Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Identification and Chromosomal Distribution
2.2. Protein Length, Sequence, and Motif Composition Analysis
2.3. Gene Structure Analysis and Cis-Element Prediction
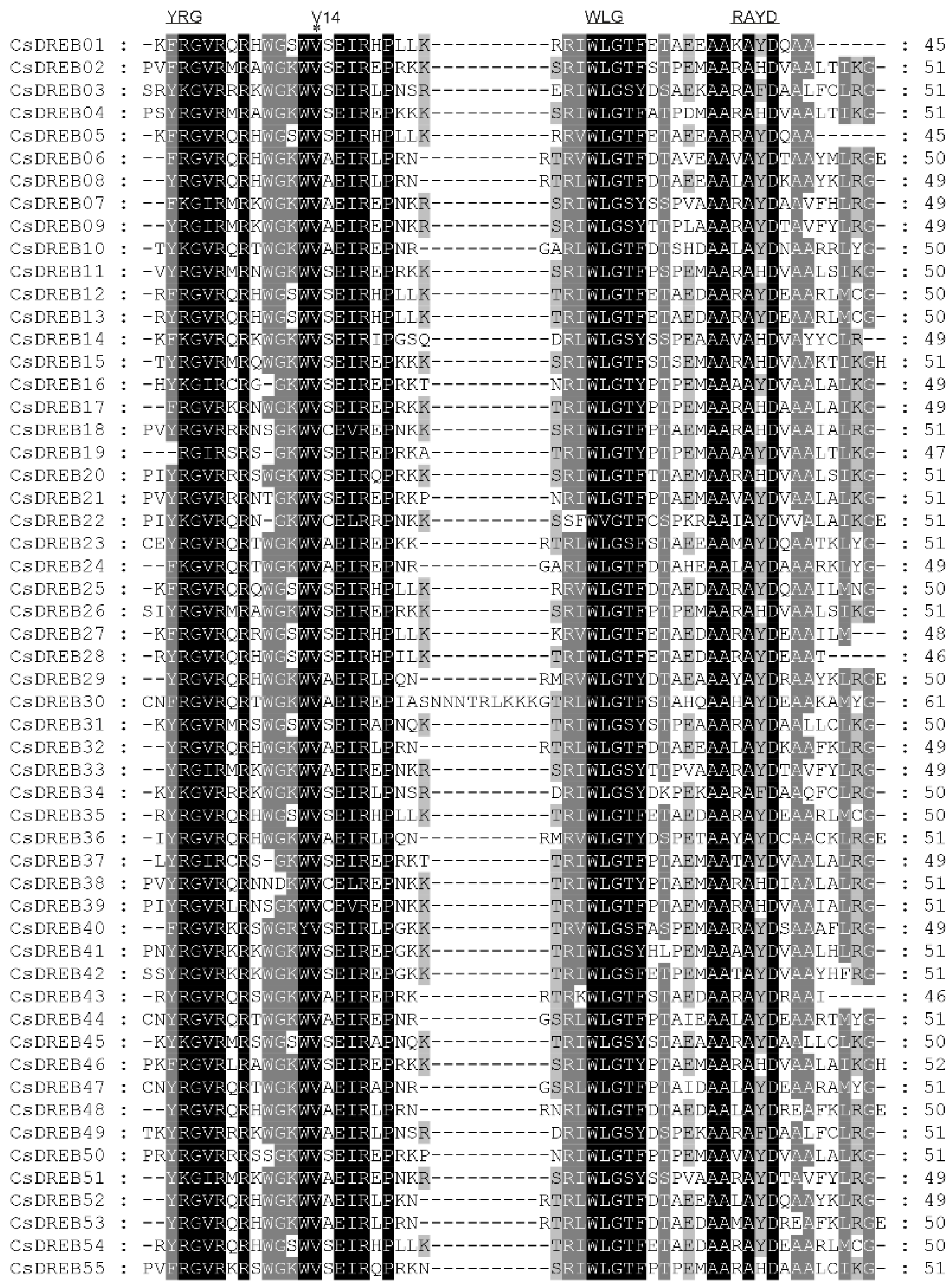
2.4. Multiple Sequence Alignment and Phylogenetic Analysis
2.5. Gene Duplication and Synteny Analysis
2.6. Transcriptome Analysis of CsDREB Genes in Cucumber
2.7. Transcriptome Analysis of CsDREBs in Response to Abiotic and Biotic Stresses
2.8. Plant Materials, Stress Treatments, and qRT-PCR Analysis
3. Results
3.1. Identification of Genes and the Comparison of Gene Characterizations in Different Cucumber Accessions
3.2. Multiple Sequence Alignment and Phylogenetic Relationships of CsDREBs
3.3. Gene Structure, Motif Composition, and Cis-Element Analysis of CsDREBs
3.4. Gene Duplication and Synteny Analysis
3.5. Expression Patterns of CsDREB in Different Tissues
3.6. Expression Profiles of CsDREB Genes under Abiotic and Biotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP2/ERF | APETALA2/ethylene response factor |
| CDS | Coding sequence |
| Chr | Chromosome |
| CM | Conserved motif |
| CT | Control treatment |
| DM | Downy mildew |
| DPI | Days post-inoculation |
| DREB | Dehydration-responsive element-binding factor |
| FC | Fold-change |
| FPKM | Fragments per kilobase of exon model per million mapped fragments |
| Glu | Glutamine |
| HMM | Hidden Markov model |
| Kb | Kilobase |
| mRNA | Messenger RNA |
| NCBI | National Center for Biotechnology Information |
| PM | Powdery mildew |
| UTR | Untranslated region |
| Val | Valine |
References
- Ohama, N.; Kusakabe, K.; Mizoi, J.; Zhao, H.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Shinozaki, K.; et al. The Transcriptional Cascade in the Heat Stress Response of Arabidopsis Is Strictly Regulated at the Level of Transcription Factor Expression. Plant Cell 2016, 28, 181–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Weisskopf, L. Airborne medicine: Bacterial volatiles and their influence on plant health. New Phytol. 2020, 226, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk between Responses to Biotic and Abiotic Stresses the Missing Link in Understanding Plant Defence. Curr. Issues Mol. Biol. 2017, 23, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Paes de Melo, B.; Carpinetti, P.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M. Abiotic Stresses in Plants and Their Markers: A Practice View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.; Zhu, C.; Hu, Z.; Liu, S.; Wu, H.; Zhou, Y. Identification and Transcriptional Analysis of Zinc Finger-Homeodomain (ZF-HD) Family Genes in Cucumber. Biochem. Genet. 2021, 59, 884–901. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Ratcliffe, O.J. A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 2000, 3, 423–434. [Google Scholar] [CrossRef]
- Wang, G.D.; Xu, X.P.; Wang, H.; Liu, Q.; Yang, X.T.; Liao, L.X.; Cai, G.H. A tomato transcription factor, SlDREB3 enhances the tolerance to chilling in transgenic tomato. Plant Physiol. Biochem. 2019, 142, 254–262. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, C.L.; Wang, G.L.; Wang, Y.X.; Qi, C.H.; You, C.X.; Li, Y.Y.; Hao, Y.J. Apple AP2/EREBP transcription factor MdSHINE2 confers drought resistance by regulating wax biosynthesis. Planta 2019, 249, 1627–1643. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Sui, N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Deyholos, M.K. Comprehensive transcriptional profiling of NaCl-Stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-Wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genomics. 2012, 287, 495–513. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Cao, Z.F.; Li, J.; Chen, F.; Li, Y.Q.; Zhou, H.M.; Liu, Q. Effect of two conserved amino acid residues on DREB1A function. Biochemistry 2001, 66, 623–627. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, G.; Liu, S.; Jia, J.; Cheng, L.; Qi, D.; Shen, S.; Peng, X.; Liu, G. Identified of a novel cis-element regulating the alternative splicing of LcDREB2. Sci. Rep. 2017, 7, 46106. [Google Scholar] [CrossRef] [Green Version]
- Lakhwani, D.; Pandey, A.; Dhar, Y.V.; Bag, S.K.; Trivedi, P.K.; Asif, M.H. Genome-wide analysis of the AP2/ERF family in Musa species reveals divergence and neofunctionalisation during evolution. Sci. Rep. 2016, 6, 18878. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ma, H.; Lin, J. Angiosperm-Wide and Family-Level Analyses of AP2/ERF Genes Reveal Differential Retention and Sequence Divergence after Whole-Genome Duplication. Front. Plant Sci. 2019, 10, 196. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.L.; Li, Y.N.; Zhang, X.P.; Song, Y.; Wang, W.; Fang, J.; Cui, W.M.; Jia, X.D. Immunotoxicologic assessment of genetically modified drought-resistant wheat T349 with GmDREB1. Zhonghua Yu Fang Yi Xue Za Zhi 2012, 46, 556–560. [Google Scholar] [PubMed]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.P.; Kim, W.T. Isolation and functional characterization of the Ca-DREBLP1 gene encoding a dehydration-responsive element binding-factor-like protein 1 in hot pepper (Capsicum annuum L. cv. Pukang). Planta 2005, 220, 875–888. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Liu, Y.; Gao, H.; Wang, Z.; Sun, G. CkDREB gene in Caragana korshinskii is involved in the regulation of stress response to multiple abiotic stresses as an AP2/EREBP transcription factor. Mol. Biol. Rep. 2011, 38, 2801–2811. [Google Scholar] [CrossRef]
- Hong, B.; Ma, C.; Yang, Y.; Wang, T.; Yamaguchi-Shinozaki, K.; Gao, J. Over-expression of AtDREB1A in chrysanthemum enhances tolerance to heat stress. Plant Mol. Biol. 2009, 70, 231–240. [Google Scholar] [CrossRef]
- Hwang, J.E.; Lim, C.J.; Chen, H.; Je, J.; Song, C.; Lim, C.O. Overexpression of Arabidopsis dehydration- responsive element-binding protein 2C confers tolerance to oxidative stress. Mol. Cells 2012, 33, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Sharoni, A.M.; Nuruzzaman, M.; Satoh, K.; Shimizu, T.; Kondoh, H.; Sasaya, T.; Choi, I.R.; Omura, T.; Kikuchi, S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011, 52, 344–360. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhou, W.; Liu, H.; Liu, P.; Li, Z.G. Genome-Wide analysis of the soybean DREB gene family: Identification, genomic organization and expression profiles in response to drought stress. Plant Breed. 2020, 139, 1158–1167. [Google Scholar] [CrossRef]
- Agarwal, P.; Agarwal, P.K.; Nair, S.; Sopory, S.K.; Reddy, M.K. Stress-Inducible DREB2A transcription factor from Pennisetum glaucum is a phosphoprotein and its phosphorylation negatively regulates its DNA-binding activity. Mol. Genet. Genomics 2007, 277, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Durmaz, E.; Akpınar, B.A.; Budak, H. The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol. Biochem. 2011, 49, 346–351. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-Wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, J.; Zhu, K.; Liu, L.; Chen, F.; Yu, D. Identification and characterization of two chrysanthemum (Dendronthema x moriforlium) DREB genes, belonging to the AP2/EREBP family. Mol. Biol. Rep. 2009, 36, 71–81. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Wang, H.; Zhao, T.; Xu, X.; Jiang, J.; Li, J. Genome-Wide identification and functional analysis of the ERF2 gene family in response to disease resistance against Stemphylium lycopersici in tomato. BMC Plant Biol. 2021, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shi, A.; Mou, B. Genome-wide identification and expression analysis of the CBF/DREB1 gene family in lettuce. Sci. Rep. 2020, 10, 5733. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.Y.; Jia, J.P.; Kong, D.C.; Zhang, Z.D.; Song, S.; Li, Y.Y.; Pang, X.M. Genome-wide identification and analysis of the DREB genes and their expression profiles under abiotic stresses in Chinese jujube (Ziziphus jujuba Mill.). J. For. Res. 2019, 30, 1277–1287. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhu, J.J.; Wei, C.J.; Guo, Q.; Bian, C.K.; Xiang, Z.H.; Zhao, A.C. Genome-wide identification and characterization of the DREB transcription factor gene family in mulberry. Biol. Plant. 2015, 59, 253–265. [Google Scholar] [CrossRef]
- Huang, X.; Song, X.P.; Chen, R.F.; Zhang, B.Q.; Li, C.N.; Liang, Y.S.; Qiu, L.H.; Fan, Y.G.; Zhou, Z.F.; Zhou, H.W.; et al. Genome-Wide Analysis of the DREB Subfamily in Saccharum spontaneum Reveals Their Functional Divergence During Cold and Drought Stresses. Front. Genet. 2020, 10, 1326. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Liu, X.H.; Wang, Z.C.; Zhang, M.Q.; Zhang, J.S. Genome-Wide identification and expression profiling of DREB genes in Saccharum spontaneum. BMC Genom. 2021, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.N.; Cheng, H.; Yan, M.K.; Priyadarshani, S.; Zhang, M.; He, Q.; Huang, Y.M.; Chen, F.Q.; Liu, L.P.; Huang, X.Y.; et al. Identification and expression analysis of the DREB transcription factor family in pineapple (Ananas comosus (L.) Merr.). PeerJ 2020, 8, e9006. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Munir, F.; Gul, A.; Amir, R.; Paracha, R.Z. Genome-Wide analysis, identification, evolution and genomic organization of dehydration responsive element-binding (DREB) gene family in Solanum tuberosum. PeerJ 2021, 9, e11647. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhang, C.J.; Duan, L.X.; Luan, Q.Q.; Li, J.L.; Yang, A.G.; Qi, X.Q.; Ren, Z.H. CsMYB60 is a key regulator of flavonols and proanthocyanidans that determine the colour of fruit spines in cucumber. J. Exp. Bot. 2019, 70, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, Q.Q.; Chen, C.H.; Liu, M.Y.; Li, Q.; Wang, L.N.; Ren, Z. CsWRKY50 mediates defense responses to Pseudoperonospora cubensis infection in Cucumis sativus. Plant Sci. 2019, 279, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Savory, E.A.; Vaillancourt, B.; Childs, K.L.; Hamilton, J.P.; Day, B.; Buell, C.R. Expression Profiling of Cucumis sativus in Response to Infection by Pseudoperonospora cubensis. PLoS ONE 2012, 7, e34954. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Chen, X.Q.; Han, J.; Lu, W.L.; Ren, Z.H. Genome-wide analysis of theWRKYgene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Hu, L.; Liu, S. Genome-Wide identification and phylogenetic analysis of the ERF gene family in cucumbers. Genet. Mol. Biol. 2011, 34, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant pan-genomes are the new reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Q.; Xin, H.; Xu, Y.; Lin, S.; Chen, X.; Yao, Z.; et al. Graph-Based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 2022, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Z.; Yan, P.; Huang, S.; Fei, Z.; Lin, K. RNA-Seq improves annotation of protein-coding genes in the cucumber genome. BMC Genom. 2011, 12, 540. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J.; Huo, H.; Wu, Z.; Yang, R.; Gong, H. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Tang, R.; Wang, L.; Chen, C.; Ren, Z. Genome-Wide identification and expression analysis of Hsf and Hsp gene families in cucumber (Cucumis sativus L.). Plant Growth Regul. 2021, 95, 223–239. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Shi, Y.; Qi, X.; Chen, X. Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5.1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling. BMC Genom. 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, H.; Huang, W.; Xu, Y.; Zhou, Q.; Wang, S.; Ruan, J.; Huang, S.; Zhang, Z. A chromosome-scale genome assembly of cucumber (Cucumis sativus L.). GigaScience 2019, 8, giz072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Ohta, M.; Matsui, K.; Hiratsu, K.; Shinshi, H.; Ohme-Takagi, M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 2001, 13, 1959–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; White, M.J.; MacRae, T.H. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur. J. Biochem. 1999, 262, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xia, H.; Liu, J.; Ma, F. The gene family of dehydration responsive element-binding transcription factors in grape (Vitis vinifera): Genome-Wide identification and analysis, expression profiles, and involvement in abiotic stress resistance. Mol. Biol. Rep. 2014, 41, 1577–1590. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X. Genome-wide identification of AP2/ERF superfamily genes and their expression during fruit ripening of Chinese jujube. Sci. Rep. 2018, 8, 15612. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.B.; Azar, S.; SanClemente, H.; Mounet, F.; Dunand, C.; Marque, G.; Marque, C.; Teulières, C. Genome-wide analysis of the AP2/ERF family in Eucalyptus grandis: An intriguing over-representation of stress-responsive DREB1/CBF genes. PLoS ONE 2015, 10, e0121041. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 1998, 16, 433–442. [Google Scholar] [CrossRef]
- Knight, H.; Zarka, D.G.; Okamoto, H.; Thomashow, M.F.; Knight, M.R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol. 2004, 135, 1710–1717. [Google Scholar] [CrossRef]
- Xiao, H.; Tattersall, E.A.R.; Siddiqua, M.K.; Cramer, G.R.; Nassuth, A. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant Cell Environ. 2008, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, J.; Deng, X.; Tan, S.; Li, L.; Li, L.; Zhou, J.; Peng, H.; Yang, G.; He, G.; et al. Expansion and stress responses of AP2/EREBP superfamily in Brachypodium distachyon. Sci. Rep. 2016, 6, 21623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef] [PubMed]









| Accession Name | Accession Group | Accession Name | Accession Group |
|---|---|---|---|
| 9930 | East Asian cultivated accession | PI183967 | Indian wild accession |
| XTMC | East Asian cultivated accession | Cuc64 | Indian wild accession |
| Cu2 | East Asian cultivated accession | W4 | Indian wild accession |
| Cuc80 | Xishuangbanna cultivated accession | W8 | Indian wild accession |
| Cuc37 | Eurasian cultivated accession | Hx14 | Indian cultivated accession |
| Gy14 | Eurasian cultivated accession | Hx117 | Indian cultivated accession |
| 9110gt | Eurasian cultivated accession |
| Protein Name | 9930 | XTMC | Cu2 | Cuc80 | Cuc37 | Gy14 | 9110gt | PI183967 | Cuc64 | W4 | W8 | Hx14 | Hx117 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsDREB01 | 223 | 223 | 223 | - | 223 | 223 | - | 223 | 223 | 223 | 223 | 223 | 223 |
| CsDREB02 | 250 | 250 | 250 | - | 251 | 251 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| CsDREB03 | 203 | 203 | 201 | - | 203 | 203 | 203 | 203 | 203 | 201 | 203 | 203 | 203 |
| CsDREB04 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 252 |
| CsDREB05 | 246 | 248 | 246 | 246 | 246 | 246 | 246 | 248 | 248 | 248 | 248 | 182 | 248 |
| CsDREB06 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 346 |
| CsDREB07 | 159 | 412 | 231 | - | 412 | 159 | 412 | 142 | 142 | 159 | 362 | 159 | 159 |
| CsDREB08 | 362 | - | - | 362 | 362 | 362 | 362 | 159 | 159 | 362 | 159 | 362 | 362 |
| CsDREB09 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 | 142 |
| CsDREB10 | 212 | 250 | 250 | - | 250 | 212 | - | 212 | 250 | 250 | 255 | 250 | 250 |
| CsDREB11 | 168 | 231 | 231 | 231 | 231 | 231 | 231 | 227 | 213 | 231 | 213 | 231 | 217 |
| CsDREB12 | 175 | 175 | 175 | 175 | 175 | 175 | - | 175 | 175 | 175 | 175 | 175 | 175 |
| CsDREB13 | 164 | 164 | 164 | 164 | 164 | 164 | 135 | 164 | 164 | 164 | 164 | 164 | 186 |
| CsDREB14 | 160 | 148 | 148 | 183 | 160 | 160 | - | 148 | 148 | 148 | 148 | 148 | 148 |
| CsDREB15 | 214 | 214 | 214 | 214 | 214 | 240 | 214 | 214 | 214 | 214 | 209 | 214 | 214 |
| CsDREB16 | 169 | 169 | 169 | 169 | 169 | 169 | - | 169 | 169 | 169 | 169 | 169 | 169 |
| CsDREB17 | 230 | 282 | 251 | 251 | 251 | 230 | - | 230 | 258 | 258 | 258 | 251 | 251 |
| CsDREB18 | 213 | 213 | 213 | 213 | 213 | 182 | 213 | 213 | 213 | 213 | 213 | 213 | 213 |
| CsDREB19 | 196 | 196 | 192 | 196 | 192 | 192 | 175 | 192 | 196 | 196 | 196 | 196 | 175 |
| CsDREB20 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | 201 | - |
| CsDREB21 | 183 | 153 | 183 | 153 | 153 | 183 | - | 183 | 153 | 153 | 153 | 153 | 153 |
| CsDREB22 | 224 | - | 229 | - | 658 | 204 | 229 | 204 | 580 | 580 | 658 | 580 | 658 |
| CsDREB23 | 333 | 333 | 333 | - | 333 | 333 | - | 333 | 333 | 276 | 333 | 333 | 333 |
| CsDREB24 | 185 | 182 | 185 | 185 | 185 | 185 | 185 | 185 | 185 | 393 | 185 | 185 | 185 |
| CsDREB25 | 194 | 194 | 194 | 194 | 194 | 194 | 232 | 194 | 194 | 194 | 224 | 194 | 194 |
| CsDREB26 | 184 | 227 | 227 | 227 | 227 | 227 | - | 226 | 226 | 227 | 226 | 227 | 181 |
| CsDREB27 | 190 | 190 | 190 | 190 | 190 | 168 | 190 | 190 | 190 | 190 | 190 | 190 | 190 |
| CsDREB28 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| CsDREB29 | 311 | 303 | 311 | 307 | 311 | 307 | 311 | 311 | 311 | 311 | 311 | 311 | 311 |
| CsDREB30 | 372 | 372 | 372 | 372 | 372 | 372 | - | 372 | 372 | 372 | 372 | 372 | 372 |
| CsDREB31 | 232 | 232 | 232 | - | 232 | 232 | 232 | 232 | 232 | 232 | 232 | 232 | 232 |
| CsDREB32 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 | 358 |
| CsDREB33 | 149 | 149 | 149 | 149 | 149 | 149 | 182 | 149 | 219 | 149 | 149 | 149 | 149 |
| CsDREB34 | 171 | 177 | 171 | - | 186 | 186 | 171 | 186 | 186 | 171 | 171 | 186 | 186 |
| CsDREB35 | 219 | 219 | 219 | 219 | 219 | 219 | 219 | 219 | 219 | 219 | 219 | - | 219 |
| CsDREB36 | 304 | 304 | 304 | 304 | 304 | 304 | 304 | 304 | 304 | 273 | 200 | 304 | - |
| CsDREB37 | 252 | 252 | 166 | 252 | 252 | 252 | 252 | 252 | 252 | 252 | 225 | 252 | 252 |
| CsDREB38 | 215 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 252 | 225 | 225 |
| CsDREB39 | 200 | 200 | 208 | - | 200 | 200 | 208 | 200 | 200 | 200 | 304 | 200 | 200 |
| CsDREB40 | 154 | 154 | 154 | 154 | 154 | 163 | 154 | 163 | 154 | 154 | 154 | 154 | 154 |
| CsDREB41 | 163 | 163 | 163 | - | 163 | 163 | 163 | 67 | 163 | 163 | 163 | 131 | 163 |
| CsDREB42 | 194 | 194 | 194 | 192 | 192 | 192 | 192 | 192 | 192 | 206 | 176 | 192 | 192 |
| CsDREB43 | 305 | 305 | 217 | - | 217 | 305 | - | 309 | - | - | - | 217 | - |
| CsDREB44 | 425 | 410 | 425 | 425 | 410 | 448 | 425 | 410 | 425 | 425 | 425 | 460 | 129 |
| CsDREB45 | 224 | - | 145 | - | 201 | 201 | 201 | 201 | 201 | 201 | 214 | 201 | 201 |
| CsDREB46 | 213 | - | 191 | 191 | 199 | 213 | - | 213 | 238 | 183 | 220 | 242 | 213 |
| CsDREB47 | 179 | 179 | 179 | 179 | 198 | 199 | 198 | 179 | 198 | 199 | 199 | 199 | 199 |
| CsDREB48 | 393 | - | 393 | 393 | 396 | 397 | - | 390 | 394 | 289 | 400 | 399 | 393 |
| CsDREB49 | 213 | 196 | 216 | 216 | 260 | 213 | 213 | 216 | 216 | 216 | 216 | 213 | 216 |
| CsDREB50 | 221 | 212 | 212 | - | 221 | 224 | 221 | 236 | 212 | 221 | 736 | 221 | 212 |
| CsDREB51 | 173 | 173 | 173 | 173 | 173 | 173 | 173 | 173 | 163 | 204 | 173 | 173 | 175 |
| CsDREB52 | 346 | 346 | 346 | 346 | 346 | 346 | 346 | 350 | 350 | 350 | 350 | 346 | 346 |
| CsDREB53 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 | 397 |
| CsDREB54 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 | 196 |
| CsDREB55 | 191 | 201 | 201 | - | 201 | 201 | - | 201 | 201 | 201 | 192 | 201 | 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Han, J.; Wang, T.; Chen, C.; Liu, J.; Xu, Z.; Zhang, Q.; Wang, L.; Ren, Z. Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber. Agriculture 2022, 12, 1879. https://doi.org/10.3390/agriculture12111879
Wang C, Han J, Wang T, Chen C, Liu J, Xu Z, Zhang Q, Wang L, Ren Z. Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber. Agriculture. 2022; 12(11):1879. https://doi.org/10.3390/agriculture12111879
Chicago/Turabian StyleWang, Can, Jing Han, Ting Wang, Chunhua Chen, Junyi Liu, Zhixuan Xu, Qingxia Zhang, Lina Wang, and Zhonghai Ren. 2022. "Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber" Agriculture 12, no. 11: 1879. https://doi.org/10.3390/agriculture12111879





