Endophytic Non-Pathogenic Fusarium oxysporum-Derived Dual Benefit for Nematode Management and Improved Banana (Musa spp.) Productivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Cultures
2.2. Mass Production of Fungal Spores and Inoculum Preparation
2.3. Tissue Cultured Banana Plants
2.4. Inoculation of Tissue Cultured Plants
2.5. Field Site and Experimental Design
2.6. Nematode Inoculation, Field Establishment and Maintenance
2.7. Data Collection
2.7.1. Plant Growth and Yield Data
2.7.2. Banana Weevil Damage
2.7.3. Nematode Infection and Endophyte Colonization from Root Samples
2.8. Data Analysis
3. Results
3.1. Endophyte Colonization of Plant Roots
3.2. Plant Growth, Survival, Toppling and Snapping
3.3. Nematode Densities
3.4. Nematode Root Necrosis Damage
3.5. Banana Weevil Damage
3.6. Plant Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Agriculture Data. Available online: http://faostat.fao.org (accessed on 20 May 2021).
- Evans, E.A.; Ballen, F.H.; Siddiq, M. Banana production, global trade, consumption trends, postharvest handling and processing. In Handbook of Banana Production, Postharvest Science, Processing Technology and Nutrition, 1st ed.; Siddiq, M., Ahmed, J., Labo, M.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–18. ISBN 9781119528234. [Google Scholar]
- Wairegi, L.W.I.; van Asten, P.J.A.; Tenywa, M.M.; Bekunda, M.A. Abiotic constraints override biotic constraints in East African Highland banana systems. Field Crops Res. 2010, 117, 146–153. [Google Scholar] [CrossRef]
- van Asten, P.J.A.; Gold, C.S.; Wendt, J.; De Waele, D.; Okech, S.H.O.; Ssali, H.; Tushemereirwe, W.K. The contribution of soil quality to yield and its relationship with other factors in Uganda. In Farmer-Participatory Testing of Integrated Pest Management Options for Sustainable Banana Production in Eastern Africa, Seeta, Uganda, 8–9 December 2003; Blomme, G., Gold, D.S., Karamura, E., Eds.; INIBAP: Montpellier, France, 2003; pp. 100–115. ISBN 2-910810-74-7. [Google Scholar]
- Vuylsteke, D.; Ortiz, R.; Ferris, S. Genetic and agronomic improvement for sustainable production of plantain and banana in sub-Saharan Africa. Afr. Crop Sci. J. 2010, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sikora, R.; Coyne, D.; Quénéhervé, P. Nematode parasites of bananas and plantains. In Plant Parasitic Nematodes in Subtropical Agriculture, 3rd ed.; Sikora, R., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Glasgow, UK, 2018; pp. 617–657. ISBN 9781786391261. [Google Scholar]
- Speijer, P.; Plowright, R.; Dusabe, J.; Coyne, D. Analysis of the pathogenic variability and genetic diversity of the plant-parasitic nematode Radopholus similis on bananas. Nematology 2013, 15, 41–56. [Google Scholar] [CrossRef]
- Price, N.S. The banana burrowing nematode, Radopholus similis (Cobb) Thorne, in the Lake Victoria region of East Africa: Its introduction, spread and impact. Nematology 2006, 8, 801–817. [Google Scholar] [CrossRef]
- Murongo, M.F.; Ayuke, O.F.; Mwine, T.J.; Wangai, K.J. Spatio-temporal distribution of banana weevil Cosmopolites sordidus [Germar] and nematodes of various genera in Uganda: A case of smallholder banana orchards in Western Uganda. J. Ecol. Nat. Environ. 2019, 11, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Nyang’au, D.; Atandi, J.; Cortada, L.; Nchore, S.; Mwangi, M.; Coyne, D. Diversity of nematodes on banana (Musa spp.) in Kenya linked to altitude and with a focus on the pathogenicity of Pratylenchus goodeyi. Nematology 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Reddy, S.K.V.; Prasad, J.S.; Speijer, P.R.; Sikora, R.A.; Coyne, D.L. Distribution of plant-parasitic nematodes on Musa in Kenya. Int. J. Banan. Plantain 2007, 16, 18–23. [Google Scholar]
- Treverrow, N. Banana Weevil Borer, 3rd ed.; CABI: Alstonville, Australia, 2003; Volume H6.AE.1, p. 3. [Google Scholar]
- Sengooba, T. Survey of Banana Pest Problem Complex in Rakai and Masaka Districts, Preliminary Trip Report; Uganda Ministry of Agriculture: Entebbe, Uganda, 1986; p. 10. [Google Scholar]
- Meldrum, R.A.; Daly, A.M.; Tran-Nguyen, L.T.T.; Aitken, E.A.B. Are banana weevil borers a vector in spreading Fusarium oxysporum f. sp. cubense tropical race 4 in banana plantations? Australas. Plant Pathol. 2013, 42, 543–549. [Google Scholar] [CrossRef]
- Were, E.; Nakato, G.V.; Ocimati, W.; Ramathani, I.; Olal, S.; Beed, F. The banana weevil, Cosmopolites sordidus (Germar), is a potential vector of Xanthomonas campestris pv. musacearum in bananas. Can. J. Plant Pathol. 2015, 37, 427–434. [Google Scholar] [CrossRef]
- Waweru, B.W.; Losenge, T.; Kahangi, E.M.; Dubois, T.; Coyne, D. Potential biological control of lesion nematodes on banana using Kenyan strains of endophytic Fusarium oxysporum. Nematology 2013, 15, 101–107. [Google Scholar] [CrossRef]
- Athman, S.Y.; Dubois, T.; Coyne, D.; Gold, C.S.; Labuschagne, N.; Viljoen, A. Effect of endophytic Fusarium oxysporum on root penetration and reproduction of Radopholus similis in tissue culture-derived banana (Musa spp.) plants. Nematology 2007, 9, 599–607. [Google Scholar] [CrossRef]
- Kato, F. Optimizing Inoculation Methods of Pest-Suppressing Root-Endophytic Fungi for Mass Application in a Commercial Banana Tissue Culture. Master’s Thesis, Makerere University, Kampala, Uganda, 2013. [Google Scholar]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J. Invertebr. Pathol. 2007, 96, 34–42. [Google Scholar] [CrossRef]
- Paparu, P.; Dubois, T.; Gold, C.S.; Adipala, E.; Niere, B.; Coyne, D. Inoculation, colonization and distribution of fungal endophytes in Musa tissue culture plants. Uganda J. Agric. Sci. 2004, 9, 583–589. [Google Scholar]
- Paparu, P.; Dubois, T.; Gold, C.S.; Niere, B.; Adipala, E.; Coyne, D.L. Improved colonization of East African Highland Musa tissue culture plants by endophytic Fusarium oxysporum. J. Crop Improv. 2006, 16, 81–95. [Google Scholar] [CrossRef]
- Paparu, P.; Dubois, T.; Coyne, D.; Viljoen, A. Dual inoculation of Fusarium oxysporum endophytes in banana: Effect on plant colonization, growth and control of the root burrowing nematode and the banana weevil. Biocontrol Sci. Technol. 2009, 19, 639–655. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol. Exp. Appl. 2008, 129, 157–165. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot. 2008, 27, 1437–1441. [Google Scholar] [CrossRef]
- Waweru, B.; Turoop, L.; Kahangi, E.; Coyne, D.; Dubois, T. Non-pathogenic Fusarium oxysporum endophytes provide field control of nematodes, improving yield of banana (Musa sp.). Biol. Control 2014, 74, 82–88. [Google Scholar] [CrossRef]
- Paparu, P.; Dubois, T.; Gold, C.S.; Niere, B.; Adipala, E.; Coyne, D. Screenhouse and field persistence of nonpathogenic endophytic Fusarium oxysporum in Musa tissue culture plants. Microb. Ecol. 2008, 55, 561–568. [Google Scholar] [CrossRef]
- Schuster, R.P.; Sikora, R.A.; Amin, N. Potential of endophytic fungi for the biological control of plant parasitic nematodes. Commun. Appl. Biol. Sci. 1995, 60, 1047–1052. [Google Scholar]
- Nankinga, C. Characterisation of Entomopathogenic Fungi and Evaluation of Delivery Systems of Beauveria bassiana for the Biological Control of the Banana Weevil, Cosmopolites sordidus. Ph.D. Thesis, University of Reading, Berkshire, UK, 1999. [Google Scholar]
- Nankinga, C.M. Potential of Indigenous Fungal Pathogens for the Control of Banana Weevil Cosmopolites sordidus (Germar), in Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 1994. [Google Scholar]
- Jaronski, S.T. Mass production of entomopathogenic fungi: State of the art. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, A.J., Rojas, M.G., Shapiro-llan, I.D., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 357–413. [Google Scholar]
- Jenkins, N.E.; Heviefo, G.; Langewald, J.; Cherry, A.J.; Lomer, C.J. Development of mass production technology for aerial conidia for use as mycopesticides. Biocontrol News Inf. 1998, 19, 21–32. [Google Scholar]
- Inglis, G.D.; Juerg, E.; Goettel, S.M. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 189–253. ISBN 978-0-12-386899-2. [Google Scholar]
- Coyne, D.L.; Nicol, J.M.; Claudius-Cole, B. Practical Plant Nematology: A Field and Laboratory Guide, 3rd ed.; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2018; p. 83. ISBN 978-978-8444-91-6. [Google Scholar]
- Coyne, D.L.; Adewuyi, O.; Mbiru, E. Protocol for In Vitro Culturing of Lesion Nematodes: Radopholus similis and Pratylenchus spp. on Carrot Discs; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2014; p. 15. ISBN 978-978-8444-44-2. [Google Scholar]
- Hallmann, J.; Subbotin, A.S. Methods for extraction, processing and detection of plant and soil nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Wallingford, UK, 2018; pp. 87–119. ISBN 978-1-78639-125-4. [Google Scholar]
- Lule, M.; Dubois, T.; Coyne, D. Trainer’s Manual: A Training Course for Banana Farmers Interested in Growing Tissue Culture Bananas; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2013; p. 138. ISBN 978-978-8444-10-7. [Google Scholar]
- NARO. Grow Bananas Better; National Agricultural Research Organisation (NARO): Kampala, Uganda, 2019; p. 72.
- Gold, C.S.; Messiaen, S. The Banana Weevil Cosmopolites sordidus. Musa Pest Fact Sheet 04; INIBAP: Montpellier, France, 2000; p. 4. [Google Scholar]
- Viljoen, A.; Mahuku, G.; Massawe, C.; Ssali, R.T.; Kimunye, J.; Mostert, G.; Ndayihanzamaso, P.; Coyne, D.L. Banana Diseases and Pests: Field Guide for Diagnostics and Data Collection; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2017; p. 73. ISBN 978-978-8444-80-0. [Google Scholar]
- Speijer, R.P.; De Waele, D. Screening of Musa Germplasm for Resistance and Tolerance to Nematodes; INIBAP: Montpellier, France, 1997; p. 47. ISBN 2-910810-16-X. [Google Scholar]
- Nelson, P.E.; Toussous, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1990; p. 206. ISBN 978-0271003498. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; American Phytopathological Society Press: St. Paul, MN, USA, 1998; p. 218. [Google Scholar]
- Warton, D.I.; Hui, F.C.K. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Lenth, V.R.; Buerkner, P.; Herve, M.; Love, J.; Singmann, H. Package ‘Emmeans’; R Foundation for Statistical Computing: Vienna, Austria, 2021; p. 88. [Google Scholar] [CrossRef]
- Brown, V.A. An Introduction to Linear Mixed-Effects Modeling in R. Adv. Methods Pract. Psychol. Sci. 2021, 4, 1–19. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org (accessed on 31 May 2021).
- Talwana, H.A.L.; Speijer, P.R.; Gold, C.S.; Swennen, R.L.; De Waele, D. A comparison of the effects of the nematodes Radopholus similis and Pratylenchus goodeyi on growth, root health and yield of an East African Highland cooking banana (Musa AAA-group). Int. J. Pest Manag. 2003, 49, 199–204. [Google Scholar] [CrossRef]
- Fogain, R. Effect of Radopholus similis on plant growth and yield of plantains (Musa, AAB). Nematology 2000, 2, 129–133. [Google Scholar] [CrossRef]
- Ssango, F.; Speijer, P.R.; Coyne, D.L.; De Waele, D. Path analysis: A novel approach to determine the contribution of nematode damage to East African Highland banana (Musa spp., AAA) yield loss under two crop management practices in Uganda. Field Crops Res. 2004, 90, 177–187. [Google Scholar] [CrossRef]
- Uwimana, B.; Zorrilla-fontanesi, Y.; Van Wesemael, J.; Mduma, H.; Brown, A. Effect of seasonal drought on the agronomic performance of four banana genotypes (Musa spp.) in the East African Highlands. Agronomy 2021, 11, 4. [Google Scholar] [CrossRef]
- Hauser, S.; van Asten, P. Methodological considerations on banana (Musa spp.) yield determinations. Acta Hortic. 2010, 879, 433–444. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. The effects of Beauveria bassiana dose and exposure duration on colonization and growth of tissue cultured banana (Musa sp.) plants. Biol. Control 2009, 49, 6–10. [Google Scholar] [CrossRef]
- Dababat, A.A. Importance of the Mutualistic Endophyte Fusarium oxysporum 162 for Enhancement of Tomato Transplants and the Biological Control of the Root-Knot Nematode Meloidogyne incognita, with Particular Reference to Mode-of-Action. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2006. [Google Scholar]
- Akello, J. Biodiversity of Fungal Endophytes Associated with Maize, Sorghum and Napier Grass and the Influence of Biopriming on Resistance to Leaf Mining, Stem Boring and Sap Sucking Insect Pests. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2012. [Google Scholar]
- Brownbridge, M.; Reay, S.D.; Nelson, T.L.; Glare, T.R. Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control 2012, 61, 194–200. [Google Scholar] [CrossRef]
- Posada, F.; Vega, E.F. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao). Mycologia 2005, 97, 1195–1200. [Google Scholar] [CrossRef]
- Russo, M.L.; Scorsetti, A.C.; Vianna, M.F.; Cabello, M.; Ferreri, N.; Pelizza, S. Endophytic effects of Beauveria bassiana on corn (Zea mays) and its herbivore, Rachiplusia nu (Lepidoptera: Noctuidae). Insects 2019, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Olivain, C.; Trouvelot, S.; Cordier, C.; Pugin, A.; Alabouvette, C. Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and nonpathogenic strains of Fusarium oxysporum. Appl. Environ. Microbiol. 2003, 69, 5453–5462. [Google Scholar] [CrossRef] [Green Version]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 2015, 29, 1725–1733. [Google Scholar] [CrossRef]
- Plett, J.M.; Martin, F.M. Know your enemy, embrace your friend: Using omics to understand how plants respond differently to pathogenic and mutualistic microorganisms. Plant J. 2018, 93, 729–746. [Google Scholar] [CrossRef] [Green Version]
- Paparu, P.; Dubois, T.; Coyne, D.; Viljoen, A. Effect of Fusarium oxysporum endophyte inoculation on the activities of phenylpropanoid pathway enzymes and Radopholus similis numbers in susceptible and tolerant East African Highland bananas. Nematology 2010, 12, 469–480. [Google Scholar] [CrossRef]
- Thangavelu, R.; Palaniswami, A.; Doraiswamy, S.; Velazhahan, R. The effect of Pseudomonas fluorescens and Fusarium oxysporum f.sp. cubense on induction of defense enzymes and phenolics in banana. Biol. Plant. 2003, 46, 107–112. [Google Scholar] [CrossRef]
- De Ascensao, A.R.D.C.F.; Dubery, I.A. Panama disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense Race four. Phytopathology 2006, 90, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganley, R.J.; Sniezko, R.A.; Newcombe, G. Endophyte-mediated resistance against white pine blister rust in Pinus monticola. For. Ecol. Manag. 2008, 255, 2751–2760. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest management via endophytic colonization of tobacco seedlings by the insect fungal pathogen Beauveria bassiana. Pest Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef]
- Akello, J.; Chabi-Olaye, A.; Sikora, R.A. Insect antagonistic bio-inoculants for natural control of leaf-mining insect pests of French beans. Afr. Crop Sci. J. 2017, 25, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Zhang, T.; Bai, S.; Zhi, Y.; Chen, J.; Wang, Z. Synergistic effect of Beauveria bassiana and Trichoderma asperellum to induce maize (Zea mays L.) defense against the Asian corn borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and larval immune response. Int. J. Mol. Sci. 2020, 21, 8215. [Google Scholar] [CrossRef] [PubMed]
- Bathina, P.; Bonam, R. Effect of endophytic isolates of Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metchnikoff) Sorokin on Plutella xylostella (L.) (Lepidoptera: Plutellidae) in cabbage. Egypt. J. Biol. Pest Control 2020, 30, 1–6. [Google Scholar] [CrossRef]
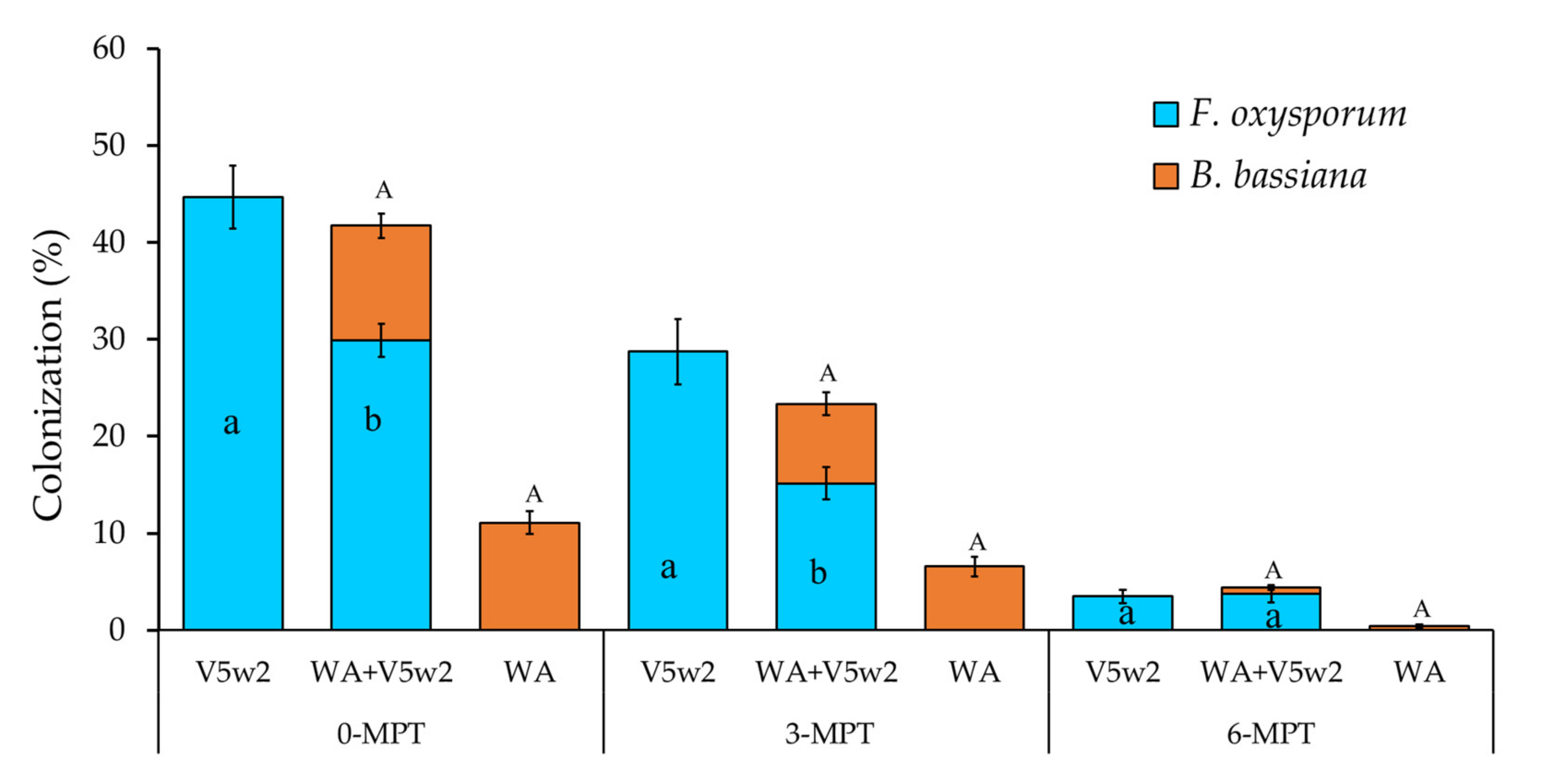
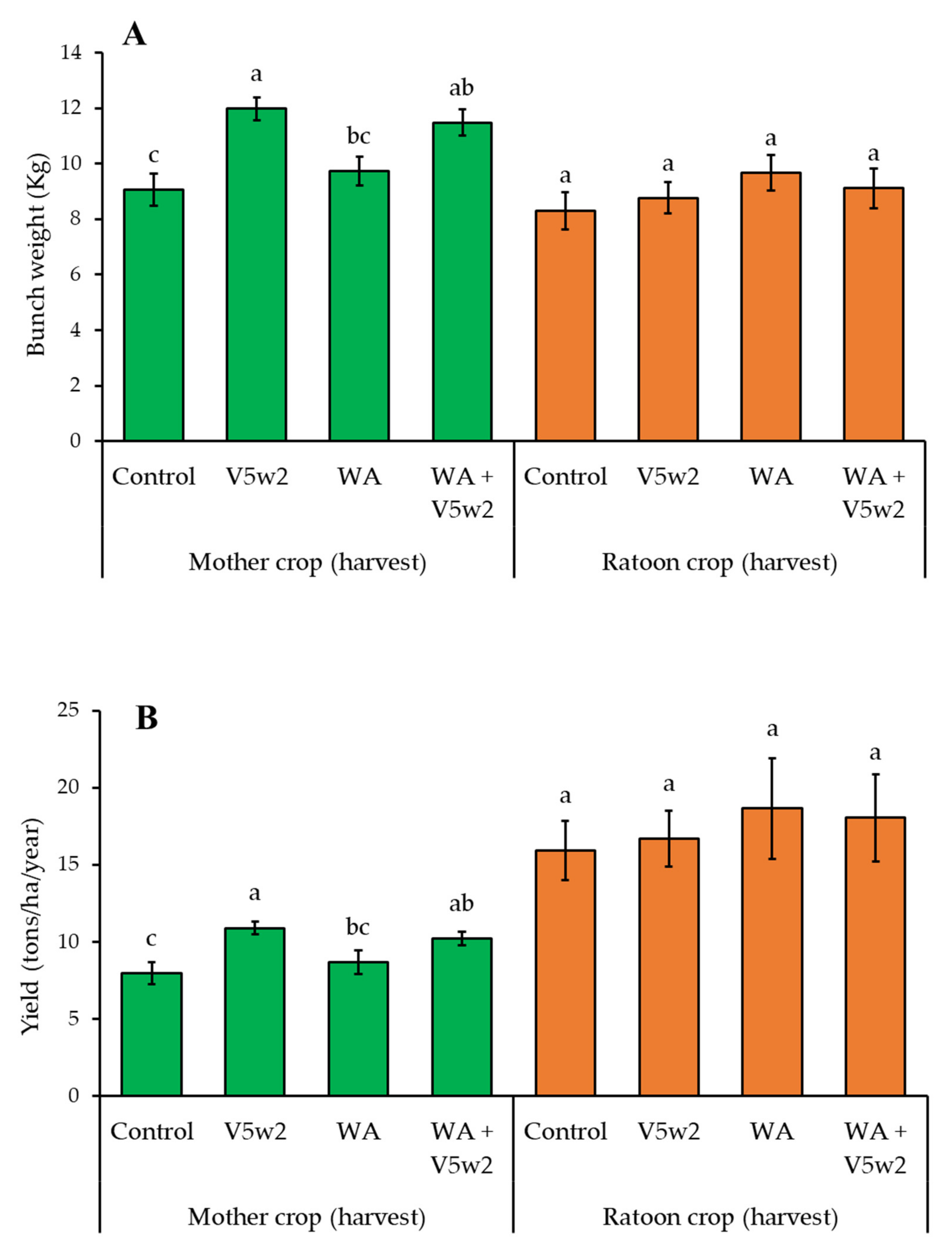
| Banana Cultivar | Treatment | Banana Growth Stage | |||||
|---|---|---|---|---|---|---|---|
| 0-MPT | 3-MPT | 6-MPT | 9-MPT | Mother Crop (Harvest) | Ratoon Crop (Harvest) | ||
| Plant height (cm) | |||||||
| Grande Naine | Control | 13.1 ± 0.3 bB | 57.6 ± 1.7 aB | 103.3 ± 3.0 aB | 162.0 ± 3.5 aB | 181.2 ± 3.9 aB | 207.9 ± 4.4 aB |
| V5W2 | 18.0 ± 0.4 aB | 58.7 ± 2.2 aB | 106.3 ± 3.1 aB | 161.3 ± 3.2 aB | 175.8 ± 3.3 aB | 202.9 ± 4.3 aB | |
| WA | 14.7 ± 0.5 bB | 58.4 ± 2.1 aB | 101.1 ± 3.2 aB | 158.4 ± 3.6 aB | 177.1 ± 4.2 aB | 197.3 ± 3.9 aB | |
| WA + V5w2 | 16.6 ± 0.3 aB | 59.5 ± 1.9 aB | 103.8 ± 3.2 aB | 166.2 ± 3.1 aB | 183.4 ± 2.8 aB | 214.9 ± 4.4 aB | |
| Mbwazirume | Control | 24.0 ± 0.6 abA | 78.9 ± 1.8 aA | 132.9 ± 2.7 aA | 217.2 ± 3.7 aA | 260.9 ± 3.2 aA | 284.7 ± 4.7 aA |
| V5W2 | 24.2 ± 0.6 abA | 77.8 ± 2.1 aA | 133.9 ± 3.3 aA | 212.3 ± 4.3 aA | 257.8 ± 3.3 aA | 284.3 ± 3.6 aA | |
| WA | 22.6 ± 0.6 bA | 79.6 ± 2.2 aA | 135.4 ± 3.2 aA | 219.9 ± 4.6 aA | 263.4 ± 2.0 aA | 291.7 ± 3.5 aA | |
| WA + V5w2 | 24.8 ± 0.5 aA | 79.1 ± 1.6 aA | 132.2 ± 2.8 aA | 217.7 ± 4.3 aA | 256.5 ± 3.4 aA | 279.9 ± 5.3 aA | |
| Girth (cm) at pseudostem base | |||||||
| Grande Naine | Control | na | 24.4 ± 0.8 aB | 42.2 ± 1.2 aB | 63.8 ± 1.3 aB | 63.3 ± 0.9 abB | 69.7 ± 1.2 abA |
| V5W2 | na | 25.2 ± 0.8 aB | 44.4 ± 1.2 aB | 63.5 ± 1.0 aB | 60.2 ± 0.6 bB | 67.3 ± 1.2 bB | |
| WA | na | 24.8 ± 0.9 aB | 41.0 ± 1.2 aB | 61.6 ± 1.1 aB | 60.8 ± 0.8 abB | 65.5 ± 1.0 bB | |
| WA + V5w2 | na | 24.8 ± 0.8 aB | 42.3 ± 1.3 aB | 64.7 ± 1.1 aB | 63.5 ± 0.9 aB | 73.7 ± 1.0 aA | |
| Mbwazirume | Control | na | 27.7 ± 0.7 aA | 49.7 ± 1.0 aA | 69.4 ± 0.9 aA | 67.0 ± 1.1 aA | 71.5 ± 1.3 aA |
| V5W2 | na | 27.7 ± 0.9 aA | 48.4 ± 1.3 aA | 68.8 ± 1.1 aA | 66.6 ± 1.0 aA | 72.0 ± 1.0 aA | |
| WA | na | 27.8 ± 0.8 aA | 48.5 ± 1.1 aA | 69.8 ± 0.9 aA | 69.0 ± 0.9 aA | 75.7 ± 1.2 aA | |
| WA + V5w2 | na | 26.8 ± 0.5 aA | 47.7 ± 1.0 aA | 67.0 ± 1.1 aA | 66.7 ± 0.9 aA | 72.7 ± 1.2 aA | |
| Girth (cm) at 100 cm above pseudostem base | |||||||
| Grande Naine | Control | na | na | 36.4 ± 0.6 aA | 45.8 ± 0.9 aA | 46.2 ± 0.8 aB | 56.9 ± 1.1 bA |
| V5W2 | na | na | 35.3 ± 0.6 aA | 44.8 ± 0.6 aA | 44.8 ± 0.7 aB | 54.6 ± 1.0 bA | |
| WA | Na | na | 35.0 ± 0.9 aA | 43.7 ± 0.7 aB | 45.6 ± 0.7 aB | 53.8 ± 0.8 bB | |
| WA + V5w2 | na | na | 35.3 ± 0.6 aA | 45.1 ± 0.8 aA | 46.8 ± 0.8 aB | 61.1 ± 1.0 aA | |
| Mbwazirume | Control | na | na | 34.2 ± 0.5 aB | 47.1 ± 0.8 abA | 48.8 ± 0.8 aA | 53.5 ± 1.3 bA |
| V5W2 | na | na | 34.1 ± 0.6 aA | 46.4 ± 0.9 abA | 47.9 ± 0.8 aA | 54.5 ± 1.0 abA | |
| WA | na | na | 35.0 ± 0.5 aA | 48.7 ± 1.0 aA | 50.1 ± 0.8 aA | 57.7 ± 1.1 aA | |
| WA + V5w2 | na | na | 33.5 ± 0.6 aA | 45.1 ± 1.0 bA | 47.6 ± 0.7 aA | 53.5 ± 1.1 bB | |
| Number of functional leaves | |||||||
| Grande Naine | Control | 6.4 ± 0.1 aA | 8.7 ± 0.2 aA | 11.8 ± 0.3 aA | 8.4 ± 0.2 abA | 0.1 ± 0.1 aB | 0.6 ± 0.2 aB |
| V5W2 | 6.5 ± 0.1 aA | 8.9 ± 0.3 aA | 12.4 ± 0.2 aA | 8.0 ± 0.2 bcA | 0.4 ± 0.1 aB | 0.4 ± 0.2 aB | |
| WA | 6.0 ± 0.1 bA | 8.6 ± 0.2 aA | 12.0 ± 0.2 aA | 7.9 ± 0.2 cA | 0.3 ± 0.1 aB | 0.6 ± 0.2 aB | |
| WA + V5w2 | 5.6 ± 0.1 bA | 8.4 ± 0.2 aA | 12.4 ± 0.2 aA | 8.6 ± 0.1 aA | 0.3 ± 0.1 aB | 0.6 ± 0.2 aB | |
| Mbwazirume | Control | 5.3 ± 0.1 aB | 7.2 ± 0.1 aB | 10.9 ± 0.1 aB | 7.5 ± 0.1 aB | 2.9 ± 0.2 aA | 1.1 ± 0.3 aA |
| V5W2 | 5.4 ± 0.1 aB | 7.0 ± 0.2 aB | 10.6 ± 0.2 aB | 7.4 ± 0.1 aB | 3.2 ± 0.2 aA | 0.9 ± 0.2 aA | |
| WA | 5.2 ± 0.1 aB | 7.0 ± 0.2 aB | 10.3 ± 0.2 aB | 7.5 ± 0.1 aA | 3.0 ± 0.2 aA | 1.7 ± 0.2 aA | |
| WA + V5w2 | 4.5 ± 0.1 bB | 6.8 ± 0.1 aB | 10.6 ± 0.2 aB | 7.4 ± 0.1 aB | 3.4 ± 0.2 aA | 1.0 ± 0.3 aA | |
| Crop Cycle | Treatment | Flowered Plants (%) | Harvested Plants (%) | Toppled Plants (%) | Snapped Plants (%) |
|---|---|---|---|---|---|
| Mother crop (harvest) | Control | 90.6 ± 3.1 aA | 73.4 ± 3.0 cA | 23.3 ± 3.2 aA | 3.3 ± 1.2 aB |
| V5w2 | 95.3 ± 2.0 aA | 93.6 ± 1.7 aA | 2.3 ± 1.6 cB | 4.1 ± 1.2 aB | |
| WA | 95.3 ± 2.3 aA | 80.4 ± 3.4 bcA | 16.5 ± 4.1 abA | 3.1 ± 1.7 aB | |
| WA + V5w2 | 92.2 ± 1.6 aA | 89.1 ± 2.6 abA | 7.8 ± 1.6 bcB | 3.1 ± 1.7 aB | |
| Ratoon crop (harvest) | Control | 88.9 ± 1.7 aA | 66.5 ± 5.1 aA | 25.7 ± 3.7 aA | 7.7 ± 1.9 aA |
| V5w2 | 93.1 ± 2.3 aA | 75.5 ± 4.8 aB | 16.5 ± 3.3 aA | 8.0 ± 2.3 aA | |
| WA | 94.0 ± 2.8 aA | 75.8 ± 6.2 aA | 18.2 ± 4.5 aA | 6.0 ± 3.2 aA | |
| WA + V5w2 | 87.9 ± 6.9 aA | 67.1 ± 8.2 aB | 24.9 ± 4.8 aA | 8.0 ± 4.3 aA |
| Growth Stage | Treatment | R. similis | H. multicinctus | Total Nematode * | Root Necrosis (%) |
|---|---|---|---|---|---|
| 3-MPT | Control | 3319 ± 645 a | 122 ± 50 a | 3528 ± 657 a | 24.2 ± 1.1 a |
| V5w2 | 1528 ± 308 b | 81 ± 30 a | 1622 ± 324 b | 12.8 ± 1.0 c | |
| WA | 2800 ± 619 ab | 116 ± 38 a | 2934 ± 626 ab | 17.7 ± 0.9 b | |
| WA + V5w2 | 2175 ± 373 ab | 109 ± 44 a | 2341 ± 395 ab | 14.5 ± 0.7 bc | |
| 6-MPT | Control | 5567 ± 780 a | 205 ± 78 a | 5780 ± 796 a | 33.0 ± 1.4 a |
| V5w2 | 2892 ± 410 b | 43 ± 16 b | 2954 ± 415 b | 26.4 ± 1.9 b | |
| WA | 3350 ± 502 b | 76 ± 24 ab | 3485 ± 512 ab | 29.5 ± 1.9 ab | |
| WA + V5w2 | 3129 ± 281 b | 112 ± 28 ab | 3281 ± 291 b | 28.0 ± 1.5 ab | |
| 9-MPT | Control | 8203 ± 1064 a | 275 ± 103 a | 8559 ± 1072 a | 34.3 ± 1.9 a |
| V5w2 | 5409 ± 610 ab | 125 ± 78 a | 5550 ± 613 b | 22.1 ± 1.4 b | |
| WA | 7238 ± 811 ab | 94 ± 34 a | 7366 ± 806 ab | 34.2 ± 2.9 a | |
| WA + V5w2 | 5113 ± 588 b | 131 ± 54 a | 5269 ± 601 b | 24.0 ± 1.7 b | |
| Mother crop (harvest) | Control | 8024 ± 509 a | 2867 ± 373 a | 11069 ± 561 a | 52.6 ± 1.6 a |
| V5w2 | 5301 ± 614 b | 1761 ± 265 a | 7202 ± 666 b | 41.8 ± 1.5 b | |
| WA | 6200 ± 646 ab | 3187 ± 534 a | 9531 ± 867 ab | 50.8 ± 1.6 a | |
| WA + V5w2 | 4489 ± 368 b | 2559 ± 317 a | 7153 ± 440 b | 42.8 ± 1.3 b | |
| Ratoon crop (harvest) | Control | 9158 ± 1063 a | 1050 ± 167 a | 10375 ± 1194 a | 49.6 ± 1.9 a |
| V5w2 | 8685 ± 813 a | 940 ± 119 a | 9679 ± 892 a | 48.3 ± 2.2 a | |
| WA | 9320 ± 1151 a | 1072 ± 169 a | 10517 ± 1288 a | 52.1 ± 1.5 a | |
| WA + V5w2 | 8148 ± 700 a | 1160 ± 152 a | 9450 ± 796 a | 49.7 ± 1.9 a |
| Growth Stage | Treatment | Outer Corm (OC) | Inner Corm (IC) | Outer Pseudostem (OP) | Inner Pseudostem (IP) |
|---|---|---|---|---|---|
| Mother crop (harvest) | Control | 5.80 ± 0.28 aB | 2.35 ± 0.25 aB | 2.98 ± 0.23 aB | 1.35 ± 0.19 aB |
| V5w2 | 6.56 ± 0.57 aB | 2.27 ± 0.46 aB | 3.43 ± 0.47 aB | 1.43 ± 0.40 aB | |
| WA | 5.28 ± 0.55 aB | 2.14 ± 0.48 aB | 2.73 ± 0.39 aB | 1.01 ± 0.25 aB | |
| WA + V5w2 | 5.68 ± 0.48 aB | 1.81 ± 0.28 aB | 2.32 ± 0.37 aB | 1.04 ± 0.27 aB | |
| Ratoon crop (harvest) | Control | 15.27 ± 1.29 aA | 5.40 ± 0.89 aA | 8.36 ± 1.85 aA | 2.96 ± 1.66 aA |
| V5w2 | 12.06 ± 2.26 aA | 3.52 ± 1.27 aA | 7.02 ± 1.83 aA | 3.54 ± 0.93 aA | |
| WA | 16.26 ± 1.77 aA | 4.77 ± 1.80 aA | 8.35 ± 2.57 aA | 2.55 ± 1.39 aA | |
| WA + V5w2 | 15.90 ± 2.56 aA | 6.57 ± 2.30 aA | 9.93 ± 2.57 aA | 3.67 ± 1.18 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisaakye, J.; Fourie, H.; Haukeland, S.; Kisitu, J.; Nakimera, S.; Cortada, L.; Subramanian, S.; Coyne, D. Endophytic Non-Pathogenic Fusarium oxysporum-Derived Dual Benefit for Nematode Management and Improved Banana (Musa spp.) Productivity. Agriculture 2022, 12, 125. https://doi.org/10.3390/agriculture12020125
Kisaakye J, Fourie H, Haukeland S, Kisitu J, Nakimera S, Cortada L, Subramanian S, Coyne D. Endophytic Non-Pathogenic Fusarium oxysporum-Derived Dual Benefit for Nematode Management and Improved Banana (Musa spp.) Productivity. Agriculture. 2022; 12(2):125. https://doi.org/10.3390/agriculture12020125
Chicago/Turabian StyleKisaakye, James, Hendrika Fourie, Solveig Haukeland, Joseph Kisitu, Solomy Nakimera, Laura Cortada, Sevgan Subramanian, and Danny Coyne. 2022. "Endophytic Non-Pathogenic Fusarium oxysporum-Derived Dual Benefit for Nematode Management and Improved Banana (Musa spp.) Productivity" Agriculture 12, no. 2: 125. https://doi.org/10.3390/agriculture12020125






