NaCl Accumulation, Shoot Biomass, Antioxidant Capacity, and Gene Expression of Passiflora edulis f. Flavicarpa Deg. in Response to Irrigation Waters of Moderate to High Salinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
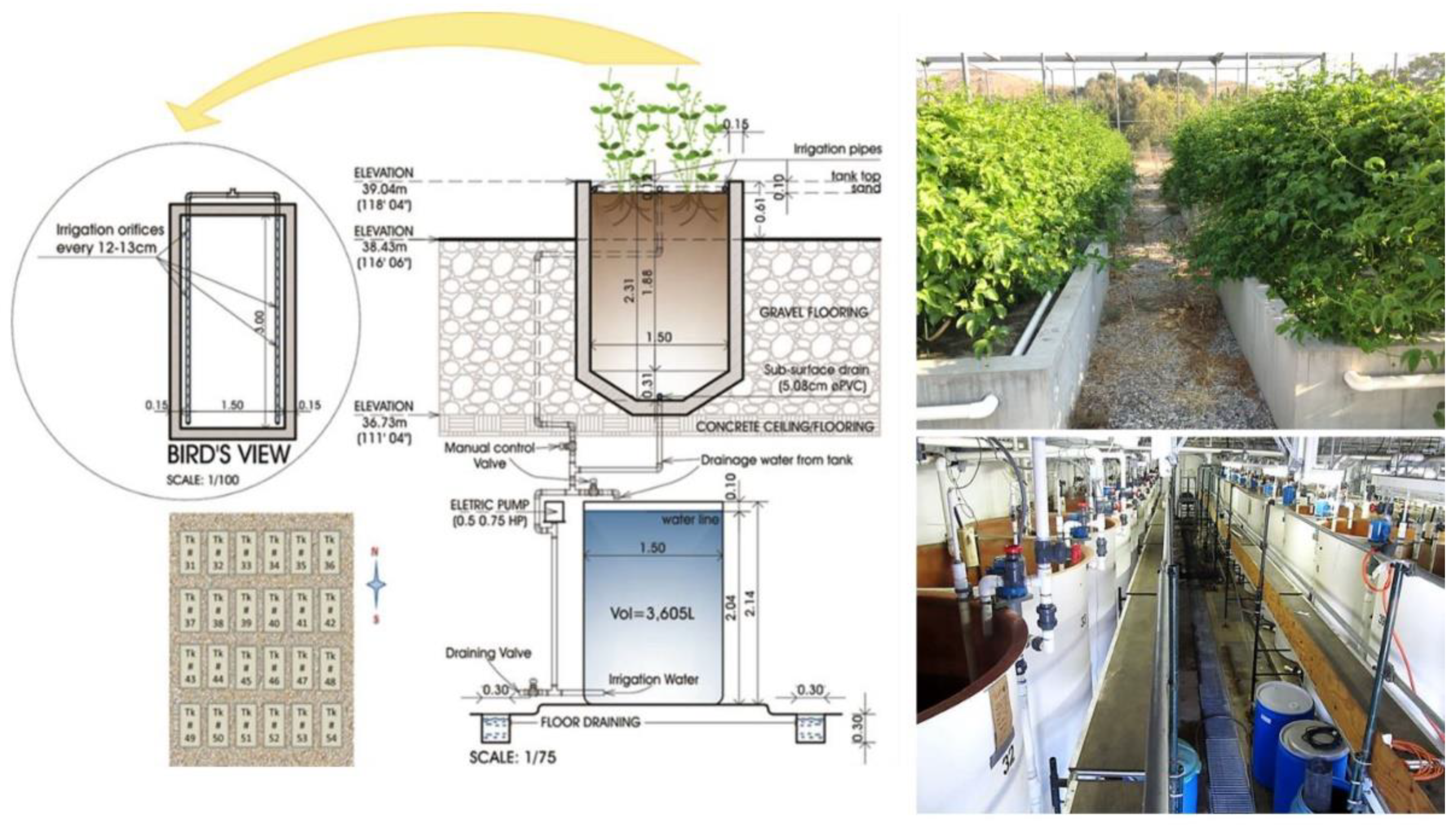
2.2. Irrigation Water Salinity and Sand Tank Cultivation
2.3. Plant Mineral Nutrition
2.4. Lysimeter Tanks
2.5. Sample Collection
2.6. Antioxidant Capacity Tests
2.7. Expression Analyses
3. Results
3.1. Effects of Leaf Na and Cl Accumulation on Shoot Biomass
3.2. Effects of Na and Cl Accumulation, Drying Method, and Leaf Age on Antioxidant Capacity
3.3. Effect of Salinity on Gene Expression
4. Discussion
4.1. Effects of Na and Cl Accumulation on Shoot Biomass
4.2. Effects of Na and Cl Accumulation, Drying Method, and Leaf Age on Antioxidant Capacity
4.3. Effect of Na and Cl Accumulation on Gene Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, J.T.C.; Petry, F.C.; Tobaruela, E.C.; Mercadante, A.Z.; Gloria, M.B.A.; Costa, A.M.; Lajolo, F.M.; Hassimotto, N.M.A. Brazilian native passion fruit (Passiflora tenuifila Killip) is a rich source of proanthocyanidins, carote-noids, and dietary fiber. Food Res. Int. 2021, 147, 110521. [Google Scholar] [CrossRef] [PubMed]
- Bernacci, L.C.; Soares-Scott, M.D.; Junqueira, N.T.V.; Passos, I.R.S.; Meletti, L.M.M. Passiflora edulis Sims: The correct taxonomic way to cite the yellow passion fruit (and of others colors). Rev. Bras. Frutic. 2008, 30, 566–576. [Google Scholar] [CrossRef]
- Sá, C.P.; Neto, R.C.A.; Negreiros, J.R.S.; Nogueira, S.R. Coeficientes Técnicos, Custos de Produção e Indicadores Economicos Para o Cultivo do Maracujá BRS Gigante Amarelo, No Acre; Embrapa: Rio Branco, Acre, Brazil, 2015. [Google Scholar]
- Altendorf, S. Minor Tropical Fruits: Mainstreaming a Niche Market; Food and Agricultural Organization: Rome, Italy, 2018; pp. 67–75. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Tropical_Fruits/Documents/Minor_Tropical_Fruits_FoodOutlook_1_2018.pdf (accessed on 10 September 2022).
- Li, H.; Zhou, P.; Yang, Q.; Shen, Y.; Deng, J.; Li, L.; Zhao, D. Comparative studies on anxiolytic activities and fla-vonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’. J. Ethnopharmacol. 2011, 133, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Newall, C.A.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines. A Guide for Health-Care Professionals; The Pharmaceutical Press: London, UK, 1996; p. 296. [Google Scholar]
- Fisher, A.A.; Purcell, P.; Couteur, D.G.L. Toxicity of Passiflora incarnata L. J. Toxicol. Clin. Toxicol. 2000, 38, 63–66. [Google Scholar] [CrossRef]
- Machado, C.F.; Jesus, F.N.; Ledo, C.A.S. Divergencia genetica de acessos de maracuja utilizando descritores quantitativos e qualitativos. Rev. Bras. Frutic. 2015, 37, 442–449. [Google Scholar] [CrossRef]
- Dias, T.J.; Cavalcante, L.F.; Pereira, W.E.; Freire, J.L.D.O.; Souto, A.G.D.L. Irrigação com água salina em solo com biofertilizante bovino no crescimento do maracujazeiro amarelo. Semin. Cienc. Agrar. 2013, 34, 1639–1652. [Google Scholar] [CrossRef]
- Freire, J.L.O.; Cavalcante, L.F.; Rebequi, A.M.; Dias, T.J.; Brehm, M.A.S.; Santos, J.B. Quality of yellow passion fruit juice with cultivation using different organic sources and saline water. IDESIA 2014, 32, 79–87. [Google Scholar] [CrossRef]
- Cavalcante, L.F.; Cavalcante, Í.H.L.; Júnior, F.R.; Beckmann-Cavalcante, M.Z.; Santos, G.P. Leaf-macronutrient status and fruit yield of biofertilized yellow passion fruit plants. J. Plant Nutr. 2012, 35, 176–191. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Jesus, O.N.; Soares, T.L.; Santos, I.S.; Oliveira, E.J.; Filho, M.A.C. Growth, physiological, ana-tomical and nutritional responses of two phenotypically distinct passion fruit species (Passiflora L.) and their hybrid under saline conditions. Sci. Hort. 2020, 263, 109037. [Google Scholar] [CrossRef]
- Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; Rios, A.O. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L. Drying affects artemisinin, dihydroartemisinic acid, artemisinic acid, and the anti-oxidant capacity of Artemisia annua L. leaves. J. Agric. Food Chem. 2010, 58, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, C.B.M.; Jesus, O.N.; Santos, E.S.L.; Corrêa, R.X.; Souza, A.P. Genetic breeding and diversity of the genus Passiflora: Progress and perspectives in molecular and genetic studies. Int. J. Mol. Sci. 2014, 15, 14122–14152. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, C.B.M.; Faleiro, F.G.; Jesus, O.N.; Santos, E.S.L.; Souza, A.P. The Genetic Diversity, Conservation, and Use of Passion Fruit (Passiflora spp.). In Genetic Diversity and Erosion in Plants: Case Histories; Ahuja, M.R., Jain, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 215–231. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Suarez, D.L.; Simunek, J. UNSATCHEM: Unsaturated water and solute transport model with equilibrium and kinetic chemistry. Soil Sci. Soc. Am. J. 1997, 61, 1633–1646. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity [oxygen radical absorbance capacity (ORAC)] of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Sandhu, D.; Liu, X.; Halvorson, J.J. Spinach (Spinacea oleracea L.) response to salinity: Nutritional value, physiological parameters, antioxidant capacity, and gene expression. Agriculture 2018, 8, 163. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Cornacchione, M.V.; Liu, X.; Suarez, D.L. Nutrient composition, forage parameters, and antioxi-dant capacity of alfalfa (Medicago sativa, L.) in response to saline irrigation water. Agriculture 2015, 5, 577–597. [Google Scholar] [CrossRef]
- Munhoz, C.F.; Santos, A.A.; Arenhart, R.A.; Santini, L.; Monteiro-Vitorello, C.B.; Vieira, M.L.C. Analysis of plant gene expression during passion fruit–Xanthomonas axonopodis interaction implicates lipoxygenase 2 in host de-fence. Ann. Appl. Biol. 2015, 167, 135–155. [Google Scholar] [CrossRef]
- Freitas, M.S.M.; Monnerat, P.H.; Vieira, I.J.C. Mineral deficiency in Passiflora alata Curtis: Vitexin bioproduction. J. Plant Nutr. 2008, 31, 1844–1854. [Google Scholar] [CrossRef]
- Hurtado-Salazar, A.; Silva, D.F.P.; Ceballos-Aguirre, N.; Ocampo-Pérez, J.; Bruckner, C.H. Promissory Passiflora L. species (Passifloraceae) for tolerance to water-salt stress. Rev. Colomb. Cienc. Hortic. 2020, 14, 44–49. [Google Scholar] [CrossRef]
- Soares, F.A.L.; Gheyi, H.R.; Viana, S.B.A.; Uyeda, C.A.; Fernandes, P.D. Water salinity and initial development of yellow passion fruit. Sci. Agric. 2002, 59, 491–497. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Fruit yield and survival of five commercial strawberry cultivars under field cultivation and salinity stress. Sci. Hort. 2019, 243, 401–410. [Google Scholar] [CrossRef]
- Suarez, D.L.; Celis, N.; Ferreira, J.F.S.; Reynolds, T.; Sandhu, D. Linking genetic determinants with salinity toler-ance and ion relationships in eggplant, tomato and pepper. Sci. Rep. 2021, 11, 16298. [Google Scholar] [CrossRef] [PubMed]
- Celis, N.; Suarez, D.L.; Wu, L.; Li, R.; Arpaia, M.L.; Mauk, P. Salt tolerance and growth of 13 avocado rootstocks related best to chloride uptake. HortScience 2018, 53, 1737. [Google Scholar] [CrossRef]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the Move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depré, S.; Thomine, S.; Filleur, S. Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [CrossRef]






| Treatment | Target ECw | Averaged Final ECw | pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | PO43− | NO3− | CO3H− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | dS m−1 | mmolc L−1 | ||||||||||
| RMW | 0.6 | 7.7 | 0.1 | 1.88 | 3.32 | 0.80 | 1.01 | 1.30 | ND | 0.38 | 3.4 | |
| T1 | 3.0 | 3.0 | 7.8 | 5.9 | 13.62 | 3.98 | 2.80 | 1.99 | 14.70 | 1.0 | 8.62 | 0.0 |
| T2 | 6.0 | 6.0 | 7.7 | 5.9 | 32.12 | 12.88 | 7.40 | 25.99 | 22.70 | 1.0 | 8.62 | 0.0 |
| T3 | 12.0 | 12.0 | 7.3 | 5.9 | 70.12 | 27.18 | 14.50 | 85.39 | 22.70 | 1.0 | 8.62 | 0.0 |
| ECw | K | Na | Cl | P | Ca | Mg | S | N | Fe | Zn | Cu | B | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g kg−1 DM | mg kg−1 DM | |||||||||||
| 3.0 | 20.2 a | 9.8 c | 10.2 c | 2.1 a | 7.8 a | 2.0 a | 3.6 a | 34.3 b | 116 a | 50.5 a | 3.7 a | 118 a | 11.5 a |
| 6.0 | 20.1 a | 18.4 b | 22.8 b | 2.5 a | 7.8 a | 2.1 a | 3.9 a | 41.6 ab | 119 a | 39.9 a | 3.6 a | 56.0 b | 13.8 a |
| 12.0 | 20.5 a | 29.4 a | 43.3 a | 2.4 a | 9.8 a | 2.7 a | 3.2 a | 43.2 a | 138 a | 51.3 a | 3.3 a | 83.3 ab | 11.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.F.S.; Liu, X.; Suddarth, S.R.P.; Nguyen, C.; Sandhu, D. NaCl Accumulation, Shoot Biomass, Antioxidant Capacity, and Gene Expression of Passiflora edulis f. Flavicarpa Deg. in Response to Irrigation Waters of Moderate to High Salinity. Agriculture 2022, 12, 1856. https://doi.org/10.3390/agriculture12111856
Ferreira JFS, Liu X, Suddarth SRP, Nguyen C, Sandhu D. NaCl Accumulation, Shoot Biomass, Antioxidant Capacity, and Gene Expression of Passiflora edulis f. Flavicarpa Deg. in Response to Irrigation Waters of Moderate to High Salinity. Agriculture. 2022; 12(11):1856. https://doi.org/10.3390/agriculture12111856
Chicago/Turabian StyleFerreira, Jorge F. S., Xuan Liu, Stella Ribeiro Prazeres Suddarth, Christina Nguyen, and Devinder Sandhu. 2022. "NaCl Accumulation, Shoot Biomass, Antioxidant Capacity, and Gene Expression of Passiflora edulis f. Flavicarpa Deg. in Response to Irrigation Waters of Moderate to High Salinity" Agriculture 12, no. 11: 1856. https://doi.org/10.3390/agriculture12111856
APA StyleFerreira, J. F. S., Liu, X., Suddarth, S. R. P., Nguyen, C., & Sandhu, D. (2022). NaCl Accumulation, Shoot Biomass, Antioxidant Capacity, and Gene Expression of Passiflora edulis f. Flavicarpa Deg. in Response to Irrigation Waters of Moderate to High Salinity. Agriculture, 12(11), 1856. https://doi.org/10.3390/agriculture12111856








