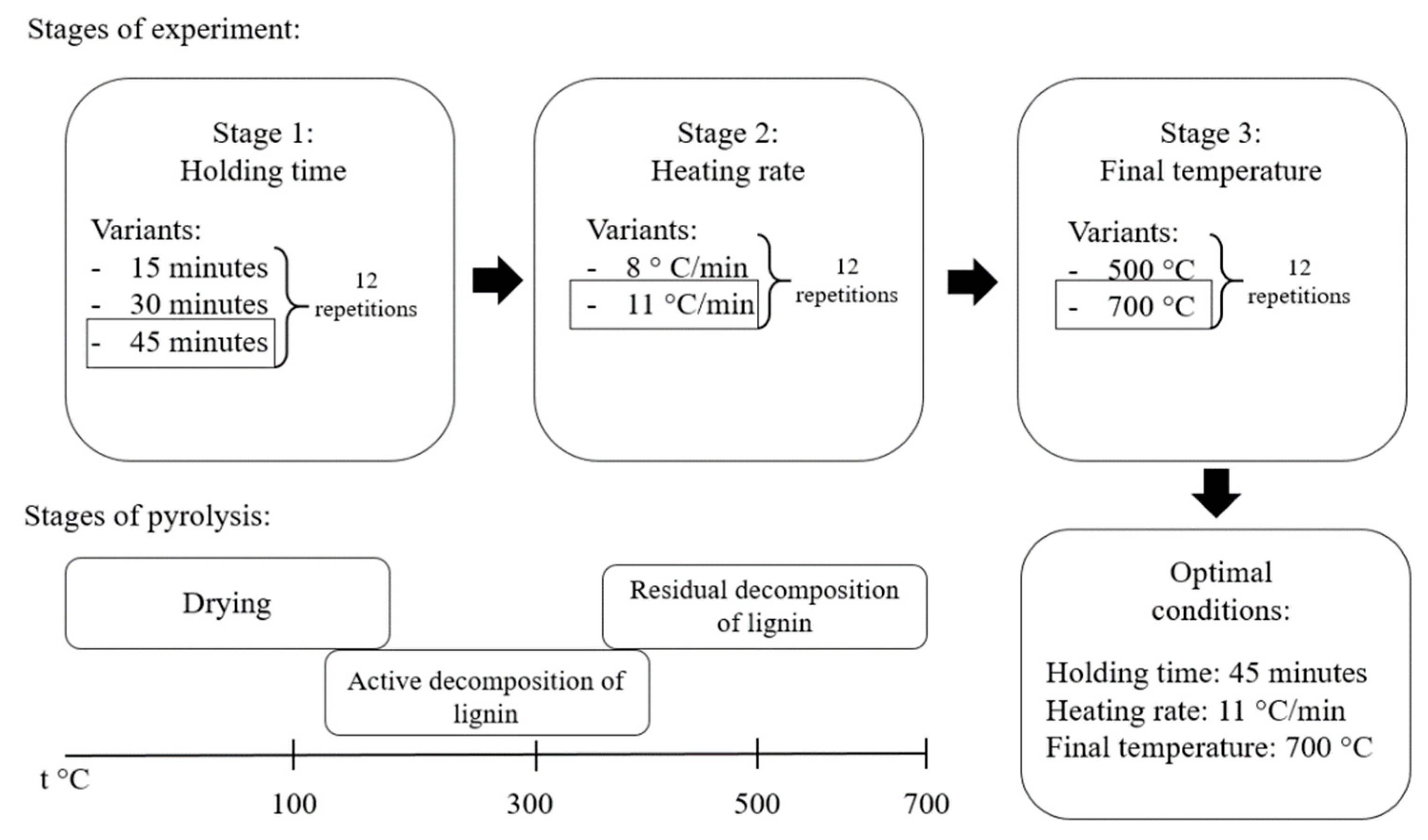

| Stage | Final Temperature, °C | Heating Rate, °C∙min−1 | Holding Time, min | Product Yield, % | Ash Content, % | SBET, m2∙g–1 |
|---|---|---|---|---|---|---|
| Stage 1 | 500 | 8 | 15 | 47.5 | 28.3 | 135 ± 9 * |
| 500 | 8 | 30 | 45.2 | 26.2 | 164 ± 11 | |
| 500 | 8 | 45 | 43.1 | 18.0 | 186 ± 13 | |
| Stage 2 | 500 | 8 | 45 | 43.1 | 17.6 | 184 ± 10 |
| 500 | 11 | 45 | 42.3 | 14.0 | 278 ± 17 | |
| Stage 3 | 500 | 11 | 45 | 42.3 | 14.3 | 279 ± 19 |
| 700 | 11 | 45 | 37.1 | 12.7 | 398 ± 21 |
| Sorption Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pore volume (cm3∙g–1) | ||||||||
| SBET (m2 g–1) | ΣV | Vmacro >50 nm | Vmeso 2–50 nm | Vmicro ˂2 nm | ||||
| 398 ± 21 * | 2.88 | 0.61 ± 0.05 | 1.59 ± 0.11 | 0.68 ± 0.04 | ||||
| Elemental analysis | ||||||||
| Content of elements and ash, % | Atomic Relations | |||||||
| C | H | N | O | Ash | H/C | O/C | (N+O)/C | C/N |
| 70.4 | 2.1 | 3.0 | 11.8 | 12.7 | 0.36 | 0.13 | 0.15 | 34.10 |
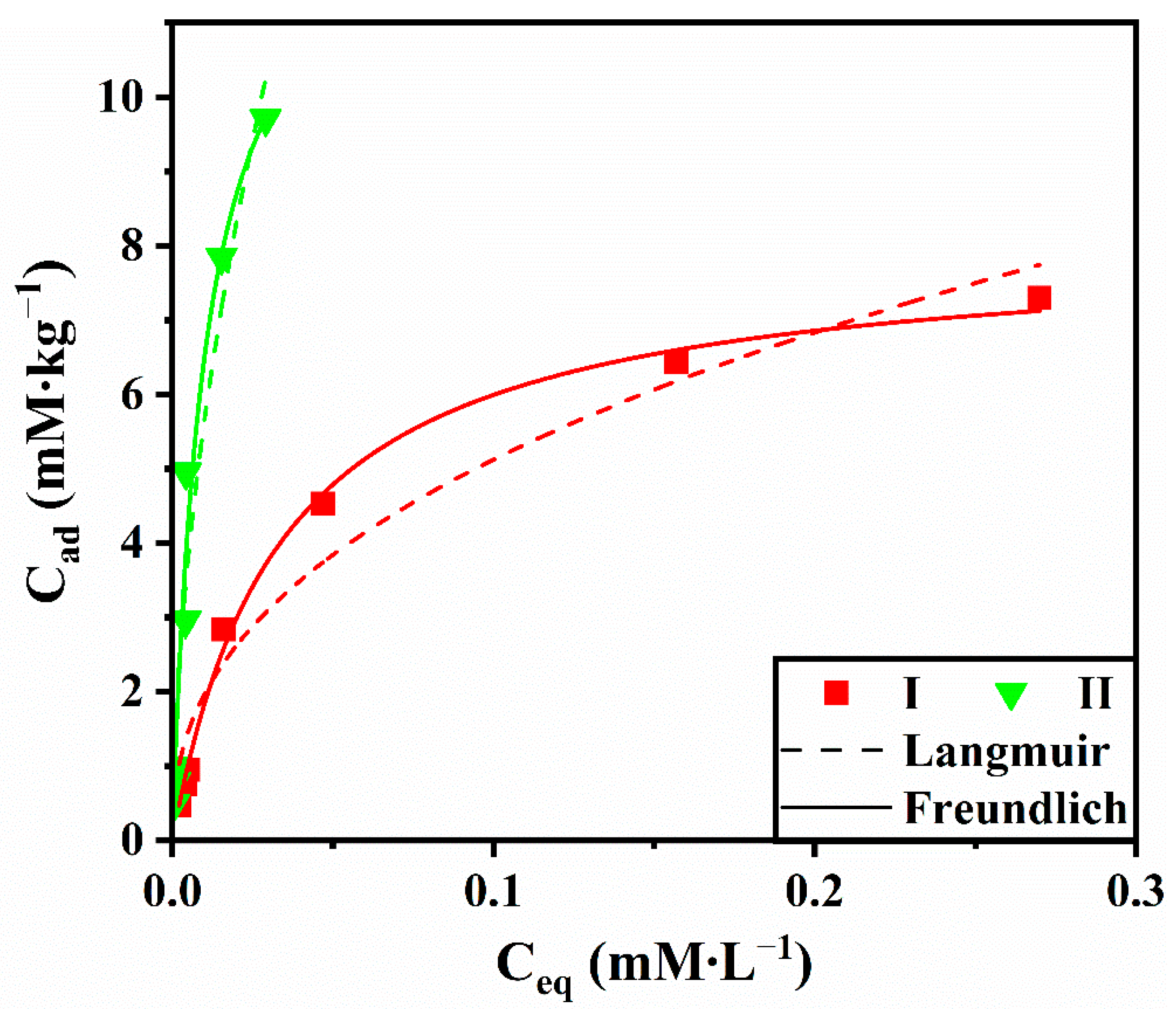
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Cm (mM∙L–1) | KL (L∙mM–1) | R2 | KF (L∙kg–1) | n | R2 | |
| Soil | 8.01 | 29.76 | 0.997 | 13.35 | 0.41 | 0.959 |
| Soil + biochar | 13.15 | 97.64 | 0.976 | 73.48 | 0.56 | 0.948 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobzenko, I.; Burachevskaya, M.; Zamulina, I.; Barakhov, A.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Tereschenko, A.; Kalinichenko, V.; et al. Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture 2022, 12, 1689. https://doi.org/10.3390/agriculture12101689
Lobzenko I, Burachevskaya M, Zamulina I, Barakhov A, Bauer T, Mandzhieva S, Sushkova S, Minkina T, Tereschenko A, Kalinichenko V, et al. Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture. 2022; 12(10):1689. https://doi.org/10.3390/agriculture12101689
Chicago/Turabian StyleLobzenko, Ilia, Marina Burachevskaya, Inna Zamulina, Anatoly Barakhov, Tatiana Bauer, Saglara Mandzhieva, Svetlana Sushkova, Tatiana Minkina, Andrey Tereschenko, Valery Kalinichenko, and et al. 2022. "Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation" Agriculture 12, no. 10: 1689. https://doi.org/10.3390/agriculture12101689
APA StyleLobzenko, I., Burachevskaya, M., Zamulina, I., Barakhov, A., Bauer, T., Mandzhieva, S., Sushkova, S., Minkina, T., Tereschenko, A., Kalinichenko, V., Khroniuk, O., & Rajput, V. D. (2022). Development of a Unique Technology for the Pyrolysis of Rice Husk Biochar for Promising Heavy Metal Remediation. Agriculture, 12(10), 1689. https://doi.org/10.3390/agriculture12101689












