Trait Variation between Two Wild Specimens of Pleurotus ostreatus and Their Progeny in the Context of Usefulness in Nematode Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mycelia and Growing Tests
2.2. Toxicity Tests
2.3. Molecular Tests
3. Statistics
4. Results
4.1. Mycelia and Molecular Tests
4.2. Growing Tests
4.3. Toxicity Tests
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffee, B.A. Wood, nematodes, and the nematode-trapping fungus Arthrobotrys oligospora. Soil Biol. Biochem. 2004, 36, 1171–1178. [Google Scholar] [CrossRef]
- Truong, B.N.; Suzuki, A.; Truong, B.N.; Okazaki, K.; Fukiharu, T.; Takeuchi, Y.; Futai, K.; Le, X.-T.; Suzuki, A. Characterization of the nematocidal toxocyst in Pleurotus subgen. Coremiopleurotus. Mycoscience 2007, 48, 222–230. [Google Scholar] [CrossRef]
- Vandenbossche, B.A.; Niere, B.; Vidal, S. Effect of Temperature on the Hatch of Two German Populations of the Beet Cyst nematodes, Heterodera schachtii and Heterodera betae. J. Plant Dis. Prot. 2015, 122, 250–254. [Google Scholar] [CrossRef]
- Almaghrabi, B.; Ali, M.A.; Zahoor, A.; Shah, K.H.; Bohlmann, H. Arabidopsis thionin-like genes are involved in resistance against the beet-cyst nematode (Heterodera schachtii). Plant Physiol. Biochem. 2019, 140, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.R.; Zare, R. Fungi as Biological Control Agents of Plant-Parasitic Nematodes. In Progress in Biological Control Series: Plant Defence: Biological Control; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 12, pp. 67–107. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Arjona, D.; Aragón, C.; Aguilera, J.A.; Ramírez, L.; Pisabarro, A.G. Reproducible and controllable light induction of in vitro fruiting of the white-rot basidiomycete Pleurotus ostreatus. Mycol. Res. 2009, 113, 552–558. [Google Scholar] [CrossRef]
- Zawadzka, A.; Janczewska, A.; Kobus-Cisowska, J.; Dziedziński, M.; Siwulski, M.; Czarniecka-Skubina, E.; Stuper-Szablewska, K. The effect of light conditions on the content of selected active ingredients in anatomical parts of the oyster mushroom (Pleurotus ostreatus L.). PLoS ONE 2022, 17, e0262279. [Google Scholar] [CrossRef]
- Kothe, E. Mating-type genes for basidiomycete strain improvement in mushroom farming. Appl. Microbiol. Biotechnol. 2001, 56, 602–612. [Google Scholar] [CrossRef]
- Larraya, L.M.; Pérez, G.; Iribarren, I.; Blanco, J.A.; Alfonso, M.; Pisabarro, A.G.; Ramírez, L. Relationship between Monokaryotic Growth Rate and Mating Type in the Edible Basidiomycete Pleurotus ostreatus. Appl. Environ. Microbiol. 2001, 67, 3385–3390. [Google Scholar] [CrossRef]
- Gupta, B.; Niranjan Reddy, B.P.; Kotasthane, A.S. Molecular characterization and mating type analysis of oyster mushroom (Pleurotus spp.) using single basidiospores for strain improvement. World J. Microbiol. Biotechnol. 2011, 27, 1–9. [Google Scholar] [CrossRef]
- Barh, A.; Sharma, V.P.; Annepu, S.K.; Kamal, S.; Sharma, S.; Bhatt, P. Genetic improvement in Pleurotus (oyster mushroom): A review. 3 Biotech 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Grundler, F.M.W. Metabolism in Nematode Feeding Sites. In Advances in Botanical Research: Plant Nematode Interactions—A View on Compatible Interrelationships; Escobar, C., Fenoll, C., Eds.; Elsevier Academic Press: London, UK, 2015; Volume 73, pp. 119–138. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: Metabolites from nematophagous basidiomycetes and non-nematophagous fungi. Appl. Microbiol. Biotechnol. 2016, 100, 3813–3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Kaneko, K.; Li, W.; Koike, K. The Toxin Produced by Pleurotus ostreatus Reduces the Head Size of Nematodes. Biol. Pharm. Bull. 2008, 31, 574–576. [Google Scholar] [CrossRef] [Green Version]
- Frangež, R.; Šuput, D.; Molgó, J.; Benoit, E. Ostreolysin A/Pleurotolysin B and Equinatoxins: Structure, Function and Pathophysiological Effects of These Pore-Forming Proteins. Toxins 2017, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Barron, G.L.; Thorn, R.G. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 1987, 65, 774–778. [Google Scholar] [CrossRef]
- Pineda-Alegría, J.A.; Sánchez-Vázquez, J.E.; González-Cortazar, M.; Zamilpa, A.; López-Arellano, M.E.; Cuevas-Padilla, E.J.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L. The Edible Mushroom Pleurotus djamor Produces Metabolites with Lethal Activity Against the Parasitic Nematode Haemonchus contortus. J. Med. Food 2017, 20, 1184–1192. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, S.K.; Singh, D.; Masurkar, P. First report of edible mushroom Pleurotus ostreatus from India with potential to kill plant parasitic nematodes. Indian Phytopathol. 2019, 72, 173–176. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, J.M.; Di Maro, A. The ribotoxin-like protein Ostreatin from Pleurotus ostreatus fruiting bodies: Confirmation of a novel ribonuclease family expressed in basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329–1336. [Google Scholar] [CrossRef]
- Žužek, M.C.; Maček, P.; Sepčić, K.; Cestnik, V.; Frangež, R. Toxic and lethal effects of ostreolysin, a cytolytic protein from edible oyster mushroom (Pleurotus ostreatus), in rodents. Toxicon 2006, 48, 264–271. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, H.W.; Yang, C.T.; Wali, N.; Shie, J.J.; Hsueh, Y.P. Sensory cilia as the Achilles heel of nematodes when attacked by carnivorous mushrooms. Proc. Natl. Acad. Sci. USA 2020, 117, 6014–6022. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-M.; Hameed, K.; Cotter, V.T.; Liao, H.-L. Isolation of mother cultures and preparation of spawn for oyster mushroom cultivation. UF/IFAS Ext. 2018, 2018, SL449. [Google Scholar] [CrossRef]
- Brown, A. Mating in mushrooms: Increasing the chances but prolonging the affair. Trends Genet. 2001, 17, 393–400. [Google Scholar] [CrossRef]
- Marlin, M.; Wolf, A.; Alomran, M.; Carta, L.; Newcombe, G. Nematophagous Pleurotus Species Consume Some Nematode Species but Are Themselves Consumed by Others. Forests 2019, 10, 404. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. 11 February 2006. In WormBook: The Online Review of C. Elegans Biology; WormBook: Pasadena, CA, USA, 2005–2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK19649/ (accessed on 30 March 2021).
- Nazrul, M.I.; YinBing, B. Differentiation of homokaryons and heterokaryons of Agaricus bisporus with inter-simple sequence repeat markers. Microbiol. Res. 2011, 166, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Heydari, R.; Pourjam, E.; Goltapeh, E.M. Antagonistic Effect of Some Species of Pleurotus on the Root-knot Nematode, Meloidogyne javanica in vitro. Plant Pathol. J. 2006, 5, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Palizi, P.; Goltapeh, E.; Pourjam, E.; Safaie, N. Potential of Oyster Mushrooms for the Biocontrol of Sugar Beet Nematode (Heterodera schachtii). J. Plant Prot. Res. 2009, 49, 27–34. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Comans-Pérez, R.J.; Sánchez, J.E.; Al-Ani, L.K.T.; González-Cortázar, M.; Castañeda-Ramírez, G.S.; Mendoza-de Gives, P.; Sánchez-García, A.D.; Millán-Orozco, J.; Aguilar-Marcelino, L. Biological control of sheep nematode Haemonchus contortus using edible mushrooms. Biol. Control 2021, 152, 104420. [Google Scholar] [CrossRef]
- Ibrahim, I.K.A.; Awd-Allah, S.; Handoo, Z.A. Life cycle and control of the cyst nematode Heterodera goldeni on rice in Egypt. Int. J. Nematol. 2014, 24, 11–17. [Google Scholar]
- Ibrahim, I.K.A.; Handoo, Z.A. Pathogenicity and control of Meloidogyne incognita on rice in Egypt. Pak. J. Nematol. 2018, 36, 123–129. [Google Scholar] [CrossRef]
- Wille, C.N.; Gomes, C.B.; Minotto, E.; Nascimento, J.S. Potential of aqueous extracts of basidiomycetes to control root-knot nematodes on lettuce. Hortic. Bras. 2019, 37, 054–059. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Uriostegui, L.A.; Sánchez-García, A.D.; Zamilpa, A.; Sánchez, J.E.; González-Garduño, R.; Mendoza-de Gives, P.; Castañeda-Ramírez, G.S.; González-Cortazar, M.; Aguilar-Marcelino, L. In vitro evaluation of hydroalcoholic extracts of mycelium, basidiomata and spent substrate of Pleurotus ostreatus against Haemonchus contortus. Trop. Subtrop. Agroecosystems 2021, 24, 62. [Google Scholar] [CrossRef]
- Braga, F.R. Predatory Activity of the Fungus Pleurotus eryngii on Ancylostoma caninum Infective Larvae. SOJ Vet. Sci. 2015, 1, 104–130. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, X.; Zheng, L.; Li, L.; Huang, R.; Zhang, K. Nematicidal metabolites from the fungus Pleurotus ferulae Lenzi. Ann. Microbiol. 2007, 57, 527–529. [Google Scholar] [CrossRef]


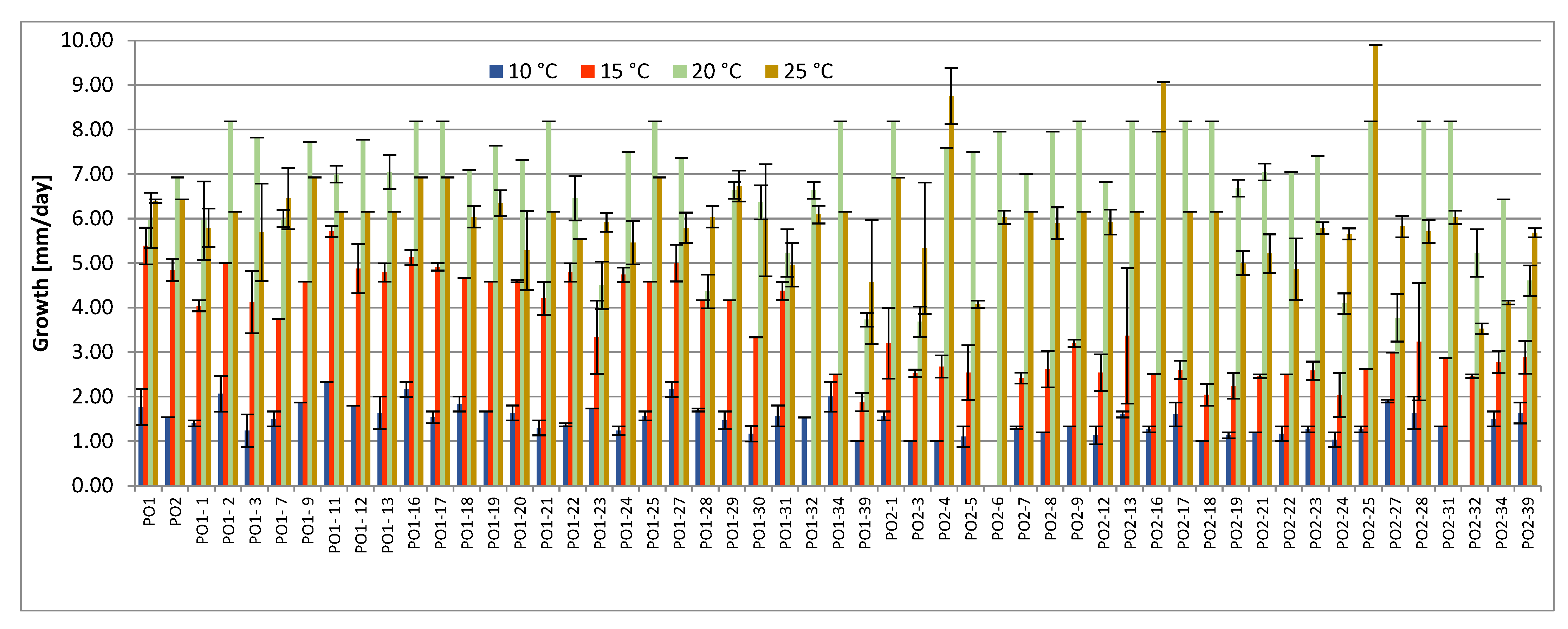

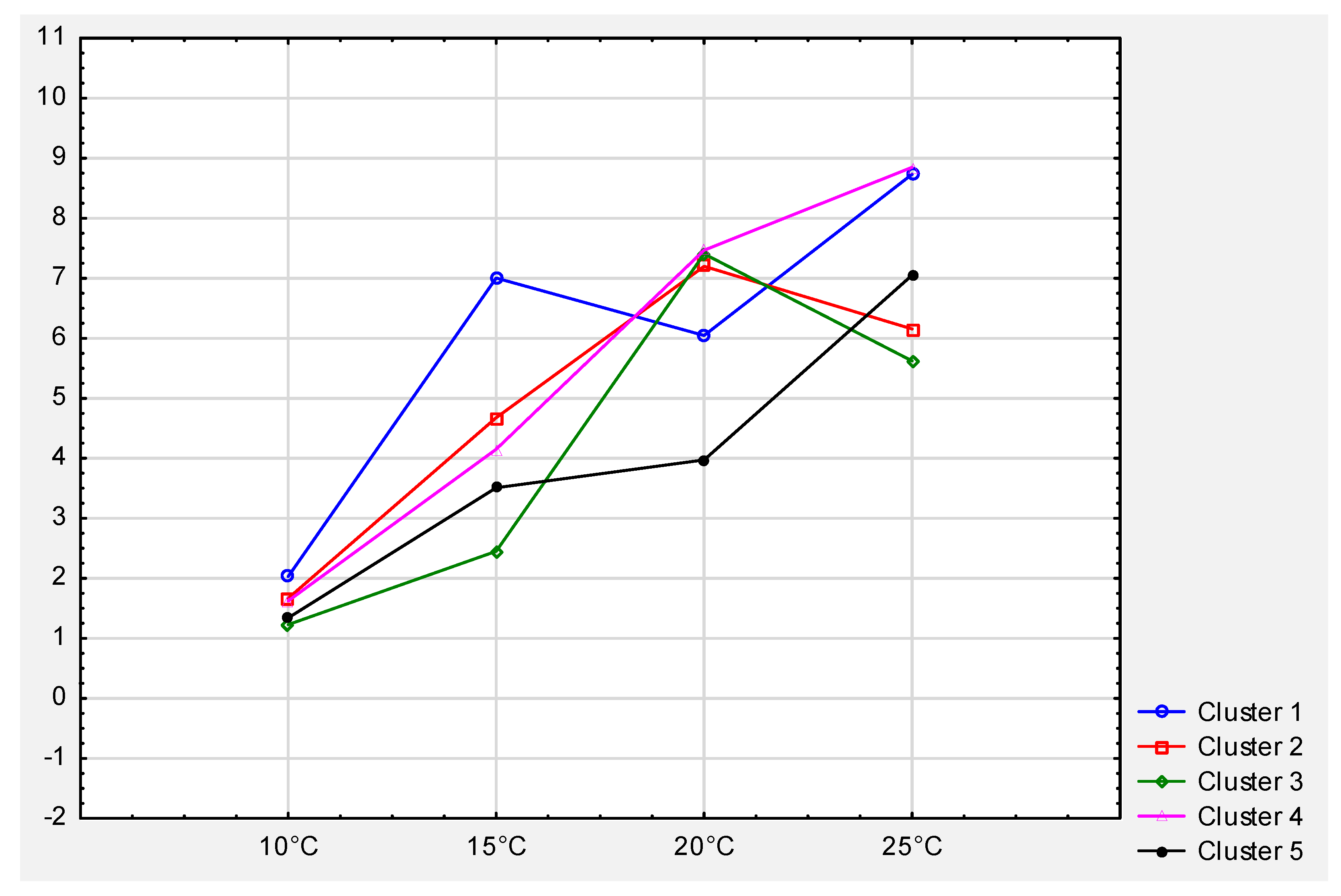
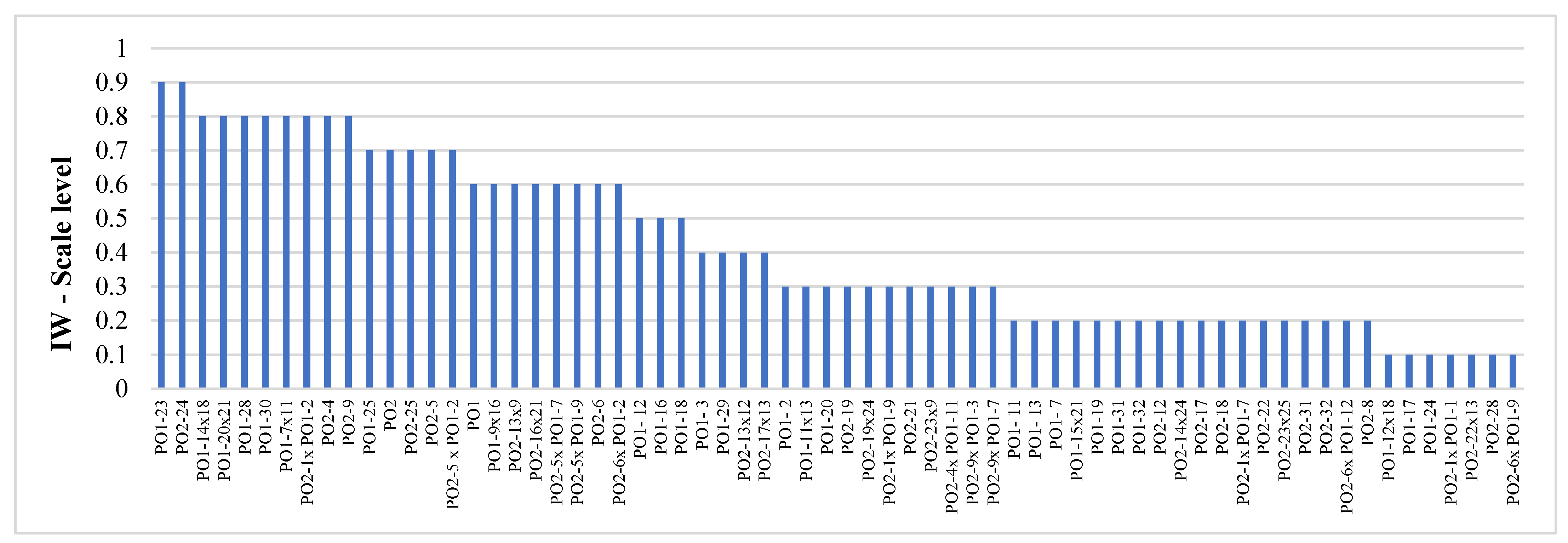

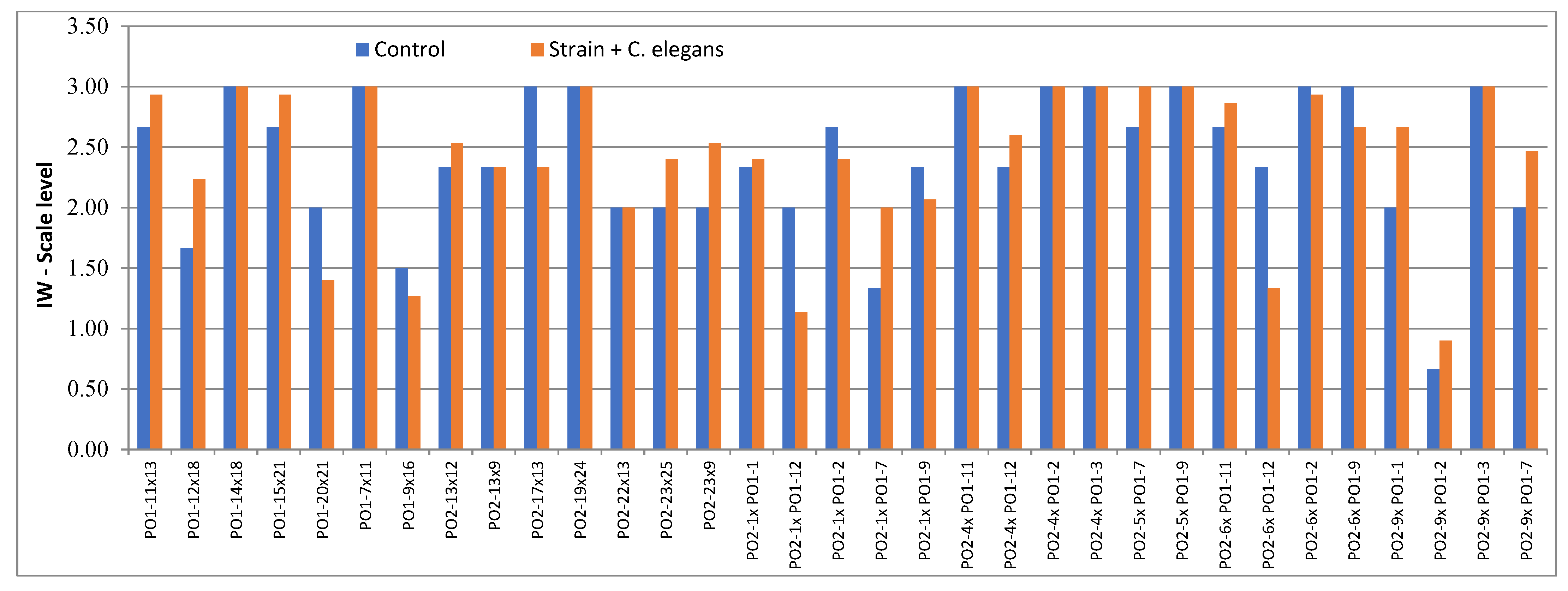
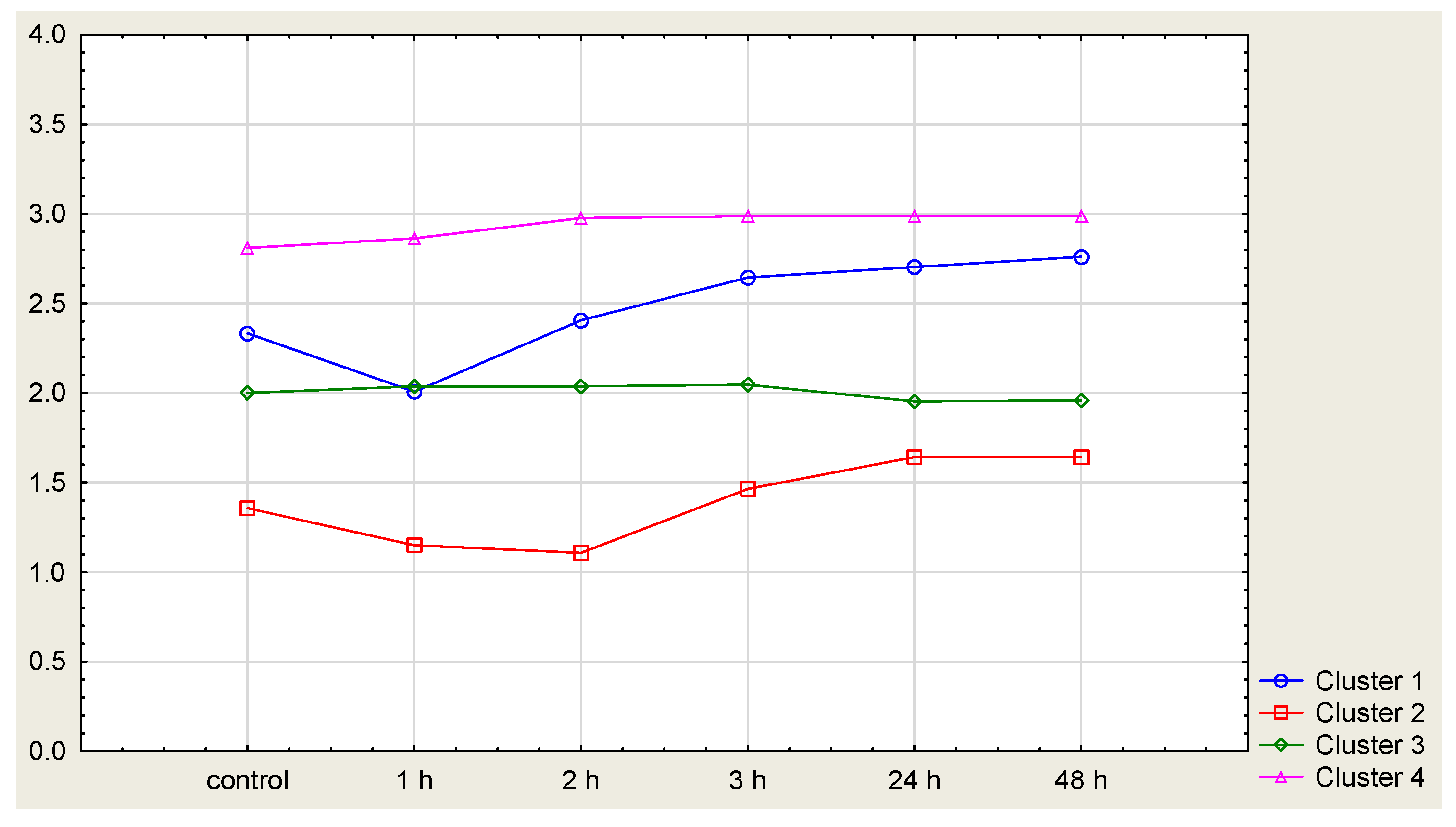
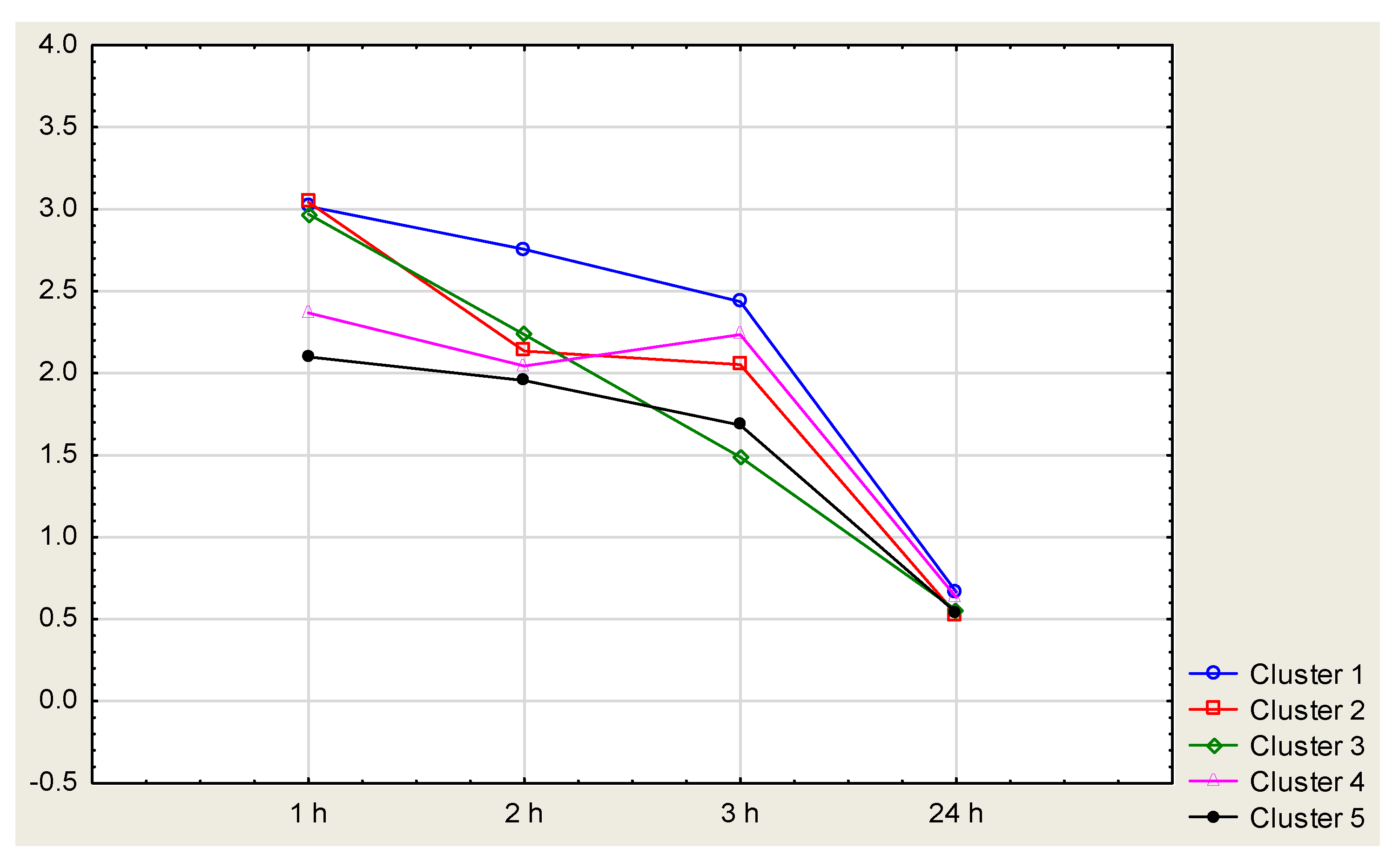
| No. | Elements of Cluster 1 | Elements of Cluster 2 | Elements of Cluster 3 | Elements of Cluster 4 | Elements of Cluster 5 |
|---|---|---|---|---|---|
| 1 | PO1-11x13 | PO1 | PO1-30 | PO2-4 | PO1-23 |
| 2 | PO1-12x18 | PO1-1 | PO1-32 | PO2-16 | PO1-28 |
| 3 | PO1-7x11 | PO1-2 | PO1-34 | PO2-25 | PO1-39 |
| 4 | PO2-13x9 | PO1-3 | PO2-1 | PO1-14x18 | PO2-3 |
| 5 | PO2-17x13 | PO1-7 | PO2-5 | PO1-15x21 | PO2-24 |
| 6 | PO2-23x25 | PO1-9 | PO2-6 | PO1-20x21 | PO2-27 |
| 7 | PO2-23x9 | PO1-11 | PO2-7 | PO1-9x16 | PO2-16x21 |
| 8 | PO2-1xPO1-1 | PO1-12 | PO2-8 | PO2-13x12 | PO2-19x24 |
| 9 | PO2-1xPO1-2 | PO1-13 | PO2-9 | PO2-22x13 | PO2-1xPO1-12 |
| 10 | PO2-4xPO1-11 | PO1-16 | PO2-12 | PO2-1xPO1-7 | PO2-1xPO1-9 |
| 11 | PO2-5xPO1-9 | PO1-17 | PO2-13 | PO2-4xPO1-2 | PO2-4xPO1-12 |
| 12 | PO2-6xPO1-2 | PO1-18 | PO2-17 | PO2-5xPO1-7 | PO2-4xPO1-3 |
| 13 | PO1-19 | PO2-18 | PO2-6xPO1-11 | PO2-9xPO1-1 | |
| 14 | PO1-20 | PO2-19 | PO2-6xPO1-12 | PO2-9xPO1-3 | |
| 15 | PO1-21 | PO2-21 | PO2-6xPO1-9 | PO2-9xPO1-7 | |
| 16 | PO1-22 | PO2-22 | PO2-9xPO1-2 | ||
| 17 | PO1-24 | PO2-23 | |||
| 18 | PO1-25 | PO2-28 | |||
| 19 | PO1-27 | PO2-31 | |||
| 20 | PO1-29 | PO2-32 | |||
| 21 | PO1-31 | ||||
| 22 | PO2 |
| No. | Elements of Cluster 1 | Elements of Cluster 2 | Elements of Cluster 3 | Elements of Cluster 4 |
|---|---|---|---|---|
| 1 | PO1-11 | PO1-2 | PO1-16 | PO1 |
| 2 | PO1-12 | PO1-39 | PO1-17 | PO1-1 |
| 3 | PO1-21 | PO2-12 | PO1-19 | PO1-3 |
| 4 | PO1-24 | PO2-17 | PO1-20 | PO1-7 |
| 5 | PO1-31 | PO2-18 | PO1-22 | PO1-9 |
| 6 | PO1-32 | PO2-21 | PO1-23 | PO1-13 |
| 7 | PO1-34 | PO2-22 | PO1-25 | PO1-18 |
| 8 | PO2 | PO2-31 | PO2-1 | PO1-27 |
| 9 | PO2-8 | PO2-32 | PO2-3 | PO1-28 |
| 10 | PO2-13 | PO1-20x21 | PO2-4 | PO1-29 |
| 11 | PO2-19 | PO1-9x16 | PO2-5 | PO1-30 |
| 12 | PO2-25 | PO2-1xPO1-12 | PO2-7 | PO2-6 |
| 13 | PO2-28 | PO2-6xPO1-12 | PO2-9 | PO2-16 |
| 14 | PO2-13x12 | PO2-9xPO1-2 | PO2-23 | PO2-24 |
| 15 | PO2-13x9 | PO1-12x18 | PO1-11x13 | |
| 16 | PO2-17x13 | PO2-22x13 | PO1-14x18 | |
| 17 | PO2-23x25 | PO2-1xPO1-7 | PO1-15x21 | |
| 18 | PO2-23x9 | PO2-1xPO1-9 | PO1-7x11 | |
| 19 | PO2-1xPO1-1 | PO2-19x24 | ||
| 20 | PO2-1xPO1-2 | PO2-4xPO1-11 | ||
| 21 | PO2-4xPO1-12 | PO2-4xPO1-2 | ||
| 22 | PO2-9xPO1-1 | PO2-4xPO1-3 | ||
| 23 | PO2-9xPO1-7 | PO2-5xPO1-7 | ||
| 24 | PO2-5xPO1-9 | |||
| 25 | PO2-6xPO1-11 | |||
| 26 | PO2-6xPO1-2 | |||
| 27 | PO2-6xPO1-9 | |||
| 28 | PO2-9xPO1-3 |
| No. | Elements of Cluster 1 | Elements of Cluster 2 | Elements of Cluster 3 | Elements of Cluster 4 | Elements of Cluster 5 |
|---|---|---|---|---|---|
| 1 | PO2-1 | PO1-7 | PO2-6 | PO2-23 | PO1-29 |
| 2 | PO2-3 | PO1-13 | PO2-9 | PO1 | PO1-30 |
| 3 | PO2-4 | PO1-20 | PO2-16 | PO1-28 | PO1-31 |
| 4 | PO2-5 | PO1-22 | PO2-19 | PO1-34 | PO1-32 |
| 5 | PO2-7 | PO1-12x18 | PO1-1 | PO1-39 | PO1-11x13 |
| 6 | PO2-8 | PO1-20x21 | PO1-2 | PO1-15x21 | PO1-14x18 |
| 7 | PO2-12 | PO1-9x16 | PO1-3 | PO2 | PO1-7x11 |
| 8 | PO2-13 | PO2-22x13 | PO1-9 | PO2-13x12 | PO2-1xPO1-1 |
| 9 | PO2-17 | PO2-1xPO1-2 | PO1-11 | PO2-13x9 | PO2-1xPO1-12 |
| 10 | PO2-18 | PO2-4xPO1-3 | PO1-12 | PO2-17x13 | PO2-1xPO1-7 |
| 11 | PO2-21 | PO2-5xPO1-9 | PO1-16 | PO2-19x24 | PO2-4xPO1-11 |
| 12 | PO2-22 | PO2-6xPO1-12 | PO1-17 | PO2-23x25 | PO2-4xPO1-2 |
| 13 | PO2-24 | PO2-9xPO1-1 | PO1-18 | PO2-1xPO1-9 | PO2-6xPO1-11 |
| 14 | PO2-25 | PO2-9xPO1-2 | PO1-19 | PO2-5xPO1-7 | PO2-6xPO1-2 |
| 15 | PO2-28 | PO2-9xPO1-7 | PO1-21 | PO2-9xPO1-3 | PO2-6xPO1-9 |
| 16 | PO2-31 | PO1-23 | |||
| 17 | PO2-32 | PO1-24 | |||
| 18 | PO2-4xPO1-12 | PO1-25 | |||
| 19 | PO1-27 | ||||
| 20 | PO2-23x9 |
| Mycelial Growth [Cluster 1] | Nematode Movement [Cluster 5] | Hyphal Knobs [Cluster 1 and Cluster 4] | Hyphal Knobs on PDA (IW = 0809) |
|---|---|---|---|
| PO1-11x13 | PO1-11x13 | PO1-11x13 | - |
| PO1-7x11 | PO1-7x11 | - | PO1-7x11 |
| PO2-1xPO1-1 | PO2-1xPO1-1 | PO2-1PO1-1 | - |
| PO2-1xPO1-2 | - | PO2-1xPO1-2 | PO2-1xPO1-2 |
| PO2-4xPO1-11 | PO2-4xPO1-11 | PO2-4xPO1-11 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudrys, P.; Nabrdalik, M.; Hendel, P.; Kolasa-Więcek, A.; Moliszewska, E. Trait Variation between Two Wild Specimens of Pleurotus ostreatus and Their Progeny in the Context of Usefulness in Nematode Control. Agriculture 2022, 12, 1819. https://doi.org/10.3390/agriculture12111819
Kudrys P, Nabrdalik M, Hendel P, Kolasa-Więcek A, Moliszewska E. Trait Variation between Two Wild Specimens of Pleurotus ostreatus and Their Progeny in the Context of Usefulness in Nematode Control. Agriculture. 2022; 12(11):1819. https://doi.org/10.3390/agriculture12111819
Chicago/Turabian StyleKudrys, Paweł, Małgorzata Nabrdalik, Patrycja Hendel, Alicja Kolasa-Więcek, and Ewa Moliszewska. 2022. "Trait Variation between Two Wild Specimens of Pleurotus ostreatus and Their Progeny in the Context of Usefulness in Nematode Control" Agriculture 12, no. 11: 1819. https://doi.org/10.3390/agriculture12111819





