Synergetic Effect of Different Plant Growth Regulators on Micropropagation of Sugarcane (Saccharum officinarum L.) by Callogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Surface Sterilization
2.3. Media Preparation
2.4. Inoculation
2.5. Callus Induction
2.6. Shoot Induction
2.7. Root Induction
2.8. Acclimatization
2.9. Statistical Analysis
3. Results
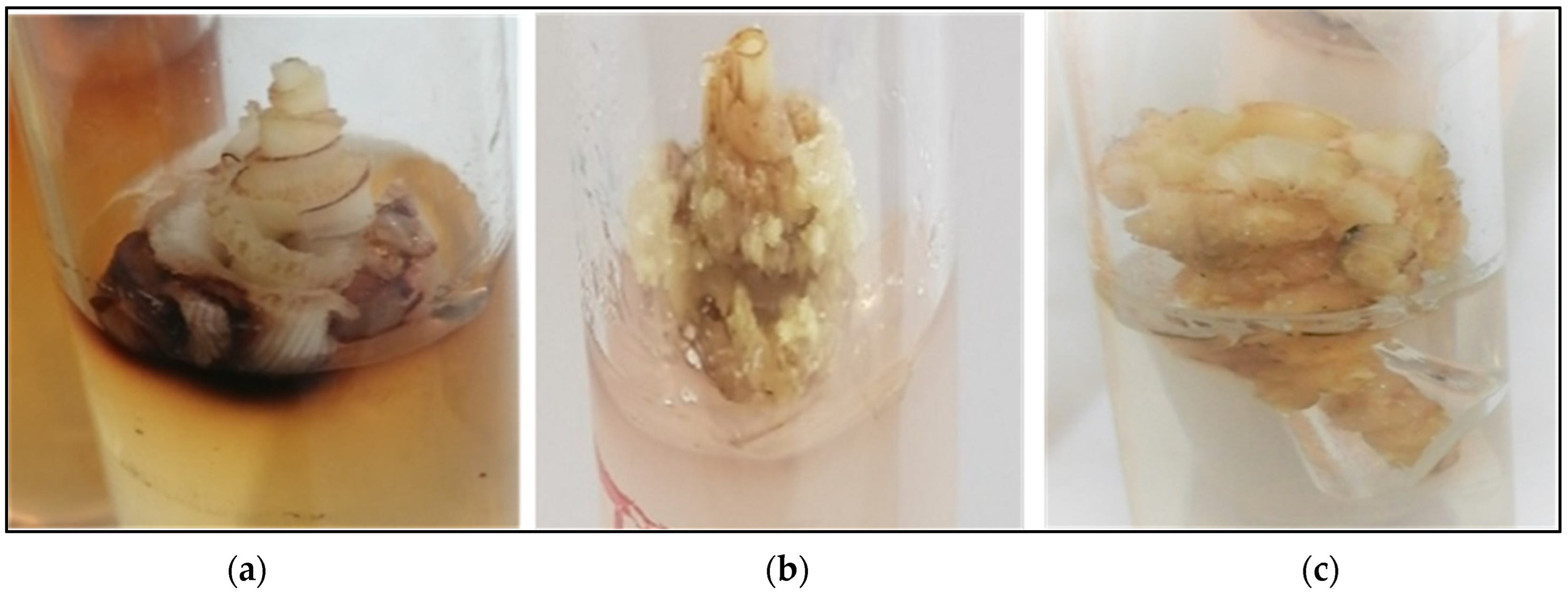
3.1. Callus Induction
3.2. Plant Regeneration from Callus Cultures of Sugarcane (YT-53, CP-77-400, NSG-59)
3.3. Root Induction
3.4. Acclimatization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomathi, R.; Krishnapriya, V.; Arunkumar, R.; Govindaraj, P.; Ram, B. Physiological Traits Imparting Drought Stress Tolerance to Promising Sugarcane (Saccharum Spp.) Clones. Plant Physiol. Rep. 2020, 25, 509–515. [Google Scholar] [CrossRef]
- Hensel, F.; Covello, A.; Gargano, A. International Seminar on Agriculture, Biodiversity, Food Security and Health; IOP Conf. Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 883, p. 012075. [Google Scholar]
- Dinesh, P.; Thirunavukkarasu, D.P.; Saraniya, A.R.; Ramanathan, T. In vitro Studies of Sugarcane Variety Co-91017 Through Micropropagation of Shoot Tip Culture. Adv. Plants Agric. Res. 2015, 2, 234–238. [Google Scholar]
- Salokhe, S. Development of an Efficient Protocol for Tissue Culture of Sugarcane. Plant Cell Biotechnol. Mol. Bio. 2021, 4, 9–21. [Google Scholar]
- Lal, M.; Tiwari, A.K.; Gupta, G.N. Commercial Scale Micropropagation of Sugarcane: Constraints and Remedies. Sugar Tech 2015, 17, 339–347. [Google Scholar] [CrossRef]
- Hailu, M.; Chimdessa, M.; Muthswamy, M. In Vitro Propagation of Selected Sugarcane (Saccharum officinarum L.) Varieties (C 86-165 and C 86-12) Through Shoot Apical Meristem. Int. J. Hortic. Agric. 2018, 3, 1–7. [Google Scholar]
- Purnamaningsih, R.; Sukmadjaja, D.; Suhesti, S.; Rahayu, S. In vitro Propagation of Six Selected Sugarcane Mutant Clones Through Leaf Explants. IOP Conf. Ser. Earth Environ. Sci. 2021, 883, 012075. [Google Scholar] [CrossRef]
- Subedi, M.; Ojha, B.R.; Ghimire, S.K.; Joshi, B.K.; Niroula, R.K.; Sah, B.P.; Poudel, A.P. Effect of sugarcane genotypes and 2, 4-dichlorophenoxy acetic acid on callus induction. J. Inst. Agric. Anim. Sci. 2015, 33, 237–242. [Google Scholar] [CrossRef]
- Kumar, D.; Sengar, R.S.; Malik, N.; Yadav, M.K.; Gupta, S.; Chand, P.; Kumar, P. In Vitro Evaluation to Intensify the Differential Morphogenetic Response Through Plant Growth Regulators and Antibiotic Supplementation in Sugarcane. Plant Physiol. Rep. 2020, 25, 335–346. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Aftab, F.; Zafar, Y.; Malik, K.A.; Iqbal, J. Plant regeneration from embryogenic cell suspensions and protoplasts in sugarcane (Saccharum spp. Hybrid cv. Col-54). PCTOC 1996, 44, 71–78. [Google Scholar] [CrossRef]
- Sengar, K.; Sengar, R.S.; Garg, S.K. The Effect of in vitro Environmental Conditions on Some Sugarcane Genotypes for Micropropagation. Afr. J. Biotechnol. 2011, 10, 17122–17126. [Google Scholar]
- Mostafa, H.H.; Wang, H.; Song, J.; Li, X. Effects of Genotypes and Explants on Garlic Callus Production and Endogenous Hormones. Sci. Rep. 2020, 10, 4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baday, S.J. Sugarcane of Rapid Multiplication by Callogenesis. JPSC 2020, 1660, 012005. [Google Scholar] [CrossRef]
- Srivong, T.; Zhu, Y.J.; Pongdontri, P.; Pliansinchai, U.; Sakuanrungsirikul, S.; Borthakur, D.; Kosittrakun, M. Optimization of Callus Induction and Plant Regeneration in Sugarcane (Saccharum spp.) for a Study of Sucrose Accumulation in Relation to Soluble Acid Invertase Expression. Chiang Mai J. Sci. 2015, 42, 797–805. [Google Scholar]
- Mayerni, R.; Satria, B.; Wardhani, D.K.; Chan, S.R.O.S. Effect of Auxin (2,4-D) and Cytokinin (BAP) in Callus Induction of Local Patchouli Plants (Pogostemon cablin Benth.). IOP Conf. Ser. Earth Environ. Sci. 2020, 583, 012003. [Google Scholar] [CrossRef]
- Kaur, A.; Malhotra, P.K.; Manchanda, P.; Gosal, S.S. Micropropagation and Somatic Embryogenesis in Sugarcane. Biotechnol. Crop Improv. 2018, 1, 57–91. [Google Scholar]
- Dalila, Z.D.; Jaafar, H.; Manaf, A.A. Effects Of 2, 4-D and Kinetin on Callus Induction of Barringtonia racemosa leaf and Endosperm Explants in Different Types of Basal Media. Asian J. Plant Sci. 2013, 12, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, P.; Ding, G.; Zhou, L.; Tang, P.; Sun, M.; Zheng, Y.; Lin, S. Optimization of Hormone Combinations for Root Growth and Bud Germination in Chinese Fir (Cunninghamia lanceolata) Clone Leaf Cuttings. Sci. Rep. 2017, 7, 5046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastuti, R.; Munawarti, A.; Firdiana, E.R. The Combination Effect of Auxin and Cytokinin on in vitro Callus Formation of Physalis angulata L.—A Medicinal Plant. AIP Conf. Proc. 2017, 1908, 040007. [Google Scholar]
- Iqbal, M.; Aamir, A.; Naima, H.N.; Umair, A.K.; Muhammad, N.A.F.; Muhammad, I.; Mubashir, H. Effect of Explants and Growth Regulators on The Expression of Callogenesis Somatic Embryogenesis and Plantlets Formation in Sugarcane (Saccharum officinarum L.). Int. J. Biosci. 2016, 9, 147–156. [Google Scholar]
- Tripathy, S.K.; Ithape, D.M. High-throughput in vitro culture system targeting genetic transformation in sugarcane. J. Crop Sci. Biotechnol. 2020, 23, 325–335. [Google Scholar] [CrossRef]
- Perez-Jimenez, M.; Cantero-Navarro, E.; Perez-Alfocea, F.; Le-Disquet, I.; Guivarc’h, A.; Cos-Terrer, J. Relationship Between Endogenous Hormonal Content and Somatic Organogenesis in Callus of Peach (Prunus persica L. Batsch) Cultivars and Prunus persica× Prunus dulcis Rootstocks. J. Plant Phys. 2014, 171, 619–624. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Zhang, Q.; Lin, H.; Xia, T.; Akhtar, K.; Xu, W. In Vitro Regeneration Potential of White Lupin (Lupinus albus) from Cotyledonary Nodes. Plants 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.R.; Muhammad, A.; Hussain, I.; Shah, S.H.; Kumar, T.; Inam, S.; Ali, G.M. Rapid in vitro Multiplication of Sugarcane Elite Genotypes and Detection of Sugarcane Mosaic Virus Through Two Steps RT-PCR. Int. J. Agric. Biol. 2012, 14, 870–878. [Google Scholar]
- Muller, D.; Leyser, O. Auxin, Cytokinin and the Control of Shoot Branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular and Physiological Aspects of in vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Abu-Romman, S.M.; Al-Hadid, K.A.; Arabiyyat, A.R. Kinetin is the most effective cytokinin on shoot multiplication from cucumber. J. Agric. Sci. 2015, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Devi, T.R.; Dasgupta, M.; Sahoo, M.R.; Kole, P.C.; Prakash, N. High Efficient de novo Root-to-shoot Organogenesis in Citrus jambhiri Lush.: Gene expression, Genetic Stability and Virus Indexing. PLoS ONE 2021, 16, e0246971. [Google Scholar] [CrossRef]
- Patel, A.A.; Patel, S.R.; Patel, C.L.; Prajapati, B.S. Effect of Media Composition on in vitro Multiplication of Sugarcane Genotypes. Indian J. Gen. Plant Breed. 2001, 61, 82–83. [Google Scholar]
- Kaur, R.; Kapoor, M. In Vitro Direct Plant Regeneration Using Shoot Tip Explants in Sugarcane (Saccharum officinarum L.) for rapid mass cloning. Agric. Sci. Digest. 2017, 37, 94–99. [Google Scholar] [CrossRef]
- Biswas, P.; Harun-Or Rashid, M.D.; Chowdhury, A.K.; Hossain, M.D.A. Direct Plant Regeneration Through Micropropagation Using Selected Explants of Sugarcane. Int. J. Plant Geosci. 2020, 8, 244–248. [Google Scholar] [CrossRef]
- Gupta, C.; Nema, S.; Sapre, S.; Tantwai, K. Studies on Induction of Somaclonal Variation in Sugarcane (Saccharum officinarum) and Validation of Mutant Using Molecular Markers. Int. J. Agric. Environ. Biotechnol. 2020, 13, 105–110. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ivy, N.A.; Mian, M.A.; Rasul, M.G.; Hossain, M.M.; Rahman, M.A. Effect of Auxin (NAA, IBA and IAA) in Root Regeneration Through in vitro Culture of Sugarcane. Int. J. Plant Biol. Res. 2018, 6, 1109. [Google Scholar]
- Silva, D.J.A.; Solis-Gracia, N.; Jifon, J.; Souza, S.C.; Mandadi, K.K. Use of Bioreactors for large-Scale Multiplication of Sugarcane (Saccharum spp.), Energy Cane (Saccharum spp.) and Related Species. In Vitro Cell. Dev. Biol. Plant 2020, 56, 366–376. [Google Scholar] [CrossRef]
- Tolera, B. Effects of Naphthalene Acetic Acid (NAA) and Indole-3-Butyric Acid (IBA) on in vitro Rooting of Sugarcane (Saccharum officinarum L.) Micro-shoots. J. Biotechnol. Biomater. 2016, 6, 215. [Google Scholar] [CrossRef]














| T | Composition | T | Composition | T | Composition |
|---|---|---|---|---|---|
| T1 | Ms + 2,4-D (2.0 mg L−1) | T7 | MS + 2,4-D (2 mg L−1) + BAP (0.5 mg L−1) | T13 | MS + 2,4-D (4 mg L−1) + BAP (1 mg L−1) |
| T2 | MS + 2,4-D (2.51 mg L−1) | T8 | MS + 2,4-D (3 mg L−1) + BAP (0.5 mg L−1) | T14 | MS + 2,4-D (5 mg L−1) + BAP (1 mg L−1) |
| T3 | MS + 2,4-D (3 mg L−1) | T9 | MS + 2,4-D (4 mg L−1) + BAP (0.5 mg L−1) | T15 | MS + 2,4 D (2 mg L−1) + Kinetin (0.5 mg L−1) |
| T4 | MS + 2,4-D (3.5 mg L−1) | T10 | MS + 2,4-D (5 mg L−1) + BAP (0.5 mg L−1) | T16 | MS + 2,4-D (3 mg L−1) + Kinetin (0.5 mg L−1) |
| T5 | MS + 2,4-D (4 mg L−1) | T11 | MS + 2,4-D (2 mg L−1) + BAP (1 mg L−1) | T17 | MS + 2,4-D (4 mg L−1) + Kinetin (0.5 mg L−1) |
| T6 | MS + 2,4-D (5 mg L−1) | T12 | MS + 2,4-D (3 mg L−1) + BAP (1 mg L−1) | T18 | MS + 2,4-D (5 mg L−1) + Kinetin (0.5 mg L−1) |
| Treatment | Composition | Treatment | Composition |
|---|---|---|---|
| T1 | MS + BAP (1 mg L−1) | T7 | MS+ BAP (0.5 mg L−1) + Kinetin (0.5 mg L−1) |
| T2 | MS + BAP (2 mg L−1) | T8 | MS + BAP (1 mg L−1) + Kinetin (1 mg L−1) |
| T3 | MS + Kinetin (1 mg L−1) | T9 | MS + BAP (0.5 mg L−1) + IBA (0.5 mg L−1) |
| T4 | MS + Kinetin (2 mg L−1) | T10 | MS + BAP (0.5 mg L−1) + IBA (0.5 mg L−1) + NAA (0.5 mg L−1) |
| T5 | IBA (1 mg L−1) | T11 | MS + BAP (1 mg L−1) + IBA (1 mg L−1) + NAA (1 mg L−1) |
| T6 | NAA (1 mg L−1) | T12 | MS + Kinetin (0.5 mg L−1) + IBA (0.5 mg L−1) + NAA (0.5 mg L−1) |
| Treatment | Composition |
|---|---|
| T1 | 1/2 MS + NAA (1 mg L−1) |
| T2 | 1/2 MS + NAA (2 mg L−1) |
| T3 | 1/2 MS + NAA (3 mg L−1) |
| T4 | 1/2 MS + NAA (1 mg L−1) + IBA (1 mg L−1) |
| T5 | 1/2 MS + NAA (2 mg L−1) + IBA (2 mg L−1) |
| Source of Variation | df | SS | % Variation Explained | MS | F | p-Value |
|---|---|---|---|---|---|---|
| Treatment | 17 | 2.389 | 41.724 | 0.141 | 34.960 | <0.001 |
| Variety | 2 | 0.250 | 4.369 | 0.125 | 31.120 | <0.001 |
| Treatment × Variety | 34 | 1.885 | 32.918 | 0.055 | 13.790 | <0.001 |
| Error | 299 | 1.202 | 20.994 | 0.004 | ||
| Total | 352 | 5.726 | ||||
| Grand Mean | 0.217 | CV% | 29.23 | R2 | 0.79 |
| (a) Source of Variation | df | SS | % Variation Explained | MS | F | p-Value |
|---|---|---|---|---|---|---|
| Treatment | 11 | 553.28 | 30.383 | 50.298 | 219.87 | <0.001 |
| Variety | 2 | 205.38 | 11.278 | 102.689 | 448.88 | <0.001 |
| Treatment × Variety | 22 | 1035.37 | 56.857 | 47.062 | 205.72 | <0.001 |
| Error | 118 | 26.99 | 1.482 | 0.229 | ||
| Total | 153 | 1821.02 | ||||
| Grand Mean | 1.682 | CV% | 28.44 | R2 | 0.985 | |
| (b) Treatment | 11 | 2507.73 | 50.095 | 227.976 | 135.54 | <0.001 |
| Variety | 2 | 229.13 | 4.577 | 114.563 | 68.11 | <0.001 |
| Treatment × Variety | 22 | 2070.59 | 41.363 | 94.118 | 55.96 | <0.001 |
| Error | 118 | 198.48 | 3.965 | 1.682 | ||
| Total | 153 | 5005.93 | ||||
| Grand Mean | 6.76 | CV% | 19.19 | R2 | 0.960 |
| (a) Source of Variation | df | SS | % Variation Explained | MS | F | p-Value |
|---|---|---|---|---|---|---|
| treat | 4 | 0.70979 | 5.208 | 0.17745 | 2.32 | 0.066 |
| Variety | 2 | 1.02792 | 7.542 | 0.51396 | 6.71 | 0.0022 |
| Treatment × Variety | 8 | 6.76283 | 49.618 | 0.84535 | 11.04 | <0.001 |
| Error | 67 | 5.12912 | 37.632 | 0.07655 | ||
| Total | 81 | 13.6297 | ||||
| Grand Mean | 0.671 | CV% | 36.37 | R2 | 0.624 | |
| (b) Treatment | 4 | 280.86 | 14.300 | 70.215 | 47.11 | <0.001 |
| Variety | 2 | 1113.03 | 56.669 | 556.517 | 373.43 | <0.001 |
| Treatment × Variety | 8 | 470.36 | 23.948 | 58.795 | 39.45 | <0.001 |
| Error | 67 | 99.85 | 5.084 | 1.49 | ||
| Total | 81 | 1964.1 | ||||
| Grand Mean | 9.25 | CV% | 13.2 | R2 | 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, Y.; Emad, M.Z.; Ali, A.; Naz, S. Synergetic Effect of Different Plant Growth Regulators on Micropropagation of Sugarcane (Saccharum officinarum L.) by Callogenesis. Agriculture 2022, 12, 1812. https://doi.org/10.3390/agriculture12111812
Saleem Y, Emad MZ, Ali A, Naz S. Synergetic Effect of Different Plant Growth Regulators on Micropropagation of Sugarcane (Saccharum officinarum L.) by Callogenesis. Agriculture. 2022; 12(11):1812. https://doi.org/10.3390/agriculture12111812
Chicago/Turabian StyleSaleem, Yasmeen, Muhammad Zaka Emad, Aamir Ali, and Shagufta Naz. 2022. "Synergetic Effect of Different Plant Growth Regulators on Micropropagation of Sugarcane (Saccharum officinarum L.) by Callogenesis" Agriculture 12, no. 11: 1812. https://doi.org/10.3390/agriculture12111812
APA StyleSaleem, Y., Emad, M. Z., Ali, A., & Naz, S. (2022). Synergetic Effect of Different Plant Growth Regulators on Micropropagation of Sugarcane (Saccharum officinarum L.) by Callogenesis. Agriculture, 12(11), 1812. https://doi.org/10.3390/agriculture12111812






