Ozone in Droplets and Mist in Inhibition of Phytopathogenic Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of Ozonated Water
2.2. Preliminary Experiments (First Phase)
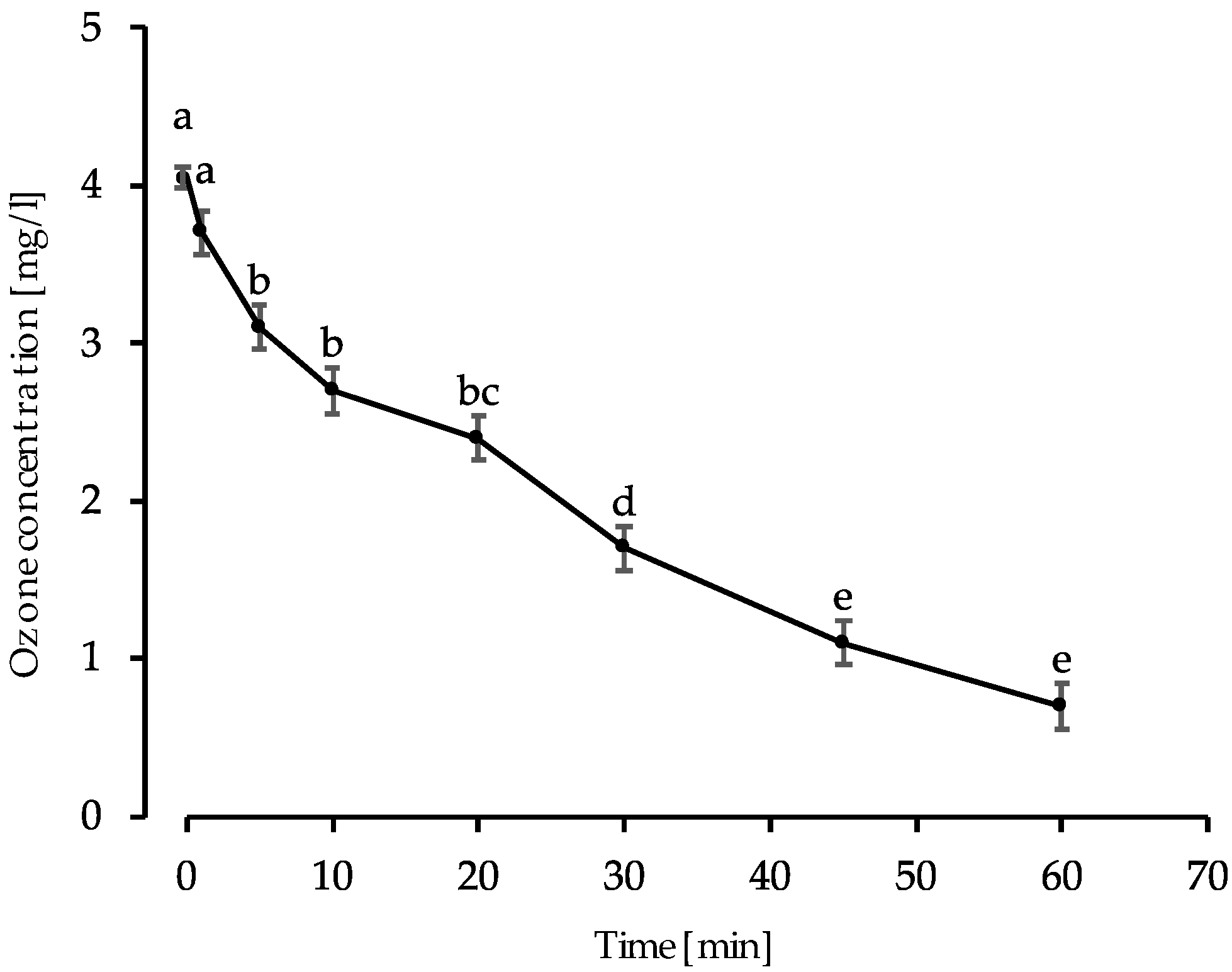
2.2.1. The Assessment of Ozone Stability in Tap Water
2.2.2. The Optimization of Microbial Reduction by Aqueous Ozone
2.3. Main Experiment (Second Phase)
2.3.1. Microbial Strains and Media
2.3.2. In Vitro Treatment of Selected Phytopathogens with Aqueous Ozone
2.4. Statistical Analysis
3. Results
3.1. The Stability of Aqueous Ozone and Reduction of Viable E. coli
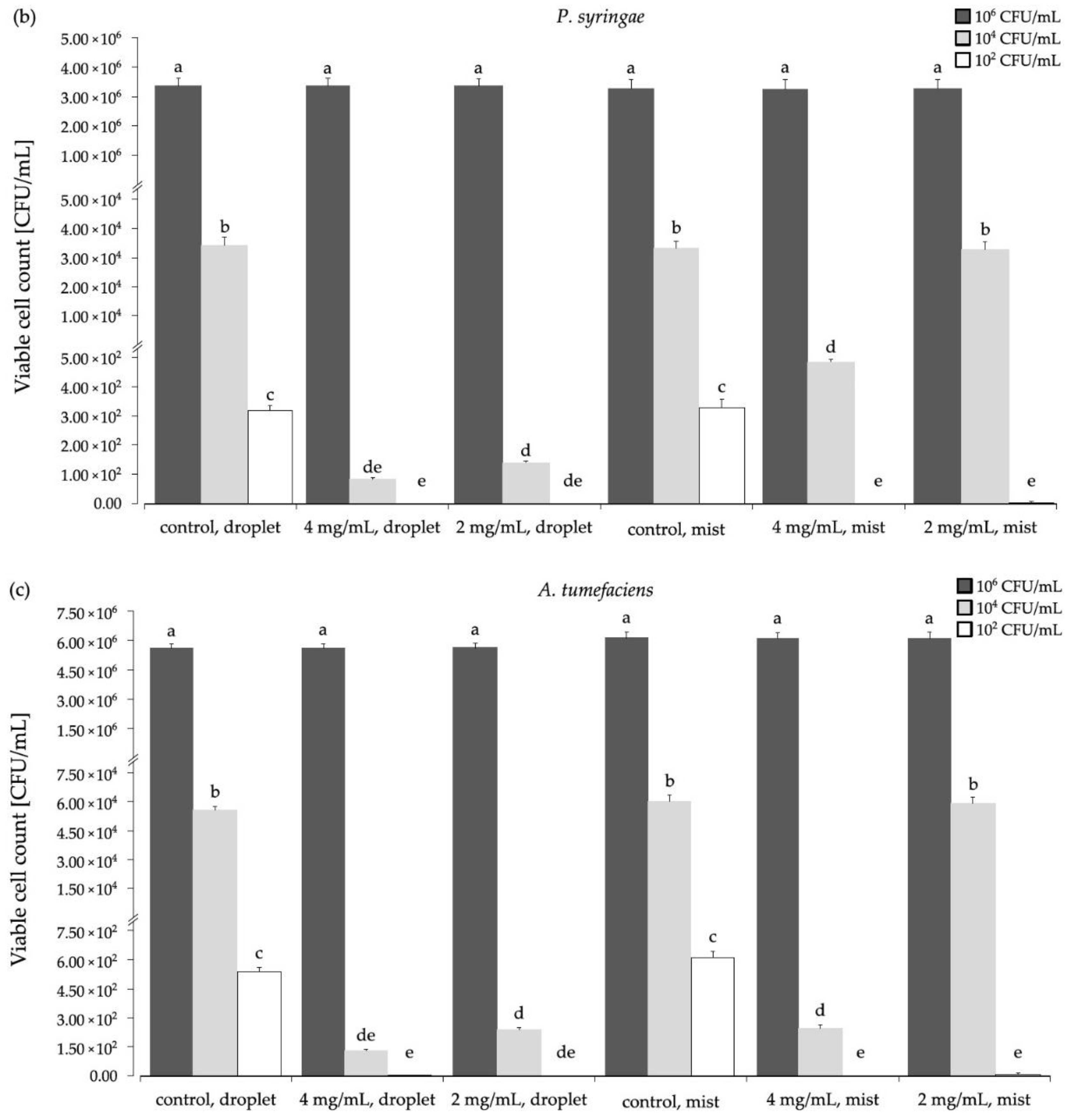
3.2. The Biocidal Effect of Aqueous Ozone on the Selected Phytopathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Bhattarai, D.R. Postharvest Horticulture in Nepal. Hortic. Int. J. 2018, 2, 458–460. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture—Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhilash, P.C.; Singh, N. Pesticide Use and Application: An Indian Scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; Lejeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Kannan, V.R.; Bastas, K.K.; Devi, R.S. Scientific and Economic Impact of Plant Pathogenic Bacteria. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; Kannan, V.R., Bastas, K.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 369–392. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in Phytopathogenic Bacteria: Do We Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef]
- Sharpe, D.; Fan, L.; McRae, K.; Walker, B.; MacKay, R.; Doucette, C. Effects of Ozone Treatment on Botrytis Cinerea and Sclerotinia Sclerotiorum in Relation to Horticultural Product Quality. J. Food Sci. 2009, 74, M250–M257. [Google Scholar] [CrossRef] [PubMed]
- Thanomsub, B.; Anupunpisit, V.; Chanphetch, S.; Watcharachaipong, T.; Poonkhum, R.; Srisukonth, C. Effects of Ozone Treatment on Cell Growth and Ultrastructural Changes in Bacteria. J. Gen. Appl. Microbiol. 2002, 48, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes, B.; Cattani Heimbecker, A.M.; de Souza Brito, G.; Costa, S.F.; van der Heijden, I.M.; Levin, A.S.; Rasslan, S. Effect of Low-Dose Gaseous Ozone on Pathogenic Bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, G.; Valdenassi, L. Use of Ozone in Water, Agriculture and Zootechnics: Relationships between Dysbiosis and Mental Disorders. Ozone Ther. 2019, 4, 8182. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Anachkov, M.; Rakovsky, S.; Zaikov, G.E. Ozone Decomposition. Interdiscip. Toxicol. 2014, 7, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Greene, A.K.; Güzel-Seydim, Z.B.; Seydim, A.C. Chemical and Physical Properties of Ozone. In Ozone in Food Processing; O’Donnell, C., Tiwari, B.K., Cullen, P.J., Rice, R.G., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2012; pp. 19–32. [Google Scholar]
- Zhang, Y.Q.; Wu, Q.P.; Zhang, J.M.; Yang, X.H. Effects of Ozone on Membrane Permeability and Ultrastructure in Pseudomonas Aeruginosa. J. Appl. Microbiol. 2011, 111, 1006–1015. [Google Scholar] [CrossRef]
- Jian, F.; Jayas, D.S.; White, N.D.G. Can Ozone Be a New Control Strategy for Pests of Stored Grain? Agric. Res. 2013, 2, 1–8. [Google Scholar] [CrossRef]
- Rice, R.G.; Graham, D.M.U.S. FDA Regulatory Approval of Ozone as an Antimicrobial Agent—What Is Allowed and What Needs to Be Understood. Ozone News 2001, 29, 22–31. [Google Scholar]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off. J. Eur. Union 2012, 49, 181–303. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0528&from=EN (accessed on 16 September 2022).
- The United States Department of Agriculture. Ozone Technical Evaluation Report. 2021. Available online: https://www.ams.usda.gov/sites/default/files/media/USDANOPTechnicalReport_Ozone.pdf (accessed on 16 September 2022).
- Díaz-López, M.; Nicolás, E.; López-Mondéjar, R.; Galera, L.; Garrido, I.; Fenoll, J.; Bastida, F. Combined Ozonation and Solarization for the Removal of Pesticides from Soil: Effects on Soil Microbial Communities. Sci. Total Environ. 2021, 758, 143950. [Google Scholar] [CrossRef]
- Prudente, A.D.; King, J.M. Efficacy and Safety Evaluation of Ozonation to Degrade Aflatoxin in Corn. J. Food Sci. 2002, 67, 2866–2872. [Google Scholar]
- Raudales, R.E.; Parke, J.L.; Guy, C.L.; Fisher, P.R. Control of Waterborne Microbes in Irrigation: A Review. Agric. Water Manag. 2014, 143, 9–28. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Brennan, C.S.; Curran, T.; Gallagher, E.; Cullen, P.J.; O’ Donnell, C.P. Application of Ozone in Grain Processing. J. Cereal Sci. 2010, 51, 248–255. [Google Scholar] [CrossRef]
- Veronico, P.; Paciolla, C.; Sasanelli, N.; De Leonardis, S.; Melillo, M.T. Ozonated Water Reduces Susceptibility in Tomato Plants to Meloidogyne Incognita by the Modulation of the Antioxidant System. Mol. Plant Pathol. 2017, 18, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Özen, T.; Koyuncu, M.A.; Erbaş, D. Effect of Ozone Treatments on the Removal of Pesticide Residues and Postharvest Quality in Green Pepper. J. Food Sci. Technol. 2021, 58, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Radhakrishnan, M.; Anandakumar, S.; Shanmugasundaram, S.; Anandharamakrishnan, C. Disinfestation Techniques for Major Cereals: A Status Report. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1125–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Kronberg, M.F.; Jaramillo Sánchez, G.; Gómez, P.L.; García-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone Washing Decreases Strawberry Susceptibility to Botrytis Cinerea While Maintaining Antioxidant, Optical and Sensory Quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef]
- Shelake, P.S.; Mohapatra, D.; Tripathi, M.K.; Giri, S.K.; Kate, A.; Kumar, M. Inactivation of Aspergillus Niger and Erwinia Carotovora in Onion (Allium Cepa L.) Bulbs Subjected to Pulsed Ozone Treatment. Postharvest Biol. Technol. 2022, 192, 111998. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Panebianco, F.; Rubiola, S.; Di Ciccio, P.A. The Use of Ozone as an Eco-Friendly Strategy against Microbial Biofilm in Dairy Manufacturing Plants: A Review. Microorganisms 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Q. Efficacy of Ozonated Water against Erwinia Carotovora Subsp. Carotovora in Brassica Campestris Ssp. Chinensis. Ozone Sci. Eng. 2017, 39, 127–136. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Zhang, K.; Gao, M.; Shi, C.; Ge, C.; Qu, D.; Zhu, J.; Shi, Y.; Han, J. Inactivation of Vibrio Parahaemolyticus by Aqueous Ozone. J. Microbiol. Biotechnol. 2018, 28, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.C.; da Silva, E.S.; Oliveira, F.O.; Rodrigues, L.D.A.P.; Neves, P.R.F.; Meira, C.S.; Moreira, G.A.F.; Lobato, G.M.; Nascimento, C.; Gerhardt, M.; et al. Ozonized Water in Microbial Control: Analysis of the Stability, In Vitro Biocidal Potential, and Cytotoxicity. Biology 2021, 10, 525. [Google Scholar] [CrossRef]
- Kim, J.-G.; Youself, A.E.; Khadre, M.A. Ozone and Its Current and Future Application in the Food Industry. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Academic Press: San Diego, CA, USA, 2003; pp. 168–218. [Google Scholar]
- Rahman, M.F.; Jasim, S.Y.; Yanful, E.K.; Ndiongue, S.; Borikar, D.; Jasim, S.Y.; Yanful, E.K.; Ndiongue, S.; Borikar, D. Advanced Oxidation Treatment of Drinking Water: Part II. Turbidity, Particles and Organics Removal from Lake Huron Water. Ozone Sci. Eng. 2010, 32, 295–304. [Google Scholar] [CrossRef]
- Hirahara, Y.; Iwata, K.; Nakamuro, K. Effect of Citric Acid on Prolonging the Half-Life of Dissolved Ozone in Water. Food Saf. 2019, 7, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Knopp, G.; Dötsch, A.; Wieland, A.; Schwartz, T. Ozone Treatment of Conditioned Wastewater Selects Antibiotic Resistance Genes, Opportunistic Bacteria, and Induce Strong Population Shifts. Sci. Total Environ. 2016, 559, 103–112. [Google Scholar] [CrossRef]
- Martinelli, M.; Giovannangeli, F.; Rotunno, S.; Trombetta, C.M.; Montomoli, E. Water and Air Ozone Treatment as an Alternative Sanitizing Technology. J. Prev. Med. Hyg. 2017, 58, E48–E52. [Google Scholar] [CrossRef] [Green Version]
- Failor, K.C.; Silver, B.; Yu, W.; Heindl, J.E. Bio Film Disruption and Bactericidal Activity of Aqueous Ozone Coupled with Ultrasonic Dental Scaling. JADA Found. Sci. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Bialoszewski, D.; Pietruczuk-Padzik, A.; Kalicinska, A.; Bocian, E.; Czajkowska, M.; Bukowska, B.; Tyski, S. Activity of Ozonated Water and Ozone against Staphylococcus Aureus and Pseudomonas Aeruginosa Biofilms. Med. Sci. Monit. 2011, 17, BR339–BR344. [Google Scholar] [CrossRef] [Green Version]
- Białoszewski, D.; Bocian, E.; Bukowska, B.; Czajkowska, M.; Sokół-Leszczyńska, B.; Tyski, S. Antimicrobial Activity of Ozonated Water. Med. Sci. Monit. 2010, 16, MT71-5. [Google Scholar] [PubMed]
- Ersoy, Z.G.; Barisci, S.; Turkay, O. Mechanisms of the Escherichia Coli and Enterococcus Faecalis Inactivation by Ozone. LWT—Food Sci. Technol. 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Sarron, E.; Cochet, N.; Gadonna-Widehem, P. Effects of Aqueous Ozone on Pseudomonas Syringae Viability and Ice Nucleating Activity. Process Biochem. 2013, 48, 1004–1009. [Google Scholar] [CrossRef]
- Younis, A.M.; Shoeib, A.A.; Elsaedy, M.A.; Osman, K.A. Efficacy of Ozone and Hydrogen Peroxide on Controlling Crown Gall Bacterium and Root Knot Nematode Infected Guava Plants in Egypt. Alex. J. Agric. Sci. 2016, 61, 517–527. [Google Scholar]
- Fan, L.; Song, J.; Hildebrand, P.D.; Forney, C.F. Interaction of Ozone and Negative Air Ions to Control Micro-Organisms. J. Appl. Microbiol. 2002, 93, 144–148. [Google Scholar] [CrossRef]
- Marsili, L.; Espie, S.; Anderson, J.G.; Macgregor, S.J. Plasma Inactivation of Food-Related Microorganisms in Liquids. Radiat. Phys. Chem. 2002, 65, 507–513. [Google Scholar] [CrossRef]
- Wani, S.; Barnes, J.; Singleton, I. Investigation of Potential Reasons for Bacterial Survival on ‘Ready-to-Eat’ Leafy Produce during Exposure to Gaseous Ozone. Postharvest Biol. Technol. 2016, 111, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Gibson, K.E.; Almeida, G.; Jones, S.L.; Wrught, K.; Lee, J.A. Inactivation of Bacteria on Fresh Produce by Batch Wash Ozone Sanitation. Food Control. 2019, 106, 106747. [Google Scholar] [CrossRef]
- Kim, J.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Epelle, E.I.; Emmerson, A.; Nekrasova, M.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.; Yaseen, M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022, 61, 9600–96101. [Google Scholar] [CrossRef]
- Kroupitski, Y.; Pinto, R.; Brandl, M.T.; Belausov, E.; Sela, S. Interactions of Salmonella Enterica with Lettuce Leaves. J. Appl. Microbiol. 2009, 106, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, T.; Li, X. Development Status and Perspectives of Crop Protection Machinery and Techniques for Vegetables. Horticulture 2022, 8, 166. [Google Scholar] [CrossRef]
- Lee, A.W.; Miller, P.C.H.; Power, J.D. The Application of Pesticide Sprays to Tomato Crops. Asp. Appl. Biol. 2000, 57, 383–390. [Google Scholar]
- Rincon, V.J.; Sanchez-Hermosilla, J.; Paez, F.; Perez-Alonso, J.; Callejon, A.J. Assessment of the Influence of Working Pressure and Application Rate on Pesticide Spray Application with a Hand-Held Spray Gun on Greenhouse Pepper Crops. Crop Prot. 2017, 96, 7–13. [Google Scholar] [CrossRef]
- Masotti, F.; Cattaneo, S.; Stuknytė, M.; Noni, I. De Airborne Contamination in the Food Industry: An Update on Monitoring and Disinfection Techniques of Air. Trends Food Sci. Technol. 2019, 90, 147–156. [Google Scholar] [CrossRef]
- Testempasis, S.; Tanou, G.; Minas, I.; Samiotaki, M.; Molassiotis, A.; Karaoglanidis, G. Unraveling Interactions of the Necrotrophic Fungal Species Botrytis Cinerea with 1-Methylcyclopropene or Ozone-Treated Apple Fruit Using Proteomic Analysis. Front. Plant Sci. 2021, 12, 644255. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Taybi, T.; Antony, E.; Singleton, I.; Borland, A.; Barnes, J. Profiling Shifts in Protein Complement in Tomato Fruit Induced by Atmospheric Ozone-Enrichment and/or Wound-Inoculation with Botrytis Cinerea. Postharvest Biol. Technol. 2013, 78, 67–75. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of Ozone Treatment on the Quality of Kiwifruit during Postharvest Storage Affected by Botrytis Cinerea and Penicillium Expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Nadas, A.; Olmo, M.; Garcia, J.M. Growth of Botrytis Cinerea and Strawberry Quality in Ozone-Enriched Atmospheres. J. Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Ozkan, R.; Smilanick, J.L.; Karabulut, O.A. Toxicity of Ozone Gas to Conidia of Penicillium Digitatum, Penicillium Italicum, and Botrytis Cinerea and Control of Gray Mold on Table Grape. Postharvest Biol. Technol. 2011, 60, 47–51. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Z.; Tu, S.; Chen, S.; Peng, J.; Tu, K. Effects of Cold Plasma, UV-C or Aqueous Ozone Treatment on Botrytis Cinerea and Their Potential Application in Preserving Blueberry. J. Appl. Microbiol. 2019, 127, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Pagès, M.; Kleiber, D.; Pierron, R.J.G.; Violleau, F. Ozone Effects on Botrytis Cinerea Conidia Using a Bubble Column: Germination Inactivation and Membrane Phospholipids Oxidation. Ozone Sci. Eng. 2015, 38, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Liew, C.L.; Prange, R.K. Effect of Ozone and Storage Temperature on Postharvest Diseases and Physiology of Carrots (Daucus Carota L.). J. Am. Soc. Hortic. Sci. 1994, 119, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, A.; Barreno, E. Response to Ozone in Two Lettuce Varieties on Chlorophyll a Fluorescence, Photosynthetic Pigments and Lipid Peroxidation. Plant Physiol. Biochem. 2004, 42, 549–555. [Google Scholar] [CrossRef]
- Seki, M.; Ishikawa, T.; Terada, H.; Nashimoto, M. Microbicidal Effects of Stored Aqueous Ozone Solution Generated by Nano-Bubble Technology. In Vivo 2017, 31, 579–583. [Google Scholar] [PubMed]
- Ouf, S.A.; Moussa, T.A.; Abd-Emegeed, A.M.; Eltahlawy, S.R. Anti-Fungal Potential of Ozone against Some Dermatophytes. Brazilian J. Microbiol. 2016, 47, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, C.; Jiang, A.; Zhang, Y.; Zhao, Q.; Hu, W. Effects of Aqueous Ozone Treatment on Microbial Growth, Quality, and Pesticide Residue of Fresh-Cut Cabbage. Food Sci. Nutr. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Graham, T.; Zhang, P.; Zheng, Y.; Dixon, M.A. Phytotoxicity of Aqueous Ozone on Five Container-Grown Nursery Species. HortScience. 2009, 44, 774–780. [Google Scholar] [CrossRef]
- Romeo-Oliván, A.; Pagès, M.; Breton, C.; Lagarde, F.; Cros, H.; Yobrégat, O.; Violleau, F.; Jacques, A. Ozone Dissolved in Water: An Innovative Tool for the Production of Young Plants in Grapevine Nurseries? Ozone Sci. Eng. 2021, 44, 521–535. [Google Scholar] [CrossRef]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The Effects of Ozone Treatments on the Agro-Physiological Parameters of Tomato Plants and the Soil Microbial Community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanuwidjaja, I.; Fuka, M.M. Ozone in Droplets and Mist in Inhibition of Phytopathogenic Microbiota. Agriculture 2022, 12, 1875. https://doi.org/10.3390/agriculture12111875
Tanuwidjaja I, Fuka MM. Ozone in Droplets and Mist in Inhibition of Phytopathogenic Microbiota. Agriculture. 2022; 12(11):1875. https://doi.org/10.3390/agriculture12111875
Chicago/Turabian StyleTanuwidjaja, Irina, and Mirna Mrkonjic Fuka. 2022. "Ozone in Droplets and Mist in Inhibition of Phytopathogenic Microbiota" Agriculture 12, no. 11: 1875. https://doi.org/10.3390/agriculture12111875



