Effects of Dietary Phosphorus Deficiency and High Phosphorus Content on the Growth Performance, Serum Variables, and Tibia Development in Goslings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Housing
2.2. Experimental Design and Diets
2.3. Growth Performance
2.4. Serum Indicators
2.5. Tibial Indicators
2.6. Isolations of Total RNA and Reverse Transcription-Polymerase Chain Reactions
2.7. Statistical Analysis
3. Results
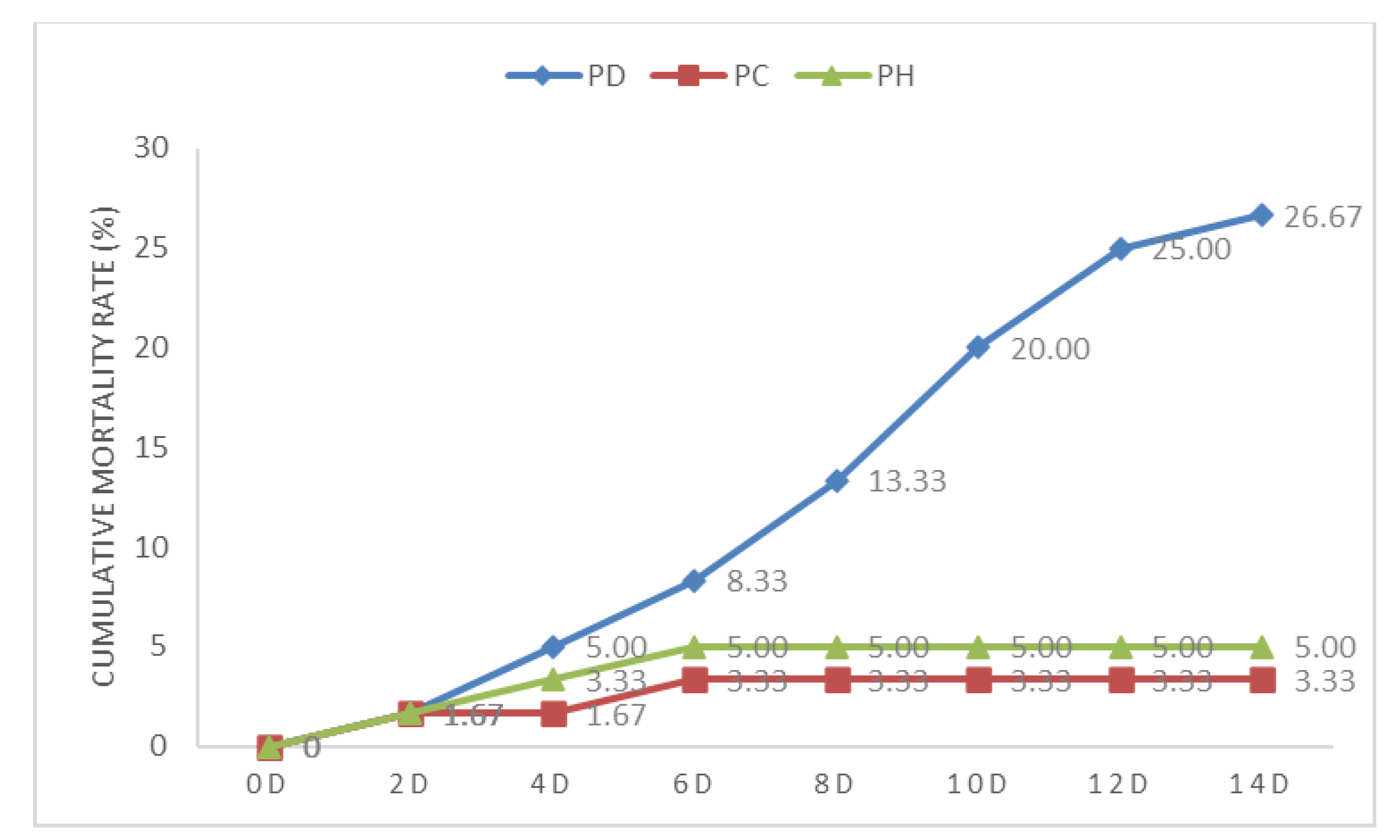
3.1. Growth Performance
3.2. Serum Ca, P Contents, and ALP Activity
3.3. Serum Hormones Contents
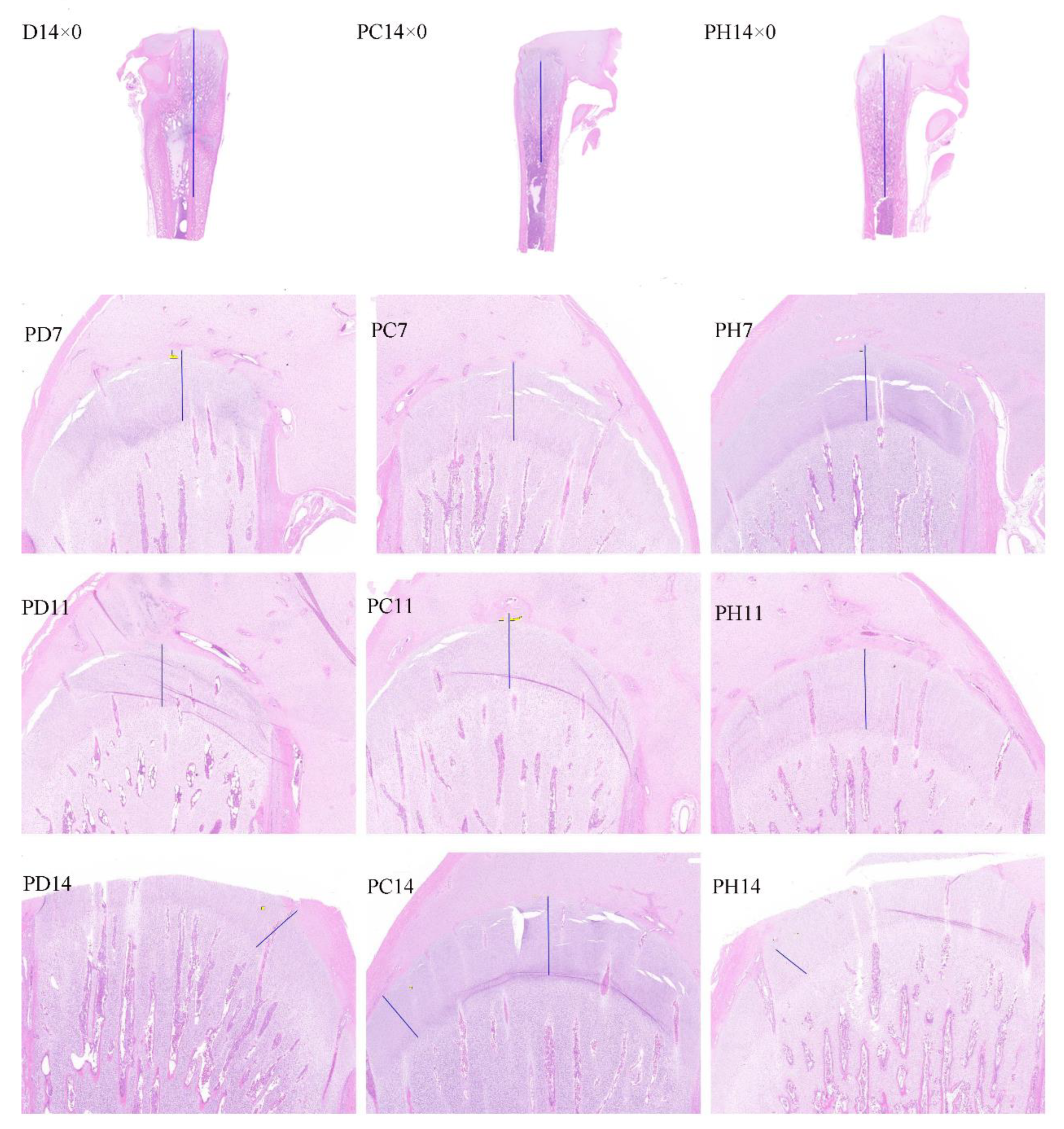
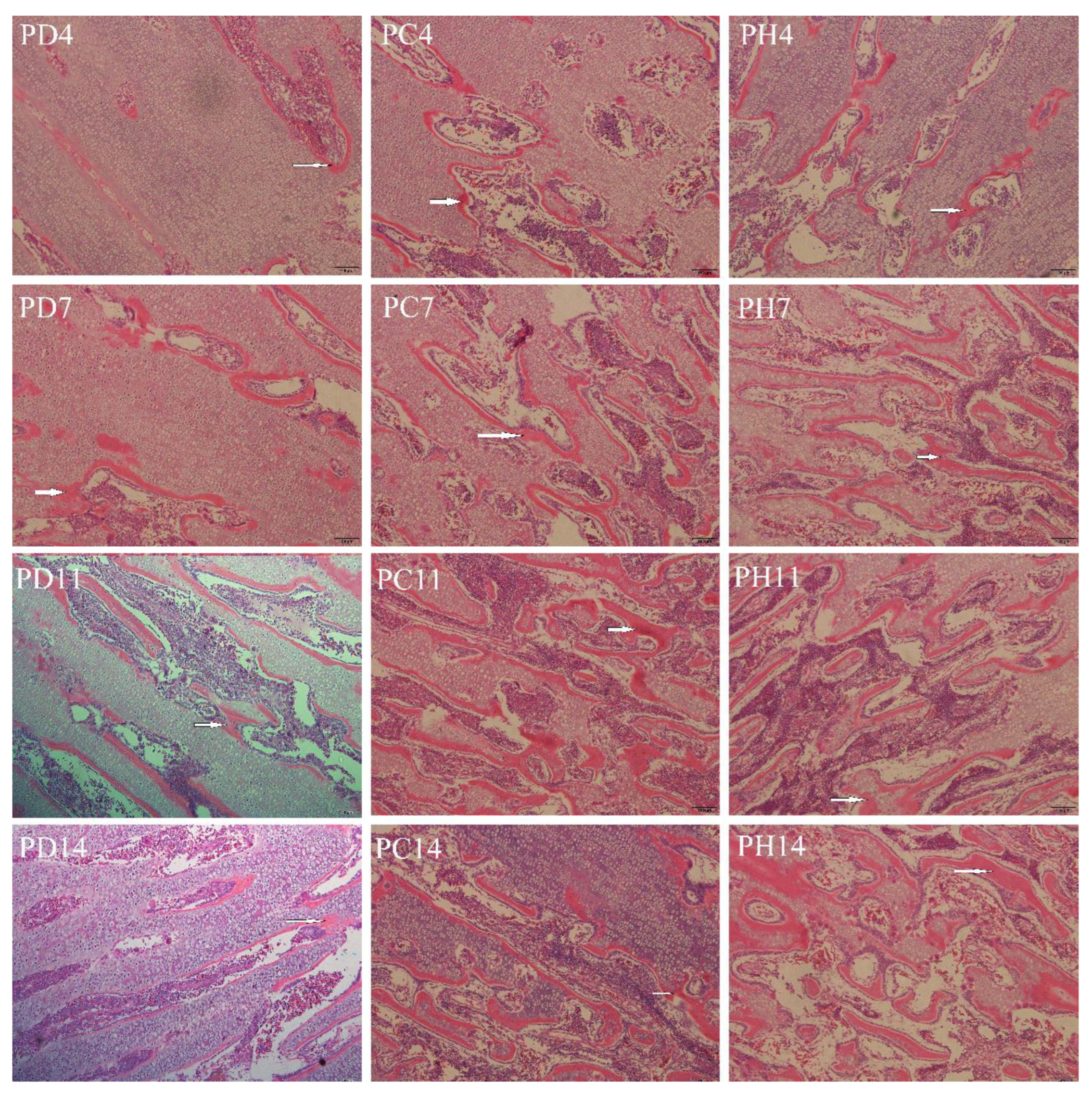
3.4. Tibial Development
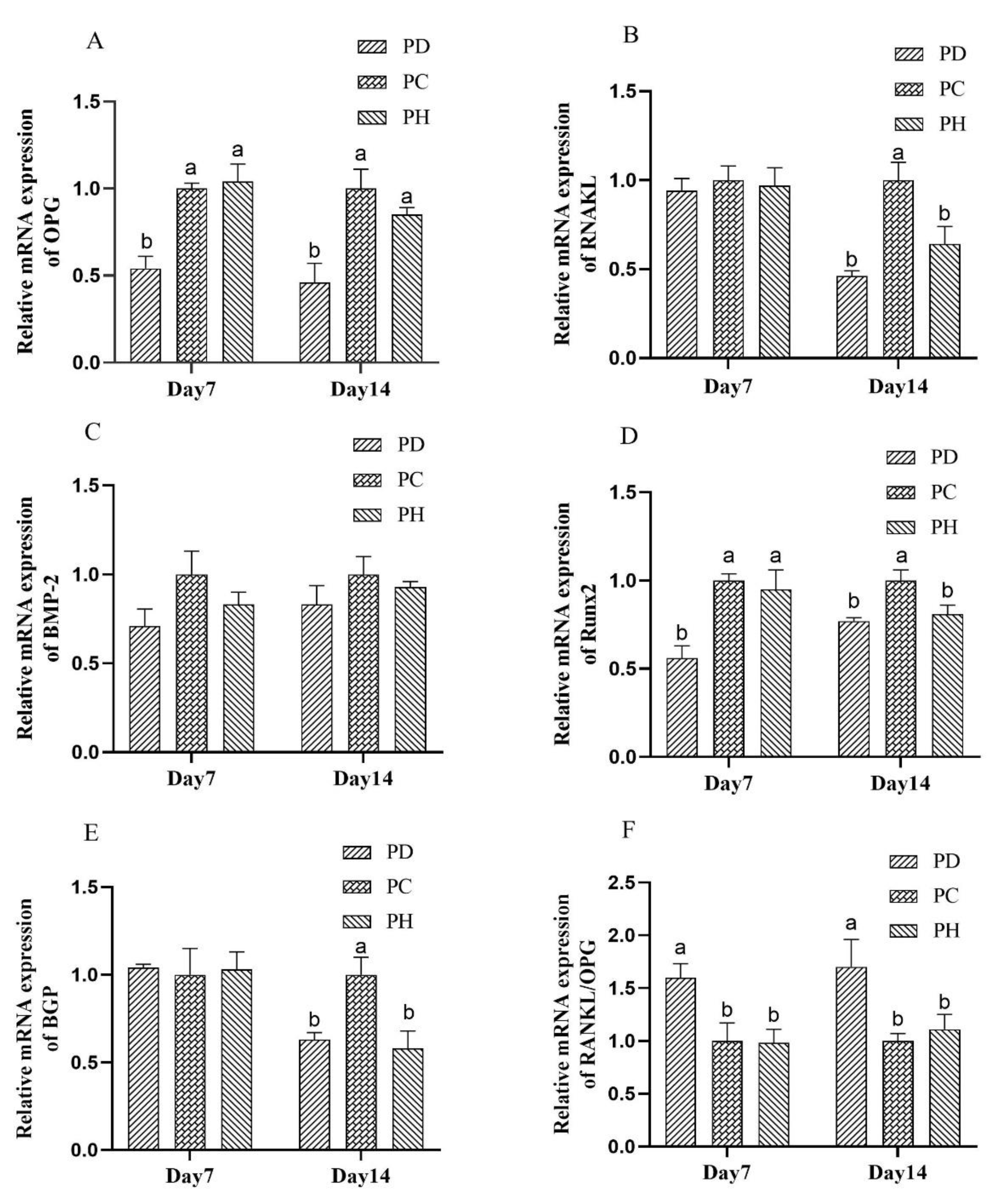
3.5. Gene Expression
4. Discussion
4.1. Growth Performance
4.2. Serum Variables
4.3. Tibia Characteristics
4.4. Gene Expression
4.5. Overall Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Calvo, M.S.; Lamberg-allardt, C.J. Phosphorus. Adv. Nutr. 2015, 6, 860–862. [Google Scholar] [PubMed] [Green Version]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Weiss, P.; Beck, L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life. Sci. 2011, 68, 205–218. [Google Scholar] [PubMed]
- Eeckhout, W.; Paepe, M.D. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anil. Feed. Sci. Tech. 1994, 47, 19–29. [Google Scholar]
- Walk, C.L.; Santos, T.T.; Bedfor, M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poul. Sci. 2014, 93, 1172–1177. [Google Scholar]
- Jing, M.; Zhao, S.; Rogiewicz, A.; Slominski, B.A.; House, J.D. Effects of phytase supplementation on production performance, egg and bone quality, plasma biochemistry and mineral excretion of layers fed varying levels of phosphorus. Animal 2021, 15, 100010. [Google Scholar] [PubMed]
- Imel, E.A.; Carpenter, T.O. Rickets: The Skeletal Disorders of Impaired Calcium or Phosphate Availability. In Pediatric Endocrinology; Humana Press: Totowa, NJ, USA, 2013; pp. 357–378. [Google Scholar]
- Ansar, M.; Khan, S.A.; Chaudhary, Z.I.; Mian, N.A.; Tipu, M.Y.; Rai, M.F. Effects of high dietary calcium and low phosphorus on urinary system of broiler chicks. Pak. Vet. J. 2004, 24, 113–116. [Google Scholar]
- Xu, L.; Li, N.; Farnell, Y.Z.; Wan, X.L.; Yang, H.M.; Zhong, X.Q.; Farnell, M.B. Effect of feeding a high calcium: Phosphorus ratio, phosphorous deficient diet on hypophosphatemic rickets onset in broilers. Agriculture 2021, 11, 1–13. [Google Scholar]
- Liu, S.B.; Liao, X.D.; Lu, L.; Li, S.F.; Luo, X.G. Dietary non-phytate phosphorus requirement of broilers fed a conventional corn-soybean meal diet from 1 to 21 d of age. Poult Sci. 2017, 96, 151–159. [Google Scholar]
- Rutherfurd, S.M.; Chung, T.K.; Morel, P.C.; Moughan, P.J. Effect of microbial phytase on ileal digestibility of phytate phosphorus, total phosphorus, and amino acids in a low-phosphorus diet for broilers. Poult Sci. 2004, 83, 61–68. [Google Scholar]
- Leytem, A.B.; Willing, B.P.; Thacker, P.A. Phytate utilisation and phosphorus excretion by broiler chickens fed diets containing cereal grains varying in phytate and phytase content. Anim. Feed Sci. Tech. 2008, 146, 160–168. [Google Scholar]
- Du, G.B.; Wang, Y.B.; Jiang, B.L. Effects of dietary high non-phytate phosphorus level on growth performance, tibia bone composition and metabolism of calcium and phosphorus of geese. China Feed. 2018, 23, 891–897. [Google Scholar]
- Li, N.; Chen, Y.J.; Xu, L.; Su, G.Q.; Wang, Z.Y.; Yang, H.M. Requirement of non-phytate phosphorus in 1- to 28-day-old geese based on growth performance, serum variables, and bone characteristics. Agriculture 2022, 12, 479. [Google Scholar]
- Zhu, Y.W.; Wang, C.Y.; Wen, J.; Wang, W.C.; Yang, L. Effect of dietary high non–phytate phosphorus level on growth performance and metabolism of calcium and phosphorus in Lion–head geese. Animal 2018, 236, 115–121. [Google Scholar] [CrossRef]
- Baker, D.H.; Oduho, G.W. Manganese utilisation in the chick: Effects of excess phosphorus on chicks fed manganese-deficient diets. Poul. Sci. 1994, 73, 1162–1165. [Google Scholar]
- Alagawany, M.; Ashour, E.A.; El–Kholy, M.S.; Mohamed, L.A.; El–Hack, M.E.A. Effect of dietary calcium and phosphorus levels on growth, carcass characteristics and liver and kidney functions of growing Egyptian geese. Poult. Sci. 2021, 100, 101244. [Google Scholar] [PubMed]
- Li, W.L.; Wang, B.W.; Liu, G.L.; Lin, Y.T. Effects of different levels of calcium and phosphorus on the production and biochemistry indexes of plasma and tibia in young Wulong geese. J. Anhui Agri. Uni. 2005, 32, 283–288. [Google Scholar]
- Wang, Z.W.; Mou, X.L.; Yang, G.W.; Li, J.K.; Liu, D.S. Effect of dietary nutrient levels on growth performance and serum parameters for northeast geese (1–28 d). J. Nucl. Agri. Sci. 2009, 23, 891–897. [Google Scholar]
- Chopin, F.; Biver, E.; Funck-Brentano, T.; Bouvard, B.; Coillaume, G.; Garnero, P.; Thomas, T. Prognostic interest of bone turnover markers in the management of postmenopausal osteoporosis. Jt. Bone Spine 2012, 79, 26–31. [Google Scholar]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar]
- Khosla, S. Minireview, The opg/rankl/rank system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yang, H.M.; Lu, J.; Li, W.Z.; Zou, J.M. Influence of whole hulled rice and rice husk feeding on the performance, carcass yield and digestive tract development of geese. Anim. Feed Sci. Tech. 2014, 194, 99–105. [Google Scholar]
- Chen, Y.J.; Yang, H.M.; Wan, X.L.; Wan, Y.; Zhang, H.; Wang, Z.Y. The effect of different dietary levels of sodium and chloride on performance and blood parameters in goslings (1–28 days of age). J. Anim. Physiol. Anim. Nutr. 2020, 104, 507–516. [Google Scholar]
- Rao, S.V.R.; Reddy, V.R.; Reddy, V.R. Non-phytin phosphorus requirements of commercial broilers and white leghorn layers. Anim. Feed Sci. Tech. 1999, 80, 1–10. [Google Scholar]
- Hu, Y.X.; Liao, X.D.; Wen, Q.; Lu, L.; Zhang, L.Y.; Luo, X.G. Phosphorus absorption and gene expression levels of related transporters in the small intestine of broilers. Brit. J. Nut. 2018, 119, 1346–1354. [Google Scholar]
- Cozannet, P.; Davin, R.; Jlali, M.; Jachacz, J.; Preynat, A.; Molist, F. Dietary metabolisable energy, digestible lysine, available phosphorus levels and exogenous enzymes affect broiler chicken performance. Animal 2021, 15, 100206. [Google Scholar] [CrossRef]
- Baradaran, N.; Shahir, M.H.; Asadi Kermani, Z.; Waldroup, P.W.; Sirjani, M.A. Effects of high non-phytate phosphorus starter diet on subsequent growth performance and carcass characteristics of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2014, 98, 643–650. [Google Scholar] [CrossRef]
- Yan, J.H.; Kerse, P.W.; Waldroup, P.W. Phosphorus requirements of broiler chicks to six weeks of age as influenced by phytase supplementation. Poult. Sci. 2001, 80, 455–459. [Google Scholar]
- Delmez, J.A.; Tindira, C.; Grooms, P.; Dusso, A.; windus, D.W.; Slatopolsky, E. Parathyroid hormone suppression by intravenous 1,25-dihydroxy vitamin D. A role for increased sensitivity to calcium. J. Clin. Invest. 1989, 83, 1349–1355. [Google Scholar] [CrossRef]
- Almaden, Y.; Hernandez, A.; Torregros, V.; Canalejo, A.; Sabate, L.; Fernandez Cruz, L.; Campistol, J.M.; Torres, A.; Rodriguez, M. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J. Am. Soc. Nephrolo. 1998, 9, 1845–1852. [Google Scholar] [CrossRef]
- Kemi, V.E.; Karkkainen, M.U.M.; Rita, H.J.; Laaksonen, M.M.L.; Outial, T.A.; Lamberg-Allardt, C.J.E. Low calcium: Phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Brit. J. Nutr. 2010, 103, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C.; Hayen, A.; Macaskill, P.; Pellegrini, F.; Craig, J.C.; Elder, G.L.; Stippoli, G.F.M. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta–analysis. JAMA J. Am. Med. Assoc. 2011, 305, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Mou, X.L.; Yang, G.W.; Li, J.K.; Liu, D.S. Effects of dietary nutritional levels on calcium, phosphorus concentrations and ALK activity in serum and calcium, phosphorus contents in tibia for geese. China Poult. 2009, 31, 16–20. [Google Scholar]
- Sommerville, B.A.; Blahos, J.; Harvey, S.; Chadwick, A.; Spencer, G.S.G. The time sequence of adaptive changes to dietary phosphorus deficiency in the chick. Horm. Metab. Res. 1985, 17, 247–250. [Google Scholar] [PubMed]
- Zhang, M.M.; Zhang, Y.H.; Mao, W.X.; Ma, Q.Q.; Gao, Y.; Liu, Y.; Liang, B.B. Correlation between BMD and TRACP, CTX-1, BALP, BGP, calcium, and phosphorus in 1084 women. Chin. J. Osteoporos. 2013, 19, 902–906. [Google Scholar]
- Namini, B.B.; Nezhad, Y.E.; Sarikhan, M.; Ahmadzadeh, A.R.; Hosseinzadeh, M.H.; Gholizadeh, B. Effects of dietary available phosphorus and microbial phytase on growth performance, carcass traits, serum minerals and toe ash content in broiler chicks. Int. J. Agric. Biol. 2012, 14, 435–439. [Google Scholar]
- Liu, P.; Oyajobi, B.O.; Russell, R.G.G.; Scutt, A. Regulation of osteogenic differentiation of human bone marrow stromal cells: Interaction between transforming growth factor-β and 1, 25 (OH)2 vitamin D3 in vitro. Calcif. Tissue Intl. 1999, 65, 173–180. [Google Scholar]
- Kumar, R. Metabolism of 1, 25-dihydroxyvitamin D3. Physiol. Rev. 1984, 64, 478–504. [Google Scholar]
- Brown, J.P.; Malaval, L.; Chapuy, M.C.; Delmas, P.D.; Edouard, C.; Meunier, P.J. Serum bone Gla-protein: A specific marker for bone formation in postmenopausal osteoporosis. Lancet 1984, 32, 1091–1093. [Google Scholar]
- Ekelund, A.; Spörndly, R.; Holtenius, K. Influence of low phosphorus intake during early lactation on apparent digestibility of phosphorus and bone metabolism in dairy cows. Livest Sci. 2006, 99, 227–236. [Google Scholar] [CrossRef]
- Qian, H.; Veit, H.P.; Kornegay, E.T.; Ravindran, V.; Denbow, D.M. Effects of supplemental phytase and phosphorus on histological and other tibial bone characteristics and performances of broilers fed semi-purified diets. Poul. Sci. 1996, 75, 618–626. [Google Scholar]
- Mohammed, A.; Gibney, M.J.; Taylor, T.G. The effects of dietary levels of inorganic phosphorus, calcium and cholecalciferol on the digestibility of phytate-P by the chick. Brit. J. Nutr. 1991, 66, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Jiang, Z.; Zhou, G.; Chen, Z.; Li, D. Non–phytate phosphorus requirements and efficacy of a genetically engineered yeast phytase in male Lingnan Yellow broilers from 1 to 21 days of age. J. Anim. Physiol. Anim. Nutr. 2011, 95, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Xing, G.Z.; Shao, Y.X.; Zhang, L.Y.; Li, S.F.; Lu, L.; Liu, Z.P.; Liao, X.D.; Luo, X.G. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilisation parameters of broilers. Poult. Sci. 2020, 99, 3207–3214. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Witten, P.E.; Hall, B.K.; Lall, S.P. Effects of dietary phosphorus on bone growth and mineralisation of vertebrae in haddock (Melanogrammus aeglefinus L.). Fish. Physiol. Biochem. 2002, 27, 35–48. [Google Scholar] [CrossRef]
- Dinev, I. Comparative pathomorphological study of rickets types in broiler chickens. Iran. J. Vet. Sci. Technol. 2011, 3, 1–10. [Google Scholar]
- Long, P.H.; Lee, S.R.; Rowland, G.N.; Britton, W.M.A. Experimental rickets in broilers: Gross, microscopic, and radiographic lesions. I. Phosphorus deficiency and calcium excess. Avian Dis. 1984, 28, 460–474. [Google Scholar] [PubMed]
- Shao, Y.; Xing, G.; Zhang, L.; Li, L.; Li, S.; Liao, X.; Luo, X. Effects of Dietary Calcium and Phosphorus Deficiency on Growth Performance, Rickets Incidence characters and tibia histological structure of broilers during 1 to 21 days of age. Chin. J. Anim. Nutr. 2019, 31, 2107–2118. [Google Scholar]
- Zhu, L.Q.; Zhu, F.H.; Zhang, Z.M.; Wang, Q.J.; Gong, Q.J. Effect of dietary available phosphorus levels on growth and tissue phosphorous and calcium contents in chicks. Chin. J. Anim. Nutr. 2001, 37, 14–16. [Google Scholar]
- Zhang, L.H.; He, T.F.; Hu, J.X.; Li, M.; Piao, X.S. Effects of normal and low calcium and phosphorus levels and 25-hydroxycholecalciferol supplementation on performance, serum antioxidant status, meat quality, and bone properties of broilers. Poult. Sci. 2020, 99, 5663–5672. [Google Scholar] [CrossRef]
- Xu, W.F.; Shi, W.M.; Yan, F. Temporal and tissue-specific expression of tomato 14-3-3 gene family in response to phosphorus deficiency. Pedosphere 2012, 22, 735–745. [Google Scholar] [CrossRef]
- Turner, R.T.; Kapelner, S.N.; Spelsberg, T.C. Tissue-specific expression of bone proteins in femora of growing rats. Am. J. Physiol.-Endoc. M. 1992, 263, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Dohi, Y.; Ohgushi, H.; Tabata, S.; Yoshikawa, T.; Dohi, K.; Moriyama, T. Osteogenesis associated with bone Gla protein gene expression in diffusion chambers by bone marrow cells with demineralised bone matrix. J. Bone. Miner. Res. 1992, 7, 1173–1180. [Google Scholar]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell. Tissue Res. 2010, 339, 189–195. [Google Scholar] [PubMed] [Green Version]
- Stricker, S.; Fundele, M.R.; Vortkamp, A.; Mundlos, S. Role of Runx genes in chondrocyte differentiation. Dev. Biol. 2002, 245, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.Z.; Wan, H.T.; Yang, J.H.; Li, Y.U.; Shen, M.L.; Yu, H.E. Effect and mechanism of guhong injection promoting fracture healing in rats. Chin. Pharm. J. 2018, 53, 1734–1741. [Google Scholar]
- Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Pinero, G.; Geoffroy, V.; Amling, M.; Karsenty, G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes. Dev. 1999, 13, 1025–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Gene. Dev. 1998, 12, 1260–1268. [Google Scholar] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Elliott, R.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar]
- Gori, F.; Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S.; Riggs, B.L. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 2000, 141, 4768–4776. [Google Scholar]
- Silva, I.; Branco, J. Rank/Rankl/opg: Literature review. Acta. Reumatol. Port. 2011, 36, 209–218. [Google Scholar]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor activator of nuclear factor κB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [PubMed]
- Bai, P.; Sun, Y.; Jin, J.; Hou, J.; Li, R.; Zhang, Q.; Yang, Y. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Resp. Res. 2011, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cowin, S.C.; Hegedus, D.H. Bone remodeling I: Theory of adaptive elasticity. J. Elast. 1976, 6, 313–326. [Google Scholar] [CrossRef]
| Items 1 | Groups 2 | ||
|---|---|---|---|
| PD | PC | PH | |
| Ingredient, % | |||
| Corn | 58.30 | 58.30 | 58.30 |
| Soybean meal, 43% CP | 31.60 | 31.60 | 31.60 |
| Wheat bran | 2.60 | 2.60 | 2.60 |
| Rice husk | 2.00 | 2.00 | 2.00 |
| Limestone | 1.93 | 1.42 | 0.77 |
| Calcium dihydrogen Phosphate | 0.00 | 1.38 | 3.12 |
| Vermiculite | 2.07 | 1.20 | 0.11 |
| DL–methionine | 0.20 | 0.20 | 0.20 |
| NaCl | 0.30 | 0.30 | 0.30 |
| Premix 3 | 1.00 | 1.00 | 1.00 |
| Total | 100.0 | 100.0 | 100.0 |
| Nutrient levels 2, % | |||
| ME (MJ/kg) | 11.24 | 11.24 | 11.24 |
| CP | 18.29 | 18.37 | 18.44 |
| Crude fiber | 4.27 | 4.27 | 4.27 |
| Calcium (Ca) | 0.76 | 0.78 | 0.78 |
| Total phosphorus (TP) | 0.35 | 0.67 | 1.05 |
| Non–phytate P (NPP) | 0.07 | 0.39 | 0.77 |
| Methionine | 0.49 | 0.49 | 0.49 |
| Lysine | 1.00 | 1.00 | 1.00 |
| Gene Name 1 | Primer Sequence (5′→3′) | Product (bp) | Accession Number |
|---|---|---|---|
| BGP | F: CGCTCCCGTTACGCTTATTT | 127 | XM_013202125.1 |
| R: AGGTGTGACAAAAAGTGTCGT | |||
| OPG | F:CATCTCAACACACTGATGGCAAG | 147 | XM_013185062 |
| R: GATGGTGTCTTGGTCTCCATTCT | |||
| RNAKL | F:ACCTGACTAAAAGAGGGCTTCAG | 102 | XM_013179680 |
| R: AGTATTTGGTGCTTCCTCCCTTC | |||
| BMP-2 | F: GCACCCAGCACGATGAAAAT | 276 | XM_013182079.1 |
| R: GACAATGGAGGGTCCGGATT | |||
| Runx2 | F: TTTACCTATACACCGCCAGTCAC | 115 | XM_013184488 |
| R: GTCCACTTTGGTTTTGGGAAGAG | |||
| β-actin | F: GCACCCAGCACGATGAAAAT | 150 | XM_013174886.1 |
| R: GACAATGGAGGGTCCGGATT |
| Item | Groups 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| PD | PC | PH | |||
| Initial body weight (g) | 101.1 | 100.7 | 100.6 | 0.19 | 0.503 |
| BW (g) | 437.2 a | 637.8 b | 663.9 b | 25.36 | <0.001 |
| ADG (g/bird·d) | 22.77 a | 37.03 b | 38.59 b | 1.77 | <0.001 |
| ADFI (g/bird·d) | 48.63 a | 68.21 b | 74.92 b | 3.03 | <0.001 |
| Feed/gain | 2.13 | 1.82 | 1.91 | 0.06 | 0.068 |
| Item | Groups 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| PD | PC | PH | |||
| Calcium (Ca, mmol/L) | |||||
| d 4 | 2.31 | 2.38 | 2.37 | 0.029 | 0.646 |
| d 7 | 2.35 | 2.29 | 2.40 | 0.041 | 0.581 |
| d 11 | 2.71 | 2.54 | 2.61 | 0.035 | 0.138 |
| d 14 | 2.51 | 2.33 | 2.48 | 0.033 | 0.054 |
| Phosphorus (P, mmol/L) | |||||
| d 4 | 2.23 | 2.45 | 2.43 | 0.063 | 0.301 |
| d 7 | 2.23 | 2.36 | 2.33 | 0.052 | 0.609 |
| d 11 | 2.33 | 2.57 | 2.46 | 0.059 | 0.247 |
| d 14 | 1.91 a | 2.37 b | 2.12 a | 0.062 | 0.002 |
| Alkaline phosphatase (ALP) activity (U/L) | |||||
| d 4 | 781.0 | 769.3 | 778.0 | 20.49 | 0.975 |
| d 7 | 1188 b | 777.8 a | 842.7 a | 48.42 | <0.001 |
| d 11 | 1125 b | 783.0 a | 947.5 ab | 49.37 | 0.008 |
| d 14 | 1114 b | 772.0 a | 936.8 ab | 53.24 | 0.020 |
| Item | Groups 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| PD | PC | PH | |||
| Parathyroid hormone (PTH) content (pg/mg) | |||||
| d 4 | 902.0 | 836.7 | 983.7 | 43.19 | 0.403 |
| d 7 | 1249 | 1054 | 1338 | 58.47 | 0.126 |
| d 11 | 1294 | 1200 | 1265 | 42.45 | 0.679 |
| d 14 | 1441 | 1359 | 1410 | 46.65 | 0.791 |
| Calcitonin (CT) content (pg/mg) | |||||
| d 4 | 17.94 | 14.48 | 18.40 | 0.774 | 0.069 |
| d 7 | 25.01 b | 19.04 a | 22.67 ab | 0.948 | 0.023 |
| d 11 | 23.61 b | 20.65 a | 27.33 c | 1.174 | <0.001 |
| d 14 | 27.41 b | 20.08 a | 25.40 b | 1.166 | 0.018 |
| 1,25-dihydroxyvitamin D3 (1,25-(OH2)D3) content (ng/mL) | |||||
| d 4 | 21.44 | 18.25 | 23.48 | 1.002 | 0.093 |
| d 7 | 28.68 | 24.37 | 30.66 | 1.223 | 0.094 |
| d 11 | 29.27 b | 22.91 a | 32.65 b | 1.339 | 0.003 |
| d 14 | 31.40 b | 27.81 a | 35.16 c | 0.967 | 0.002 |
| Bone-Gla-protein (BGP) content (ng/mL) | |||||
| d 4 | 15.63 | 16.72 | 14.24 | 0.450 | 0.069 |
| d 7 | 13.39 | 15.73 | 13.13 | 0.541 | 0.089 |
| d 11 | 14.93 | 16.01 | 14.41 | 0.455 | 0.362 |
| d 14 | 13.90 b | 15.53 c | 12.32 a | 0.418 | 0.002 |
| Osteoprotegerin (OPG) content (pg/mg) | |||||
| d 4 | 588.8 | 510.3 | 566.1 | 16.24 | 0.124 |
| d 7 | 636.1 | 612.5 | 639.1 | 14.09 | 0.725 |
| d 11 | 698.7 | 596.7 | 664.6 | 21.65 | 0.147 |
| d 14 | 767.1 | 717.2 | 780.3 | 21.83 | 0.488 |
| Item | Groups 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| PD | PC | PH | |||
| Width (cm) | |||||
| d 4 | 0.34 | 0.35 | 0.34 | 0.003 | 0.177 |
| d 7 | 0.40 | 0.41 | 0.41 | 0.006 | 0.704 |
| d 11 | 0.46 | 0.46 | 0.48 | 0.008 | 0.512 |
| d 14 | 0.48 a | 0.56 b | 0.55 b | 0.012 | 0.008 |
| Length (cm) | |||||
| d 4 | 5.03 | 5.15 | 5.00 | 0.034 | 0.174 |
| d 7 | 5.58 | 5.73 | 5.75 | 0.059 | 0.481 |
| d 11 | 6.46 a | 6.94 b | 6.99 b | 0.074 | 0.001 |
| d 14 | 7.05 a | 8.07 b | 8.10 b | 0.143 | <0.001 |
| Fresh weight (g) | |||||
| d 4 | 1.00 | 1.06 | 1.05 | 0.013 | 0.133 |
| d 7 | 1.66 | 1.72 | 1.76 | 0.060 | 0.494 |
| d 11 | 2.70 a | 3.20 b | 3.23 b | 0.091 | 0.016 |
| d 14 | 3.83 a | 4.95 b | 4.92 b | 0.187 | 0.010 |
| Strength (N) | |||||
| d 4 | 17.86 a | 25.53 b | 26.02 b | 1.079 | <0.001 |
| d 7 | 21.78 a | 47.10 b | 50.52 b | 3.509 | <0.001 |
| d 14 | 39.62 a | 174.5 b | 170.1 b | 15.71 | <0.001 |
| Item | Groups 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| PD | PC | PH | |||
| Ash content (%) | |||||
| d 4 | 30.7 a | 37.7 b | 38.3 b | 1.13 | 0.003 |
| d 7 | 29.6 a | 40.5 b | 42.0 b | 1.44 | <0.001 |
| d 11 | 31.5 a | 42.7 b | 43.9 b | 1.44 | <0.001 |
| d 14 | 29.5 a | 44.7 b | 45.3 b | 1.87 | <0.001 |
| Phosphorus (P) content (%) | |||||
| d 4 | 4.63 a | 7.55 b | 7.60 b | 0.37 | <0.001 |
| d 7 | 4.82 a | 8.40 b | 8.34 b | 0.43 | <0.001 |
| d 11 | 5.01 a | 8.68 b | 8.81 b | 0.44 | <0.001 |
| d 14 | 4.61 a | 8.76 b | 8.69 b | 0.48 | <0.001 |
| Calcium (Ca) content (%) | |||||
| d 4 | 11.36 a | 17.26 b | 17.23 b | 0.76 | <0.001 |
| d 7 | 12.18 a | 17.63 b | 18.14 b | 0.82 | <0.001 |
| d 11 | 11.24 a | 17.56 b | 18.41 b | 0.82 | <0.001 |
| d 14 | 9.21 a | 18.03 b | 18.72 b | 1.08 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; He, J.; Chen, H.; Chen, Y.; Chen, L.; Yang, H.; Xu, L.; Wang, Z. Effects of Dietary Phosphorus Deficiency and High Phosphorus Content on the Growth Performance, Serum Variables, and Tibia Development in Goslings. Agriculture 2022, 12, 1908. https://doi.org/10.3390/agriculture12111908
Li N, He J, Chen H, Chen Y, Chen L, Yang H, Xu L, Wang Z. Effects of Dietary Phosphorus Deficiency and High Phosphorus Content on the Growth Performance, Serum Variables, and Tibia Development in Goslings. Agriculture. 2022; 12(11):1908. https://doi.org/10.3390/agriculture12111908
Chicago/Turabian StyleLi, Ning, Jiayi He, Hao Chen, Yuanjing Chen, Lei Chen, Haiming Yang, Lei Xu, and Zhiyue Wang. 2022. "Effects of Dietary Phosphorus Deficiency and High Phosphorus Content on the Growth Performance, Serum Variables, and Tibia Development in Goslings" Agriculture 12, no. 11: 1908. https://doi.org/10.3390/agriculture12111908
APA StyleLi, N., He, J., Chen, H., Chen, Y., Chen, L., Yang, H., Xu, L., & Wang, Z. (2022). Effects of Dietary Phosphorus Deficiency and High Phosphorus Content on the Growth Performance, Serum Variables, and Tibia Development in Goslings. Agriculture, 12(11), 1908. https://doi.org/10.3390/agriculture12111908





