1. Introduction
The neuropeptide kisspeptin (Kp) along with gonadotropin releasing hormone (GnRH), gonadotropins, and sex steroids shapes, the physiological mechanisms implicated in mammalian reproduction. The general organization and the overarching principles of this so-called hypothalamo-pituitary-gonadal axis are broadly conserved. However, several species do display peculiarities, which seemingly set them apart. The horse belongs to this category. These peculiarities complicate the management of horse reproduction and call for the development of novel tools and methodologies to control breeding.
In the horse, at odds with what is observed in most mammals, the luteinizing hormone (LH) surge reaches peak levels one day after ovulation, rather than preceding it [
1]. Furthermore, in contrast to what is observed in woman [
2] and ewe [
3], administration of Kp, under different physiological states and using various dosing regimens, failed to trigger follicle maturation and ovulation [
4,
5,
6,
7].
Up to now, attempts to induce ovulation in mares were based on the use of the shortest native form of Kp: Kp10 (last C-term, 10 amino acids of the peptide). Single injection, multiple injections, as well as perfusion of various durations and doses were tested. Even though Kp was able to stimulate gonadotropins release, none of the approaches triggered ovulation [
4,
5]. Several reasons have been adduced to explain this lack of effect. A potential explanation may be that the sequence of the equine Kp10 (eKp10) that was used in these experiments and bears one amino acid substitution in position two compared to Kp10 in mouse or human is less effective in stimulating GnRH secretion. This may reflect decreased activity of the molecule when bound to its cognate receptor, KISS1R. A second and complementary explanation may pertain to the half-life of Kp10, which is known to be extremely short, and to the possibility that Kp10 isoform may not be the most prominent in vivo. It might be that longer isoforms have increased half-life, hence prolonged action at KISS1R. Therefore, directly pitting the impact of eKp10 against that of other Kp10 and testing the impact of degradation-resistant analogs of Kp are both valid approaches to further our understanding of mare neuroendocrinology of breeding, which might further result in the development of useful molecules for breeding control.
Recently we have designed a Kp analog based on the sequence of the ovine Kp10 (oKp10), which is strictly conserved in most non-primate mammals with few exceptions, including the horse. This analog, called C6, showed improved pharmacological properties compared to native oKp10: increased potency, improved resistance to degradation, and more prolonged in vivo action [
8]. We have shown that this molecule synchronizes and triggers ovulations in small ruminants [
8,
9]. Grounded in these observations, we decided to design a similar analog based on the eKp10 sequence (eC6) and to compare its effect with that of the analog based on oKp10 sequence (oC6) in the mare. Our aims were to establish, in the mare, whether these Kp10-derived molecules have the same efficacy when their sequence is based on either eKp10 or oKp10, and to take advantage of the improved pharmacological properties of C6 to test whether a prolonged action could indeed effectively increase the release of gonadotropins and trigger ovulation.
Supporting the hypothesis that eKp10 sequence is less active, oC6 stimulated gonadotropins release better than eC6. Nevertheless, oC6 failed to trigger ovulation during the breeding season, which confirms that stimuli required to elicit ovulation in the mare might differ from those required in other domestic mammals.
2. Materials and Methods
2.1. Synthesis of Kp10 Analogs
eC6 and oC6 were synthesized following a protocol similar to the one described earlier for oC6 [
9]. Fmoc-based Solid-Phase Peptide Synthesis (SPPS) was carried out on a Prelude synthesizer from Gyros-Protein Technologies. Syntheses were performed at a 25 μmol scale on Rink Amide ChemMatrix resin. Protected amino acids (0.25 mmol, 10 equiv.) were coupled using HCTU (98 mg, 0.238 mmol, 9.5 equiv.) and iPr2NEt (87 μL, 0.5 mmol, 20 equiv.) in NMP (3 mL) for 30 min. Capping of potential unreacted amine groups was achieved by treatment with acetic anhydride (143 μL, 1.51 mmol, 60 equiv.), iPr2NEt (68 μL, 0.39 mmol, 15.5 equiv.) and HOBt hydrate (6 mg, 0.044 mmol, 1.8 equiv.) in NMP (3 mL) for 7 min. Fmoc group was removed by three successive treatments with 20% piperidine in NMP (3 mL) for 3 min. Standard side-chain protecting groups were used for Nα-Fmoc amino acids: Arg (Pbf), Arg (Me,Pbf), Asn (Trt), Ser (tBu), Trp (Boc) and Tyr (tBu). Isoglutamate residues (iGlu) were incorporated through the coupling of Fmoc-Glu-OtBu. N-terminal palmitamide (Palm) were introduced by coupling hexadecanoic acid using a slightly modified protocol, differing in the solvent used (a 3:1 dichloromethane/NMP mixture), and the duration (2 h). Leu8 (Kp10 numbering) was introduced through the coupling of (S)-2-azido-4-methylpentanoic acid (N3Leu-OH) [
10] and the triazole heterocycle used as a Gly7-Leu8 peptide bond surrogate (Ψ [Tz]) was generated through solid-supported copper-catalyzed azide/alkyne cycloaddition (CuAAC): N-Fmoc propargylamine [
11] (0.1 mmol, 4 equiv.) and CuBr.Me2S (21 mg, 0.1 mmol, 4 equiv.) were dissolved in NMP (2.5 mL) under an argon atmosphere. After addition of
iPr
2NEt (17 μL, 0.4 mmol, 4 equiv.), the mixture was transferred into a syringe fitted with a frit containing azidopeptide resin (0.025 mmol.) swollen in NMP. The green suspension was stirred by syringe rotation for 18 h at room temperature and the resin was washed successively with NMP, CH
2Cl
2, 1 M pyridine hydrochloride in 95:5 CH2Cl2/MeOH, CH
2C
l2 and DMF. The crude triazolo-lipopeptides were released from the resin with TFA/H2O/iPr3SiH/phenol, 87.5/5/2.5/5 for 3 h, and the peptide was precipitated by ten-fold dilution into an ice-cold 1:1 diethyl ether/petroleum ether mixture, recovered by centrifugation, and washed 3 times with diethyl ether. The crude peptides were purified by RP-HPLC (LaChromElite system equipped with a Nucleosil C18 column (300 Å, 5 μm, 250 × 10 mm, 3 mL min
−1 flow rate). HPLC analyses were carried out on a Chromaster system equipped with a chromolith High Resolution RP-18e column (150 Å, 10 × 4.6 mm, 3 mL·min
−1 flow rate). Elution solvents A and B were 0.1% TFA in H
2O and 0.1% TFA in MeCN, respectively. MS analyses were carried out on a single quadrupole Agilent 6120 mass spectrometer (ESI + mode). The experimental (obs.) and calculated (calcd.) m/z values correspond either to the [M + H]+ monoisotopic ions or to the [M + 2H]
2+ or [M + 2H]
3+ average masses. Purified peptides were quantified by UV spectrophotometry at λ = 280 nm for activity tests, using the reported molar absorption coefficients: ε280 (Trp) = 5500 L·mol
−1·cm
−1, ε280 (Tyr) = 1490 L·mol
−1·cm
−1 [
12].
2.2. Characterization of Analogs
oC6: Palm-iGlu-1Tyr-Asn-Trp-Asn-Ser-Phe-GlyΨ [Tz] Leu-Arg (Me)-10Tyr-NH2
HPLC purification: 57% B (isocratic) over 30 min, tR = 14.0 min.
HPLC analysis gradient: 45–90% B over 5 min, tR = 3.29 min.
MS: [M + H]+ calcd. for C86H123N20O18: 1723.9, obs.: 1723.8; [M + 2H]2+ calcd. for C86H124N20O18: 863.0, obs.: 862.8; [M + 3H]3+ calcd. for C86H125N20O18: 575.7, obs.: 575.4.
Characterization data are consistent to those previously reported [
9].
eC6: Palm-iGlu-1Tyr-Arg-Trp-Asn-Ser-Phe-GlyΨ [Tz] Leu-Arg (Me)-10Tyr-NH2
HPLC purification gradient: 50–90% B over 20 min, tR = 14.0 min.
HPLC analysis gradient: 50–90% B over 5 min, tR = 2.23 min.
MS: [M + H]+ calcd. for C88H129N22O17: 1766.0, obs.: 1766.0; [M + 2H]2+ calcd. for C88H130N22O17: 884.1, obs.: 883.8; [M + 3H]3+ calcd. for C88H131N22O17: 589.7, obs.: 589.5.
2.3. Calcium Mobilization Assay
To assess the potency and efficacy of the C6 analogs, a HEK-293 cell line stably transfected with hKISS1R and a Ca
2+ mobilization assay previously described [
13] were used. Briefly, cells were grown in DMEM (with GlutaMAX, high glucose, and without pyruvate), 10% fetal calf serum, 1% penicillin, 1% streptomycin, 200 μg·mL
−1 Geneticin, and HEPES (25 mM). The dynamics of intracellular Ca
2+ mobilization were monitored using Fluo4 NW Ca
2+ assay kit. Cells were plated 48 h before the experiment into 96-well blackplate (Dutscher, Brumath, France) at a density of 40,000 cells/well. On the day of the experiment, test compounds were diluted from stock solution in nonbinding plates (VWR, Strasbourg, France) to 20× the final desired concentration (ranging from 1 pM to 1 μM). Basal fluorescence was measured 5 times at 7 s interval with a plate reader (PolarStar Optima, BMG Labtech). Immediately after basal reading, test compounds were added to each well and intracellular Ca
2+ dynamics monitored for 7 min. To generate concentration activity curves, mean basal fluorescence was subtracted to the value obtained after stimulation. The area under the curve (AUC) was calculated and plotted against concentrations. Data were fitted to a sigmoid curve generated with GraphPad Prism 5, and the EC
50 was automatically calculated.
2.4. Animals
All experiments with mares were conducted in accordance with the French national law (authorizations 37–118 and G 37-175-2 of the French Ministry of Agriculture) implementing the European Communities Council Directive 86/609/EEC and validated by the local Animal Ethics Committee (File numbers: 201907251345605 and 2019101117278451). Experiments were performed using adult (4–16 yr; 200–350 kg) Welsh pony mares from the UEPAO-INRAE (Unité Expérimentale PAO, INRAE, 2018. Animal Physiology Experimental Facility, DOI: 10.15454/1.5573896321728955E12) herd of Nouzilly, France (latitude 47° N). Animals were kept indoors in groups of two and exposed to the natural photoperiod. They were fed with wheat straw and given water ad libitum. A hay supplement was also provided twice daily.
2.5. Intravenous Perfusion and Blood Collection
After application of cold spray on the neck, an intravenous silicone catheter (inner diameter 1.2 mm, outer diameter 2.0 mm; Nutricath S; Vygon; France) was introduced into the jugular vein and fixed with two stitches. For each sample, 3–5 mL of blood was collected and 2.5 mL of sterile saline solution (NaCl 0.9%; B|Braun; France) with heparin (0.1%; Héparine choray® 2500 UI/5 mL; Sanofi; France) was injected to flush the catheter. Blood samples for progesterone assay were collected using heparin vacutainers. Samples were centrifuged at 4000 rpm for 10 min at room temperature. Plasma was stored at −20 °C.
2.6. Experimental Design
Experiments 1 and 2 were performed during the nonbreeding (anoestrus) season (December–January). A mare was considered acyclic when serum concentration of progesterone had been <1 ng·mL
−1 for at least four consecutive weeks based on bi-weekly blood sampling. Experiment 3 was performed during the breeding season (May–June). During the breeding season, luteolysis was induced by intramuscular administration of 250 mg of prostaglandin F2 alpha (PGF2α) (cloprosténol, Estrumate
®, Intervet, France), which was administered 5–6 days after ovulation. Three to five days after PGF2α treatment, ovaries were scanned daily by ultrasonography to track follicular growth in order to decide the appropriate time to deliver oC6 or eC6. The ultrasonography was equipped with a 7.5 MHz linear probe (Exapad, ECM, Angoulême, France). See
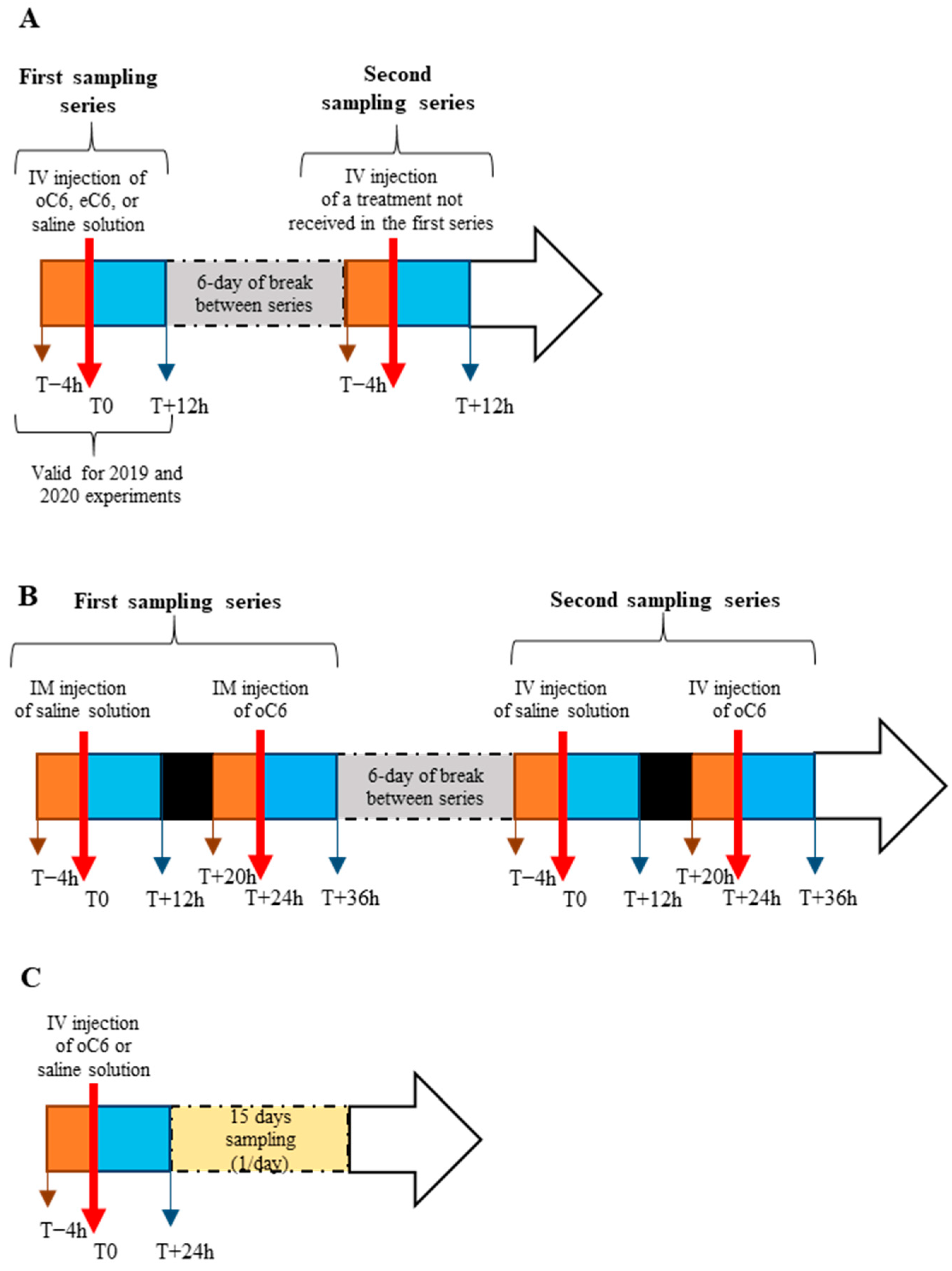
Figure 1A for a scheme of the experimental protocols.
Experiment 1 (non-breeding season). The goal of this experiment was to test the effect of an intravenous (iv) administration of either 150 nmol of oC6 or eC6 (0.259 and 0.265 mg respectively) on plasma gonadotropins level.
Figure 2A,B report the structure of o and eC6. Eleven mares were used, and the experiment was performed in two consecutive years (2019, 2020). In 2019, 4 mares received an iv dose of 150 nmol of eC6 in 2 mL of saline solution. In 2020, 7 mares were used in two experiments separated by 6 days and received 150 nmol of eC6 diluted in 2 mL, 150 nmol of oC6 diluted in 2 mL, or 2 mL of vehicle (saline solution). Hence, in 2020, each mare received two of the three treatments: vehicle and eC6, vehicle and oC6, or eC6 and oC6. To summarize, 9 mares received the eC6, 5 received the oC6, and another 4 got the saline solution. Four hours before the treatment, blood samples were collected every hour. After treatment, blood was collected every 30 min during the first 8 h, then hourly until the end of the experiment.
Experiment 2 (non-breeding season). The aim of this experiment was to compare the effect of an iv versus an intramuscular (im) injection of 150 nmol of oC6. The experiment was performed on 7 mares and included two parts separated by a 6-day interval. Mares were divided into two groups. In the first part of the experiment, the first group received an iv administration of 2 mL of saline and twenty-four hours later, an iv injection of 150 nmol of oC6; the second group received an im administration of 2 mL of saline and twenty-four hours later an im injection of 150 nmol of oC6. In the second part of the experiment, treatments were switched between groups (see
Figure 1B for a scheme of the experimental protocol).
Blood samples were collected every hour during four hours before treatment, and after treatment every 30 min during the first 8 h and every hour until the experiment ended, 12 h post-treatment.
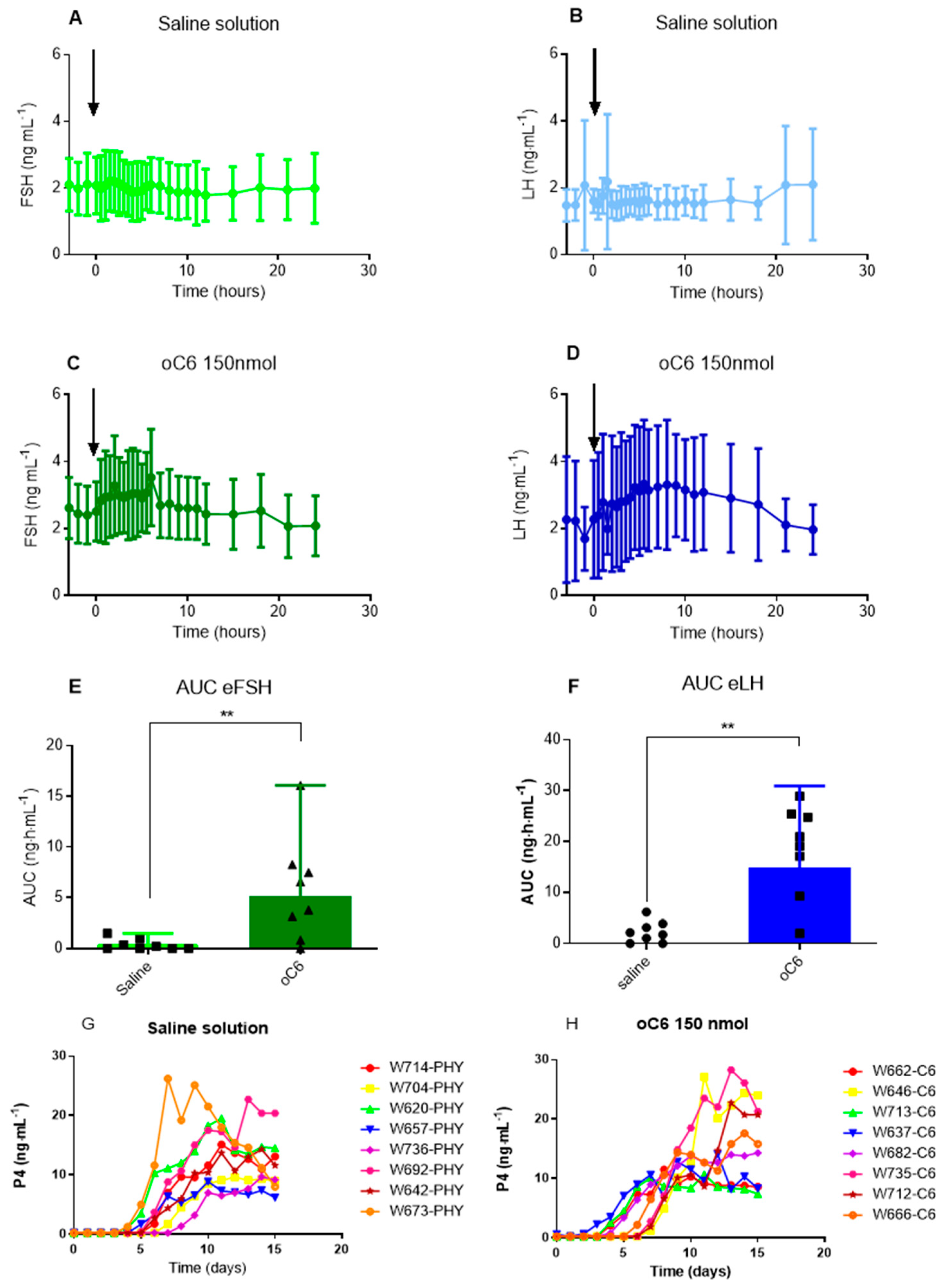
Experiment 3 (breeding season). The aim of this experiment was to assess the effect of an iv administration of 150 nmol of oC6 during the pre-ovulatory phase. When a follicle diameter reached 34–37 mm in size and a uterine oedema was observed, mares were randomly assigned to one of two groups (n = 8/group): treated group (single intravenous injection of 150 nmol of oC6) and control group (intravenous injection of saline solution). Blood was sampled every hour during 4 h before injection, every 30 min during the first 8 h post-injection, every hour until 14 h post-injection, and every 3 h until the end of the experiment (24 h post-treatment). Blood samples were collected once daily during fifteen days post-treatment to assess progesterone plasma concentration (see
Figure 1C for a scheme of the experimental protocol). Ovaries were scanned daily by ultrasonography to track follicular growth and ovulation.
2.7. Hormone Assays
Plasma LH concentration was assayed by RIA following a procedure adapted from Guillaume et al. [
14]. All samples were run in duplicates. Standards were prepared using plasma from a mare vaccinated against GnRH with an equine reference hormone (eLH, NHPP AFP 5130A; Dr. A.F. Parlow). The eLH was labelled with 125I (PerkinElmer NEN Radiochemicals) using Iodogen (Pierce, Interchim). The antibody (anti-LH AFP-240580; Dr. A.F. Parlow) was used at a final dilution of 1:440,000. The detection limit of the assay was 0.25 ng·mL
−1. The mean intra- and interassay coefficients of variation (CV) for plasma containing 3 ng·mL
−1 of LH (corresponding to 50% bond) were 4% and 10%, respectively.
Plasma FSH concentration was assayed by sandwich ELISA. All samples were run in duplicates. Standards were prepared in a solution of DPBS, BSA 0.2%, Tween 20 0.01% using as a standard the eFSH CY558. Plates were coated and incubated overnight with 100 µL of mouse monoclonal anti αeCG antibody (1:50,000; #a89A2-001 gift from Y. Combarnous; [
15]) in carbonate/bicarbonate (pH = 10) buffer a +4 °C. Overcoating was performed by one hour incubation with 150 µL of Seablock (UP40301A; Interchim) at room temperature (RT). One hundred microliters of plasma diluted to ½ in DPBS, BSA 0.2%, and Tween 20 0.01% were incubated overnight at 20 °C. Four washes of 2 min were performed after each incubation. Samples were incubated in rabbit polyclonal anti eFSHβ (100 µL; 1:40,000, #803 kind gift from Y. Combarnous; [
15]) for one hour at RT. Incubation was terminated by the washing step followed by a second incubation for one hour at RT with a goat polyclonal anti rabbit IgG coupled to peroxidase (100 µL, 1:40,000, Jackson Immunoreasearch). Finally, an incubation of 20 min with the tetramethylbenzidine ELISA substrate standard solution (100 µL, UP664780, Interchim) was performed at RT in the dark. The reaction was stopped by adding 50 µL of sulphuric acid 2N. Absorbance was measured at 450 and 620 nm using a Sunrise instrument associated with the Magellan V5 software, and the difference between values was used for concentration calculation. The detection limit was 0.3 ng·mL
−1. The intra-assay CV for PBS containing 10 ng·mL
−1 eFSH is 1.93%.
Plasma concentration of progesterone (P4) was measured by a sandwich ELISA following a procedure previously described [
8]. The detection limit was 0.25 ng·mL
−1. The intra-assay CV for PBS containing 3.5 ng·mL
−1 of P4 is of 3.7%.
2.8. Statistical Analyses
For repeated measures, the statistical analysis was performed using an ANOVA on repeated measures followed by a Dunnett multiple comparisons test when a difference was observed (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7). The AUC was calculated with the software GraphPad V6 using the trapezoid rule and subtracting the basal value obtained by the pre-treatment sampling. For comparison of AUC one-way ANOVA followed by Tukey’s multiple comparisons test (
Figure 3), Kruskal-Wallis test followed by Dunn’s post hoc test (
Figure 4,
Figure 5 and
Figure 6), or Mann-Whitney test (
Figure 7) were used. Progesterone data analysis was performed with the Gehan-Breslaw-Wilcoxon Test. Data are expressed either as mean ± SD or as median and interquartile range. Statistical analyses were performed with the GraphPad V6 software.
4. Discussion
The Kp system is a key element of mammalian reproductive system stimulating ovulation in several mammalian species. Yet, previous works in the mare have fallen short to show that this is the case for this species, casting doubts about Kp system holding a central role in eliciting mare ovulation. Our results obtained using Kp analogs further illustrate the difficulty of stimulating ovulation in the mare through the kisspeptin system but clarify some issues and suggest possible ways forward to solve this conundrum.
The first attempt to test Kp effect on gonadotropins secretion and ovulation was realized with the rat Kp10 isoform, the sequence of which is identical to that of the ovine Kp10 (YNWNSFGLRY-NH
2). The molecule was injected into light-horse mares in dioestrous at different concentrations (1, 500, and 1000 µg per mare). Even though higher concentrations increased LH and FSH plasma levels, this increase was short lasting (less than 4 h) and there was no effect on ovulation timing [
16]. The injection of human Kp10 (YNWNSFGLRF-NH
2) produced similar results with stimulation of LH secretion but no ovulation (7). Further studies investigated if repeated administrations of the equine Kp10 (eKp10, YRWNSFGLRY-NH
2) would be more effective in triggering ovulation. A first group of mares received 0.5 mg eKp10 every 4 h from day 16 post-ovulation until ovulation occurred, or up to day 25 post-ovulation. A second group received the same treatment, but it started at the beginning of oestrus and in the presence of a follicle of >30 mm in diameter. Under these experimental conditions, no effect was observed on either LH or FSH plasma concentration and on ovulation timing [
5]. In another set of experiments, performed during the breeding season, mares were injected with eKp10 (0.5 mg iv) every 4 h for 2 days. This repeated administration procedure did not trigger an LH surge, nor did it sustain an increase in serum LH throughout the experimental period [
17]. We also performed several attempts to induce ovulation using eKp10 at different dosing regimens (bolus injection, short- and long-term perfusion), different concentrations (0.5, 1, 3, and 6 mg per hours) and under different physiological states (early, middle and late follicular phase). Even though we were able to stimulate gonadotropins release, all treatments nevertheless failed to trigger ovulation [
4]. Several hypotheses could be put forth to explain this lack of efficacy in triggering ovulation: (i) a difference in the general endocrinology governing ovulation in the mare (ii) the neuroanatomy and functioning of the mare’s GnRH system, and (iii) the efficacy and the pharmacokinetics of Kp10 and in particular that of eKp10.
Concerning this last point, eKp10 differs from oKp10 by a single amino acid substitution in position two, where an Asn is substituted by an Arg. This mutation resides outside the last five amino acids in the C-terminal position, which are thought to be the Kp10 pharmacophore, which is the minimal requested region essential for the activity of the molecule [
18]. Nevertheless, we have previously shown that in vitro, when tested on HEK-293 cells transfected with the human KISS1R, eKp10 is less active than oKp10 [
19]. A similarly reduced activity compared to oKp10 was also observed when administered to ewes and LH plasma concentration used as a biomarker of efficacy [
19].
We have previously shown that the Kp analog oC6, designed on the scaffold of the ovine Kp10 sequence, induces ovulation in the ewe and in the goat [
8,
9]. This molecule has an improved pharmacokinetics profile compared to oKp10. To explore further if the problem with the lack of efficacy would reside in the sequence of eKp10, we prepared a C6 modified according to the eKp10 sequence, eC6, and compared its effect to that of oC6. When tested in a Ca
2+ mobilization assay in HEK-293 cells, expressing the hKISS1R, the two molecules showed similar potency and efficacy. However, when tested for their capacity to increase plasma gonadotropins concentration in the mare, oC6 was more effective than eC6. Inconsistency of in vitro and in vivo results might be dependent on the peculiarities of the horse reproductive and Kp systems or pertain to specific receptor-ligand interactions. Considering this last aspect, it has been shown that compounds’ kinetics properties, especially the retention time on the receptor, might have a significant impact on their in vivo effect. Actually, the retention time may even be of greater importance for in vivo drug activity than its affinity [
20]. This information is not available for the compounds we used, and it would be an interesting point for further investigations in order to understand the reason of the discordance between in vivo and in vitro results.
Neither of the two analogs induced any significant increase in plasma LH concentration when tested during the non-breeding season. LH plasma concentration during the non-breeding season is at the lower limit of sensitivity of our assay. For practical purposes, the points below assay sensitivity were set equal to the lower detection limit of the assay. By doing this, we may have erased possible differences in the lower concentrations and overlooked an increase in LH. It should also be mentioned that equine LH is released in various isoforms that could vary through the oestrous cycle [
21]. Hence, the antibodies used for RIA may have a different affinity for the different isoforms and may prevent detection of subtle changes in LH concentration. On the other hand, during the non-breeding season, and during the transition to the breeding season LH level is at its year lowest, whereas FSH is higher and declines prior to the first ovulation [
22]. Hence, it is possible that a strong physiological inhibition of LH is in place at this time of the year, rendering it difficult to stimulate LH increase.
Because im injection could assure a longer action of the injected drug and would be a preferred route of administration in a farm setting, we compared the effect of an im injection with that of an iv injection. However, the im injection produced a smaller increase in gonadotropins compared to the iv administration. It should be mentioned that oC6 aqueous solution at the concentration used for the injection tends to form a gel. When injecting im, the gel formation might significantly slow the passage into the blood of the molecule. Conversely, when injected in the blood, the dilution effect would facilitate the free circulation of the molecule. Hence, at the dose we used, the quantity of C6 reaching the blood circulation at any given time after the im injection could have been too low to trigger an increase in gonadotropins. The use of a higher dose might have a more pronounced effect, which should be tested in further experiments.
To check if oC6 would trigger ovulation during the breeding season we decided to treat mares when a pre-ovulatory follicle was detected. Compared to other species, the mare has an unusually irregular duration of the follicular phase ranging from 2 to 14 days, and a highly variable pre-ovulatory follicle diameter ranging from 31 to 59 mm in size. We decided to inject C6 when the follicle diameter ranges from 34–37 mm, a size that should be appropriate to elicit and synchronize ovulations in most mares. Under these conditions, treatment with oC6 increased both LH and FSH plasma concentration and all mares ovulated. However, transrectal ultrasonography and progesterone plasma level revealed that ovulation timing in the treated group was not different from that of the control mares.
Should we conclude from these results that stimulation of the Kp system is not required to induce ovulation in the mare? Answering this question is not trivial and could have important implications for a possible use of Kp analogs to manage reproduction in the mare. Several elements should be taken into account when considering the Kp system and the horse ovulation endocrinology. One might argue that this is explained by the various peculiarities of the mare endocrine reproductive system, even though the exact reason(s) as to why this may preclude the actioin of Kp are not forthcoming [
23]. However, there are alternative, and not mutually exclusive, explanations that revolve around the existence of multiple endogenous Kp isoforms, each having its own level of activity in vivo. Even though data on this topic are limited they might have interesting implications. It has been shown that administration of human Kp54 (hKp54) in the mouse triggers a much longer increase in LH compared to hKp10 [
24]. Based on experiments showing that hKp54 could activate GnRH neurons, as demonstrated by colocalization with the immediate early gene c-Fos, it has been proposed that hKp54, but not hKp10, would pass the blood brain barrier [
24]. In male rats, hKp54 more potently stimulates LH and testosterone secretion than hKp14 and hKp10 [
25]. Furthermore, Kp52, which is the longest endogenous isoform in rodents, was more active than human and rodents’ Kp10 in stimulating LH release in male rats [
26]. It is therefore possible that: (i) different Kp isoforms would not be equivalent in their capacity to stimulate the reproductive system and (ii) different isoforms might have different roles depending on the species. It should also be pointed out that even though Kp10 has been the most widely used pharmacological tool for exploring the physiology of the Kp system, there is no formal proof that this is the endogenous form naturally used in mammals to modulate reproductive physiology. Therefore, it could be that processing of the Kp precursor, leading to different isoforms abundance in different species, may account for a species-specific role of a particular isoform in the reproductive physiology of a given species. Further experiments will be required to identify the various isoforms in the horse, the complement of which remains completely unknown, in order to probe this hypothesis more rigorously.