Abstract
The study was conducted to evaluate the influence of silicon application on the botanical composition of grass–legume mixtures as well as the nutritional value of individual species and mixtures. The two-factor field experiment was established in a split-block arrangement. The two grass–legume mixtures, consisting of Dactylis glomerata, Festulolium braunii and Trifolium pratense or Medicago x varia and grass mixture—Dactylis glomerata, Festulolium braunii and Lolium perenne, were sown on arable land. Silicon foliar and mineral NPK fertilization was applied in three variants: NPK (non-Si-fertilized), NPK+Si (Herbagreen), NPK+Si (Optysil). Changes in the botanical composition in the sward of mixtures during the study period were influenced by the competitiveness of individual plants and weather conditions, whereas the effect of foliar fertilization with silicon was only slightly marked. The nutritional value of individual species of grasses and legumes varied over the years and also depended on the application of silicon. The greatest content of crude protein (CP), irrespective of the species and variant of silicon fertilization, was noticed in years with no drought. Our research confirmed the beneficial effect of silicon fertilization, independent of weather conditions, on the CP content and on the reduction of crude fibre (CF), neutral detergent fibre (NDF) and acid detergent fibre (ADF) in plants, especially when Herbagreen was applied. The nutritional value of the mixtures under applied silicon fertilization depended on botanical composition. In the conditions of low rainfall, a large share of F. braunii and an approximately 20% contribution of T. pratense resulted in a higher content of CP. These results may be used in the fertilization practices of temporary grassland for enhancing nutritional value, especially crude protein content, and on the reduction of fibre in the sward.
1. Introduction
The use of mixtures of grasses with legumes on arable land is important for an additional source of feed for ruminants. In sustainable agriculture, it is particularly important to cultivate grass–legumes mixtures, which are a valuable source of protein in animal nutrition and enable the production of forage under conditions of low nitrogen fertilization [1]. Temporary grasslands established on arable land provide valuable fodder, especially on farms with a small area of permanent meadows or those with low efficiency [2,3]. The increasingly frequent extreme weather conditions associated with global climatic changes, especially long-term droughts, have an impact on changes in the productivity of grasslands [4,5]. The cultivation of simple grass–legume mixtures (2–4 components) becomes more important in conditions of intensive, short-term utilization and the demand for fodder with a higher content of nutrients [6,7]. The components of temporary grass–legume mixtures in temperate climate are Medicago sativa L., Medicago x varia T. Martyn, Trifolium pratense L. and highly productive grass species, including Festulolium braunii (Richt.) A. Camus, all species of the genus Lolium and Dactylis glomerata L. [8,9]. These mixtures, compared to pure-sown species, are characterized by higher and more stable yields, especially in the case of more frequent periods of drought [10]. In addition, they are characterized by a greater concentration of energy and a more balanced ratio of protein to energy components [11,12]. The cultivation of grass–legume mixtures is also of great importance for the protection and improvement of the environment. They enrich the soil with organic matter and nutrients and improve the soil structure and its physicochemical properties [13,14,15]. The increase of interest in growing forage legumes, including Trifolium pratense, is due to increasing energy costs and the deteriorating environmental effects of synthetic nitrogen use [16]. Medicago is also a valuable plant from an economic point of view because of its favourable chemical composition, with content of protein, fibre and carotenoids [17,18]. Lolium species are the most important grasses sown in temperate Europe; among them, Lolium perenne is known to be highly productive and digestible, and is widely sown [16,19,20,21]. At the same time, this species is short-lived, remains in the sward for 2–3 years and is sensitive to unfavourable weather conditions, especially low temperatures in winter [22]. Festulolium braunii, an intergeneral hybrid derived from Festuca pratensis and Lolium multiflorum, has a high yielding potential, similar to L. multiflorum, and a better persistence and wintering, similar to F. pratensis, due to a more developed root system [23]. In turn, Dactylis glomerata is considered a permanent and competitive component of fodder grass mixtures [22,24,25].
In order to obtain the highest and best-quality crops, due to progressive climate change, more attention is being paid to the possibility of using various growth regulators or biostimulators [26,27,28,29]. Silicon is one of the components that stimulate plant growth and development [30,31]. It is not recognized as an essential element for plant growth, but the beneficial effects of this element on the growth, development, yield and disease resistance have been observed in a wide variety of plant species [32]. Silicon is deposited in the cell walls of plants, strengthens their structure and increases the stiffness of leaves and shoots, and also contributes to the faster formation of the root system by young plants, the development of hair roots and an increase in root mass [33,34]. The effect of fertilization with this nutrient is visible mainly under stress conditions [35,36]. There are some studies regarding the beneficial role of silicone in plants subjected to drought stress, mainly concentrating on water metabolism, photosynthesis and some physiological traits [37,38]. Plants are less susceptible to lodging and drought [39], and also temperature extremes (by effectively maintaining transpiration) and are more resistant to disease (especially fungi) [40] and pest damage, resulting in higher yields [41]. It has also been shown that silicon increases nutrient uptake (N, P, K) [42] and plant salinity tolerance, and moreover reduces the effects of heavy metal toxicity [43]. In plants growing under salt-stress conditions, added silicon helps in maintaining an adequate supply of essential nutrients and reduces sodium uptake and its transport to shoots [44]. Water deficit adversely affects photosynthesis, uptake and transport of essential nutrients and causes overproduction of reactive oxygen species (ROS), which lead to serious disorders in plant metabolism and damage in membranes. Silicon plays an important role in the resistance of plants to water stress, preventing damage and repairing it quickly, e.g., elimination of ROS and prevention of oxidative stress. The addition of silicon can enhance the activity of antioxidant enzymes and the concentration of antioxidant metabolites in plants growing under water stress [45]. For this reason, silicon is more often considered an essential ingredient for optimal plant yield [31].
Silicon fertilization (soil and foliar) is mainly used in the cultivation of monocotyledonous: Zea mays L. [46] or Triticum aestivum L. [47] and Hordeum vulgare L. [48], as well as several dicotyledonous species, especially Glycine max (L.) Merr. [49] or other plants in tropical and subtropical climate countries, where soils are characterized by low abundance in available silicon [50,51].
The results of the latest research indicate that the inclusion of silicon in fertilization is also justified in countries with a temperate climate [52,53]. In Poland, a positive effect of this element was found on the growth, development and yielding of sugar beet [54,55], and recently also of spring Triticum aestivum in organic farming [56]. The studies of Radkowski et al. [57] did not show a greater impact of foliar application of silicon fertilizers on the yield, but it did have an impact on the floristic and chemical composition of the meadow sward, especially on the content of protein in the silages. A trend towards a higher protein content might be caused by the higher share of legume plants in the botanical composition of the meadow plants and the increased protein content in these plants. In turn, Mastalerczuk et al. [58] showed a beneficial effect of the use of silicon on the yield of grass and clover sward as well as a higher content of crude protein and better digestibility of organic matter in organic farming. Higher crude protein content, independent of weather conditions, was noticed under multicomponent Herbagreen application.
Research conducted under silicon fertilization on the species of grasses and legumes used for grassland does not give clear results. Regardless of these studies, the available literature still provides very little data on the effect of silicon fertilization on the nutritional value of individual species of grasses and legume plants, as well as on the quality of the sward of mixtures used on grasslands. In order to obtain knowledge on the given issues, we conducted studies to investigate (i) changes in species composition of temporary grass–legume mixtures under foliar silicon fertilization and (ii) the influence of silicon application on the nutritional value and digestibility of individual grass and legume species, as well as grass–legume mixtures.
2. Materials and Methods
2.1. Experimental Design
The research was conducted in the years 2015–2017 at the Experimental Field in Miedniewice belonging to the Experimental Station of the Warsaw University of Life Sciences in Skierniewice (51°58′05″ N, 20°11′22″ E), central Poland. The two-factor field experiment was established on 9 May in 2014 on Luvisol soils of the texture of loamy sand [59]. Plot size was 29.3 m2. Row sowing was carried out with a Vredo seeder. The soil was slightly acidic (pHKCl = 5.8), content of the organic carbon (C org) was (mg/kg of soil) 72.0 and the nitrogen (N) was 9.5. The content of available nutrients was as follows (mg/kg): P—30.8 (low), K—139.0 (high), Mg—109.0 (very high). Before sowing, fertilization was applied (kg/ha): N—30 (ammonium nitrate, 34%), P—35 (triple superphosphate), K—50 (potassium salt). Annual NPK fertilization in the years of utilization amounted to (kg/ha): N—90 (in three parts—in spring and after the 1st and 2nd cut), P—35 (once in spring), K—100 (in two equal parts—in spring and after the 1st cut). The first experimental factor consisted of two grass–legume mixtures and a mixture consisting of grasses:
- −
- M1—Dactylis glomerata L. (cv. Berta), Festulolium braunii (Richt.) A. Camus, cv. Sulino, Trifolium pratense L., cv. Rozeta (55%, 30%, 15%, were sown at the rate of 12.0 kg/ha, 12.0 kg/ha, 3.0 kg/ha, respectively).
- −
- M2—Dactylis glomerata L., cv. Berta, Festulolium braunii (Richt.) A. Camus, cv. Sulino, Medicago x varia T. Martyn, cv. Radius (55%, 30%, 15%, were sown at the rate of 12.0 kg/ha, 12.0 kg/ha, 3.0 kg/ha, respectively).
- −
- M3—Dactylis glomerata L., cv. Berta, Festulolium braunii (Richt.) A. Camus, cv. Sulino, Lolium perenne L., cv. Gagat (45%, 30%, 25%, were sown at the rate of 9.5 kg/ha, 10.0 kg/ha, 9.3 kg/ha, respectively).
The second testing factor consisted of fertilizers containing silicon—Herbagreen and Optysil. Silicon foliar fertilization was used in three variants: non Si-fertilized (control), Si (Herbagreen), Si (Optysil). Herbagreen was applied in a dose of 4.0 kg/ha and Optysil—1.0 L/ha as a solution diluted in 300 L of water. The plots were sprayed using a field sprayer in two equal parts for each regrowth, i.e., four and two weeks before each cut. The fertilizer Optysil contains silicon (16.8% SiO2) and 2% of chelated iron, while Herbagreen contains silicon (17% SiO2), calcium (36.7% CaO), magnesium (2.4% MgO), iron (3.4% Fe2O3), titanium (0.5% TiO2) and other ingredients. The sward was mowed three times. The first cut was made during the heading stage of the grass species dominating in the sward (27 May 2015; 25 May 2016; 2 June 2017). The second and third harvest were taken after 6–8 weeks in relation to the previous cut.
2.2. Botanical Composition and Nutritive Value Analyses
The botanical composition of the sward of mixtures and the nutritional value of individual species of grasses and legumes, as well as mixtures in the first cut, were assessed. Samples of 500 g of green mass, taken from each plot, were subjected to botanical analysis. The sown species of grasses and legumes, as well as other species (grasses and dicotyledonous), were separated. Then, after drying and weighing, the share of the components of each mixture in the first cut was determined. The nutrient content was evaluated in individual plant species and in the sward of mixtures (in dry matter): organic matter (OM; g/kg), crude protein (CP; g/kg), crude fibre (CF; g/kg), crude ash (CA; g/kg), crude fat (CFa; g/kg), nitrogen-free extract (NFE; g/kg), neutral detergent fibre (NDF; g/kg), acid detergent fibre (ADF; g/kg), acid detergent lignin (ADL; g/kg), water soluble carbohydrates (WSC; g/kg), non-fibre carbohydrates (NFC; g/kg), organic matter digestibility (OMD; %), dry matter digestibility (DMD; %). Parameters were analysed using the NIRS method on a near-infrared spectrometer, NIRFlex N-500 (Büchi Labortechnik AG, Flawil, Switzerland), with ready-made calibrations.
2.3. Weather Conditions
Basic meteorological data were measured at the meteorological station at the research site (Table 1). The weather conditions in the period from spring to the harvest of the first cut were distinctly differentiated, especially due to precipitation.

Table 1.
Temperature (°C) and precipitation (mm) in the experiment site during the growing seasons, 2015–2017, from April to May (to the first cut).
In the first year of utilization, in the period from April to May, the precipitation was only 83 mm (30 mm lower than the average in the three-year study period), which adversely affected plant growth. There was also an uneven distribution over the decades, especially low rainfall occurred in the second decade of May, before the mowing, which could have a negative impact on the dry mass of yield and the quality. In the following year, the sum of rainfall exceeded the average for the given study period, although initially they were very small (only about 20 mm in April). However, quite high rainfall in May (total 95 mm), increasing in the following decades, contributing to the improvement of plant growth conditions. In the last year, precipitation was the highest during the study period (by 23 mm compared to the 3-year average) and was similar in both months. However, the lack of rainfall in the second decade and low precipitation in the third decade of May negatively influenced the conditions of plant growth before the harvest of the first regrowth.
2.4. Statistical Analysis
The field experiment was established in a split-block arrangement with three replications. It included three blocks of 9 plots (27 plots in total). The data were analysed using the TIBCO StatisticaTM 13.3.0 software. Three-factor analysis of variance (ANOVA) was used to evaluate nutritional components concentration. Differences between means were assessed with the Tukey’s multiple comparison test (p ≤ 0.05). Pearson correlation coefficients (PCC) were calculated to measure relationship between tested traits.
3. Results
3.1. Botanical Composition
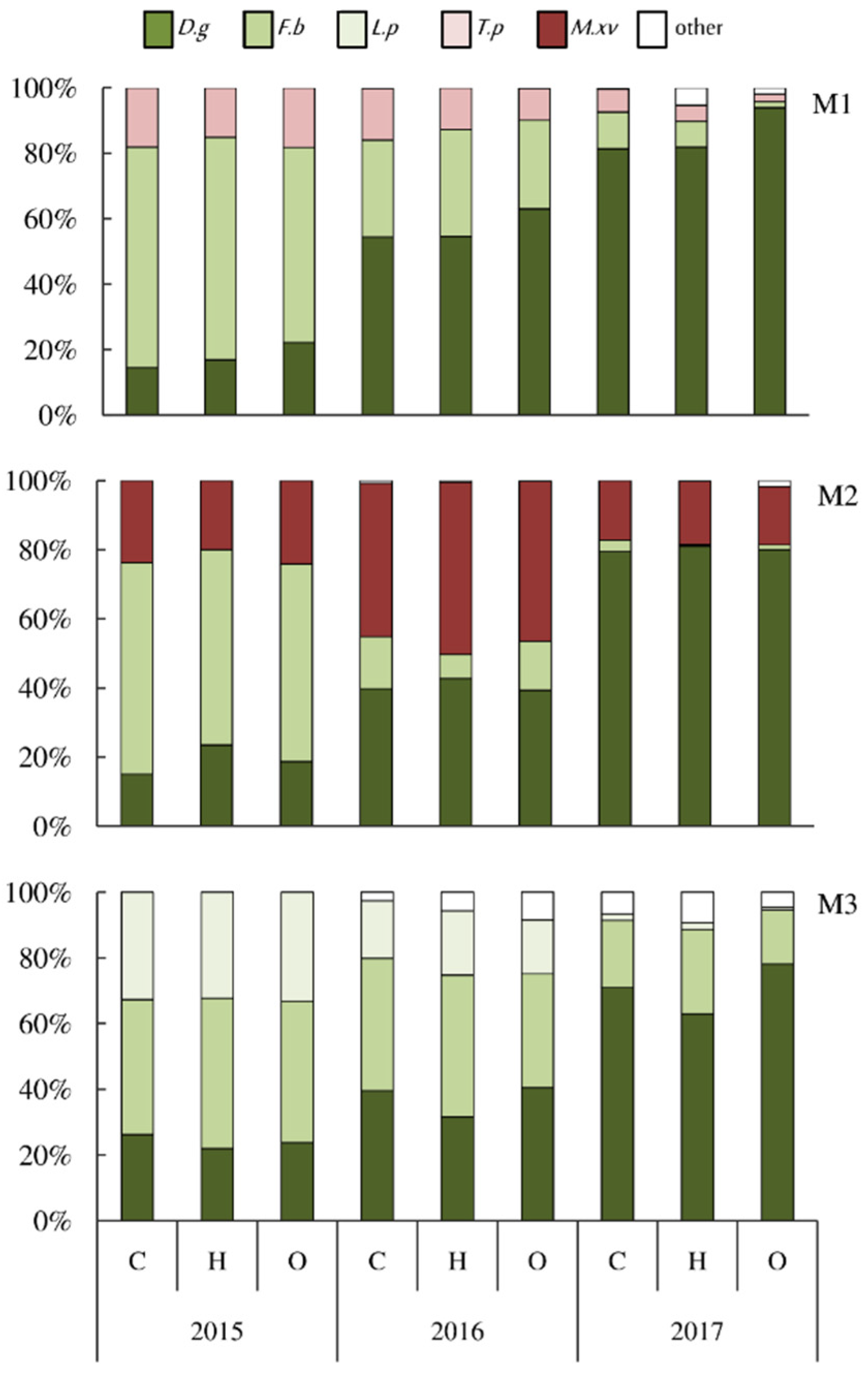
The botanical composition of the assessed mixtures varied over the years (Figure 1). In the sward of the mixture with T. pratense (M1) in the first year of utilization, F. braunii dominated (average 65%), while the share of D. glomerata was only 18%. In the following years, however, there was a clear increase in the share of D. glomerata in the sward (up to 57% and 86%, respectively) at the expense of F. braunii, which in the third year of utilization was only 7% in the sward. The share of T. pratense also decreased in subsequent years, from 17% to 5%. Similar relationships with regard to the share of D. glomerata and F. braunii in the sward were also found for the M2 mixture (M.x varia was a component). The share of D. glomerata in the following years doubled, and in the third year of use it was 80%, while F. braunii decreased to just 2%. There was also a double increase in the share of M.x varia in the second year (up to 47%), and this species dominated in the sward together with D. glomerata. However, in the last year of the study, the share of M.x varia in the sward remained at the level of 17% due to the dominance of D. glomerata.

Figure 1.
Botanical composition of the sward of grass–legume mixtures (M1—D. glomerata, F. braunii, T. pratense; M2—D. glomerata, F. braunii, M.x varia) and grass mixture (M3—D. glomerata, F. braunii, L. perenne) in the first cut depending on the silicon fertilization (C—control, H—Herbagreen, O—Optysil) during 3-years of utilization. D.g—D. glomerata, F.b—F. braunii, L.p—L. perenne, T.p—T. pratense, M. xv—M.x varia.
In the grass mixture (M3), in the first year of utilization, F. braunii and L. perenne were the dominant species (43% and 33% in the sward, respectively). While in the following year their share decreased, especially L. perenne, and consequently D. glomerata with F. branii accounted for as much as 76% in the sward. These species also dominated in the sward in the last year of the study (92% in total), because L. perenne had a negligible share.
The study showed that the influence of foliar fertilization with silicon on the botanical composition of the mixtures was only slightly marked (Figure 1). It was more visible in the mixture M1, in which T. pratense was present in addition to D. glomerata and F. braunii. It was found that the share of D. glomerata in subsequent years of utilization increased when foliar silicon fertilization was applied, especially in the form of Optysil fertilizer (by 8 to 12% in relation to the control). This was similar to the M2 mixture when silicon was used, especially in the form of multicomponent Herbagreen fertilizer. In turn, in the grass mixture (M3), a greater share of F. braunii in the sward was observed, especially when Herbagreen was used, while the remaining grass species did not show a clear reaction to silicon fertilization.
3.2. Nutritional Value
The nutritional value of individual species of grasses and legumes changed over the years of the research, and also depended on the silicon application (Table 2). The greatest content of crude protein (CP), irrespective of the species and variant of silicon fertilization, was found in plants in the second year of utilization. The crude fibre (CF) content as well as its neutral detergent fibre (NDF) and acid detergent fibre (ADF) fractions were also varied. In 2016, when rainfall occurred in all the decades before the mixtures were harvested (May), the plants had the lowest content of these components (and the highest of CP). As a result, the digestibility of organic matter (OMD) and dry matter (DMD) were the highest during the study period. The highest amounts of nitrogen-free extract (NFE), as well as water soluble carbohydrates (WSC) in plants were found in the years with less rain falling in May, especially in the first year of utilization (approx. 500 g/kg and 137 g/kg of DM, respectively). Similar regularities were with regard to the NFC, crude fat (CFa), CA, ADL and NFC content.

Table 2.
The influence of silicon application on nutrients content (g/kg DM) and digestibility (%) of mixtures.
The research showed that silicon application influenced the content of CP, CF, NDF ADF and the digestibility of dry and organic matter. The content of CP in plants increased significantly, whereas the content of CF, NDF and ADF decreased, especially when multi-component Herbagreen was applied. At that time, the digestibility of plants was improved. The research also showed that plant species which were the components of the mixtures clearly differed in their chemical composition. D. glomerata was characterized by the highest content of OM (916.1 g/kg DM), and also had significantly higher CF (including NDF and ADF, which were highly correlated) than the other species. The CP content in plants of this species was slightly lower compared to L. perenne, but clearly lower in relation to F. braunii (on average by about 9 g/kg DM). As a result, the digestibility (OMD and DMD) of D. glomerata plants was the worst compared to other species of grasses and legumes. F. braunii plants showed not only significantly higher CP, but also crude ash (CA) content (the relationship between these parameters was significant, r = 0.67). In turn, L. perenne, with the highest content of NFE, WSC and non-fibre carbohydrates (NFC), was characterized by the highest digestibility of plants among the assessed grass species. The correlations between WSC and NFE and WSC and NFC were positive and highly significant (r = 0.90 and r = 0.64, respectively).
Among legume plants, M.x varia compared to T. pratense was distinguished by a significantly higher content of CP and CA (on average by 24% and 27%, respectively), but at the same time it contained more CF (including NDF and ADF) and ADL. As a result, the digestibility of M.x varia plants was worse than T. pratense. Moreover, M.x varia was characterized by the lowest content of organic matter (OM) compared to the other assessed species.
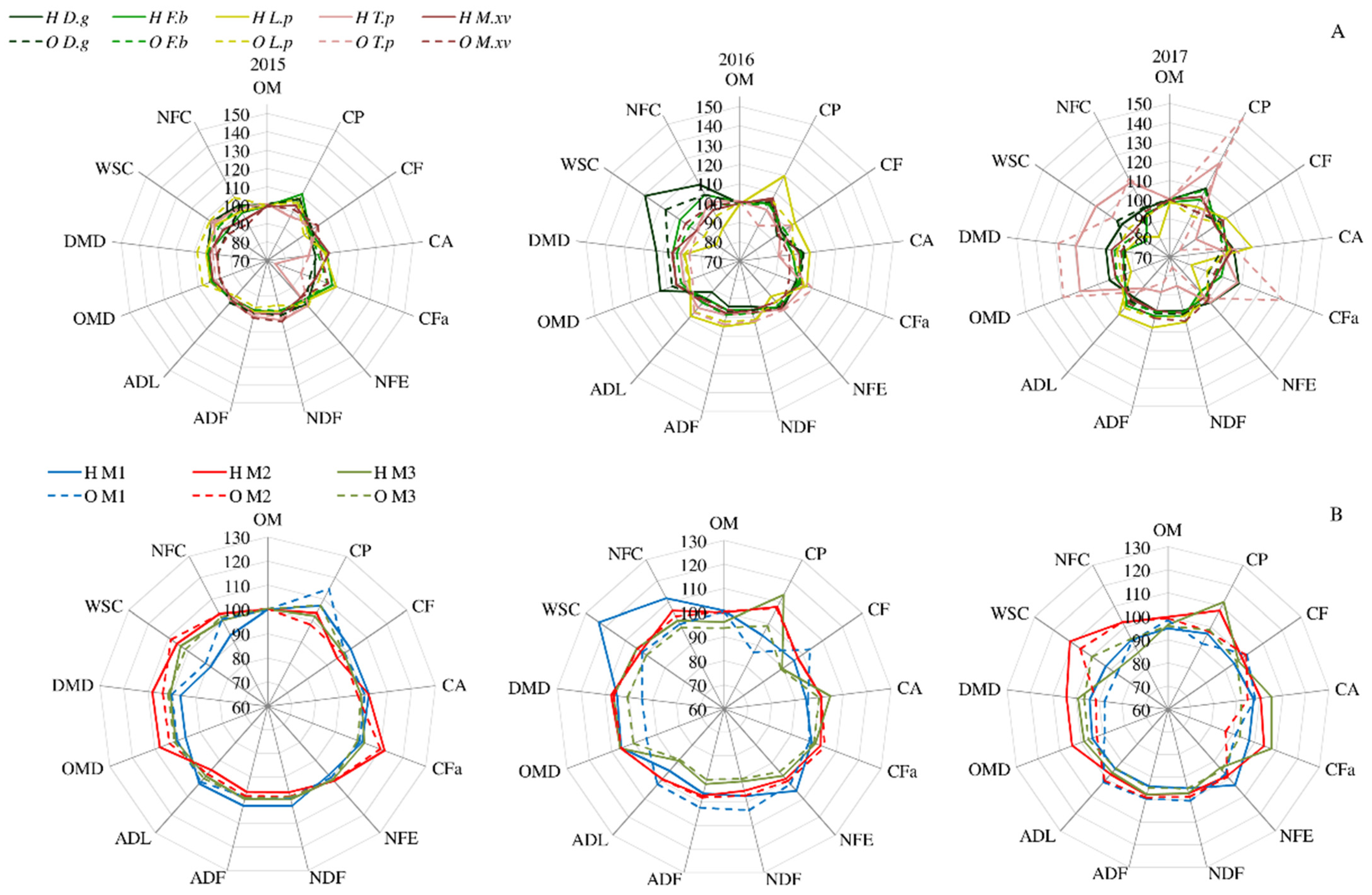
Statistical analyses showed significant interactions between the studied factors (year, silicon fertilization, species and mixtures) according to all evaluated parameters. The interactions between the applied silicon fertilization and the content of nutrients in individual species changed over the years (Figure 2A). In 2015, a higher CP content was found in silicon-fertilized grasses, especially in F. braunii when Herbagreen was used. L. perenne showed a higher content of CFa under Herbagreen application, while WSC and NFC values were higher under Optysil fertilization, and plants were characterized by the best digestibility. In turn, in legume plants, the highest amount of CA was found in M.x varia when Herbagreen was applied, and the lowest CFa in T. pratense, regardless of the silicon fertilizer. In the following year (2016), there was a clear effect of silicon fertilization, especially of Herbagreen, on L. perenne plants, which contained more CP, CA and CFa, but also more CF (and NDF, ADF, ADL), and as a result showed less digestibility. Strong negative correlations (r = −0.97) were demonstrated between CF and OMD, and also between CF and DMD (independent on silicon application). Simultaneously, D. glomerata under silicon fertilization showed the highest content of WSC and NFC, and as a result, the best digestibility among the assessed species. In the third year of use (2017), T. pratense contained significantly more CP and CFa, as well as NFC and WSC, and was characterized by the highest digestibility of plants under both silicon fertilizers. The beneficial effect of Herbagreen application on CFa content, as well as on the digestibility of plants of D. glomerata, was also noticeable. The nutritional value of the mixtures depended on the species composition (Table 2). Grass–legume mixtures (M1 and M2) were characterized by better chemical content compared to the mixture consisting only of grass species (M3). The plants of the M3 mixture contained significantly less CP (especially in comparison with the M2 mixture with M.x varia) and as well as CA. Simultaneously, the content of CF (also NDF, ADF) and as well as ADL was significantly lower, but WSC and NFC were higher than in mixtures M1 and M2. As a result, the digestibility of plants of the M3 mixture was better than that of mixtures with legumes. Moreover, significant interactions between assessed factors in addition to chemical parameters were found.

Figure 2.
Changes of nutritional parameters content (A) in species (D. glomerata, F. braunii, L. perenne, T. pratense, M.x varia) and (B) in mixtures (M1—D. glomerata, F. braunii, T. pratense; M2—D. glomerata, F. braunii, M.x varia; M3—D. glomerata, F. braunii, L. perenne) depending on silicon fertilization (H—Herbagreen, O—Optysil) and year of utilization. Nutrients: OM—organic matter, CP—crude protein, CF—crude fibre, CA—crude ash, CFa—crude fat, NFE—nitrogen-free extract, NDF—neutral detergent fibre, ADF—acid detergent fibre, ADL—acid detergent lignin, WSC—water soluble carbohydrates, NFC—non-fibre carbohydrates, OMD—organic matter digestibility, DMD—dry matter digestibility. Values of each parameter were treated in scale—the mean value of control is 100%.
In the first year, silicon fertilization had a positive effect on the value of plants in grass–legume mixtures (Figure 2B). In the M1 mixture, the CP content increased significantly, especially when Optysil was applied, as did the content of NDF, ADF and ADL. Meanwhile, in the M2 mixture, silicon fertilization had a positive effect on the content of CFa, WSC and NFC, which improved the digestibility of plants.
In the next year (2016), the beneficial effect of silicon fertilization on the chemical composition of plants in the M3 mixture consisting only of grasses was noticeable. It was found that, under Herbagreen application, the plants contained more CP and CA and less NFE, NDF, ADF and ADL compared to the grass–legume mixtures. The effect of silicon fertilization was also marked by a significant increase in WSC and NFC in plants of the M1 mixture. In the last year (2017) of the research, the beneficial effect of silicon fertilization on the CP and CA content in plants of the M3 mixture was also found, especially when Herbagreen was used. According to the M2 mixture, the application of silicon resulted in a higher content of WSC and NFC, and as a result a better digestibility of plants.
4. Discussion
In the sward of all mixtures, clear changes in the botanical composition were found during the study period. This was influenced by the competitiveness of individual plants and weather conditions. Regardless of the mixture, there was an increase in the share of D. glomerata in the subsequent years of the study (on average from 20% to 79%) (Figure 1). According to previous studies [24,25] this species is characterized by a very good development after sowing and significant competitiveness in relation to other components of mixtures in the following years of utilization. In our research, we found a particularly high proportion of D. glomerata in the mixture with T. pratense (86% in the third year of the study). In turn, the share of F. braunii in the sward of the assessed mixtures was the highest in the first year and decreased in the following years. Therefore, in the M2 mixture, only in the first year of the research could it clearly limit the share of T. pratense due to its dominance in the sward (65%). It is confirmed in the data of the literature [60] that F. braunii shows highly competitive ability in relation to T. pratense. The drought in April 2016 (total precipitation, 19.5 mm) may have contributed to the decrease in the share of F. braunii in the sward. According to the research results of Staniak [60,61], long-term drought in the year of full utilization reduced the share of this species in the yield, which indicates the sensitivity of this hybrid to drought. The share of T. pratense also decreased significantly in the next two years of research, which proves the low persistence of this species. Abberton and Marshall [62] concluded that the low durability of T. pratense is the main disadvantage of this species because most plants fall out of the sward in the third or fourth year after sowing. Regardless of these results, according to Hejduk and Kno [16], this species will remain the main legume plant in most temperate regions of the world due to its outstanding forage quality, rapid development after sowing and high competitiveness.
Staniak et al. [63] also confirm the possibility of cultivating F. braunii with Medicago. In studies of such mixtures, a greater potential of yielding under drought conditions was found in comparison to pure legume sowing. In our study, we found a significant increase in the share of Mx varia in the sward in 2016, when little rainfall occurred in spring (April). According to Staniak [60,61], this species dominates in such conditions in the sward, as it is more resistant to drought due to a better developed and deeper root system compared to grasses. Moreover, in the opinion of Jelinowska and Staniak [64], the formation of a deep and extensive root system by Medicago in the initial period of growth causes its slower growth of the above-ground part, which increases the competition of grasses. As Sulivan and Hatfield [65] report, T. pratense is the most important crop on moist, less fertile and acidic soils, while Medicago predominates in drier regions on deep soils with neutral reaction.
In the sward of a mixture consisting only of grass species (M3), a significant increase in the share of D. glomerata was observed in the following years of utilization. In the third year of the research, it was almost three times higher than in the first year. On the other hand, the share of F. braunii was twice as small and L. perenne was seen only in a very small amount. This is confirmed in the literature [22,25].
Regardless of the biological properties of individual species (Table 2), the literature data show that the productivity and botanical composition, especially of temporary grassland, are influenced markedly by nitrogen and water availability [2]. In our research, we found that insufficient rainfall and its unfavourable distribution over the plant growing period, until the first cut, caused changes in the botanical composition of the tested mixtures. According to Staniak [60,61], the share of individual components in the yield is largely modified by weather conditions. Grass–legume mixtures are less sensitive to unfavourable environmental conditions in comparison to pure sowing, due to the limitation of interspecific competition, different habitat requirements of individual components and development rhythms, as well as morphological differences in the root systems [64]. Serajchi et al. [66] reported that an ideal forage mixture composed of grasses and legumes has the potential to adopt to a greater range of environmental conditions and may provide a more reliable forage yield with nutritional quality under different environmental conditions. Moreover, in forage mixtures, legumes provide nitrogen to accompanying grass through atmospheric nitrogen fixation, which reduces fertilizer requirements [67].
The research of Radkowski et al. [57] showed the effect of foliar silicon fertilization on the botanical composition of the sward of a permanent meadow. In our research, we did not find a significant influence of silicon fertilization on the botanical composition of the vegetation of the tested mixtures. This could have been of some importance for species sensitive to water scarcity, such as F. braunii or T. pratense. According to Sacała [39] silicon improves water management in plants. This beneficial effects of silicon fertilization might be a result of better and more effective osmoregulation, improved water balance, reduced water loss by transpiration, assurance of a proper supply of necessary nutrients, reduced intake of toxic ions and improved performance of antioxidant mechanisms. Kaya et al. [46] and Epstein [68] also report that silicon plays a large number of diverse roles in plants, especially when the plants are under stressful conditions. At the same time, Kaya et al. [46] emphasizes that the beneficial function of silicon is often minimal or even is not revealed under mild or optimal water conditions.
Our research showed that the chemical composition of plants was varied, depending on the year, species and mixture, as well as on silicon application. Peoples [69] points out that the nutritional value of a plant depends on many factors, including, among others, the type of soil on which it is grown, the amount of precipitation and fertilizer doses, as well as its development stage during the harvest. In our study, the plant digestibility was significantly worse in the last year of the study (2017), which was connected with the highest CF, NDF and ADF content, and also significantly lower WSC content as compared to other years (Table 2). In this year, precipitation was the highest, but before plant harvesting, there was insufficient rain, which could have adversely affected the quality of the plants (Table 1). The CF content is influenced by many factors, including plant development stage, leaf–stem ratio, environmental conditions and nutrient availability [70]. Under drought conditions, most authors recorded a decrease in the content of CF and its fractions NDF and ADF in grasses [71,72], as well as in legumes [73].
Our study showed that T. pratense plants contained a high amount of CP (although it was significantly less than Mx varia) and were characterized by a significantly lower content of CF, as well as NDF and ADF, than other species, which, with a significantly higher content of ADL, determined their best digestibility. According to Black et al. [74], T. pratense provides forage high in protein and digestibility, which facilitates high intake by animals. In turn, Mx varia had a significantly higher contents of CP and CA, while it had a lower WSC content compared to other plant species. Similar regularities were found by Staniak and Harasim [75] in the studies on M.x varia and F. braunii. The chemical composition and nutritive value of many forage species summarized by INRA [76] showed that T. pratense and Medicago have typically high concentrations of CP; however, they contain relatively low concentrations of WSC, compared to Lolium perenne. According to Bruinenberg et al. [77], legumes contain less NDF than grasses, because as plants mature, the proportion of stems increases more slowly than in grasses. The WSC content in temperate swards is variable and normally low. Through plant breeding and gene manipulation, an increase of WSC concentration in L. perenne has been obtained, and this contributed to a slight increase in the digestibility of this species [78,79].
Medicago had significantly higher contents of CP and lower WSC than Festulolium [75]. This is confirmed by our research, not only in relation to these species, but also to the chemical composition of the mixture (M2) in 2016, in which M.x varia was dominant in the sward (Figure 1 and Figure 2B). As CP concentrations were considerably higher in legumes than in grass species, CP concentrations of mixtures depended on the contribution of the legume in the sward of mixtures. Staniak [60] also stated that the nutritional value of the feed from the tested mixtures depended on the botanical composition of the sward.
According to the literature data [80], D. glomerata is characterized by a high content of structural carbohydrates. Generative shoots dominate in the yield structure of this species, and leaf blades constitute a small share [25,81]. This explains the high content of the NDF fraction in the dry matter of this grass, as well as in the M1 and M2 mixtures in the third year of use, when this species had a greater share than in the mixture of grasses (M3). Fariaszewska et al. [72] observed a tendency for lower NDF and ADF content in L. perenne and F. braunii under mild drought stress. Drought also caused an increase in CP and WSC content, as well as slightly increasing the digestibility of all cultivars. Additionally, according to Kuchenmeister et al. [73], WSC content in grasses and legumes depends on species, cultivation method (monoculture, mixture) and water availability. Under severe stress conditions, the authors showed a tendency of increasing WSC content with a simultaneous decrease in CF. The content of CF in the feed is primarily influenced by the species composition of the meadow sward and the development phase of its components (harvest time) [82]. Moreover, it has been shown that high air temperature and rainfall deficit generally increase the abundance of structural carbohydrates in plants [83]. From the point of view of the usefulness of meadow and pasture swards in the feeding of ruminants, it is important to determine the content of individual fractions of CF (NDF, ADF, ADL) [12], which are more often balanced in the ration.
Radkowski et al. [57] showed that silage produced from the sward fertilized with silicon was characterized by a higher content of total protein and a higher nutritional value of protein and energy value compared to silage produced from the sward not fertilized with this element. According to the authors, a trend towards a higher protein content might be caused by the higher share of legumes in the botanical composition of the meadow and the increased protein content in these plants. Our three-year research confirmed the beneficial effect of silicon fertilization, independent of weather conditions, on the CP content and on the reduction of CF, NDF and ADF in plants, especially when Herbagreen was applied. As a result, plants were characterized by better digestibility (higher OMD and DMD values). The response of individual plant species to the applied silicon fertilization varied over the years. It was the same with regard to mixtures, but it depended on the species composition of the sward. Under the conditions of applying silicon in 2015 (low rainfall), a large share of F. braunii in the sward and contribution of T. pratense in the sward of the M1 mixture (approx. 20%) resulted in a higher content of CP. A clear influence of silicon on the value of T. pratense was found in 2017 (heavy rainfall), but the small share of this species in the sward did not affect the chemical composition of the mixture. As reported by Kurdali et al. [84], a reduced ability to fix atmospheric nitrogen due to water shortage negatively affects the nitrogen balance in the plant. The use of silicon in the fertilization of the well-known Cicer arietinum improved the fixation or atmospheric nitrogen in plants exposed to water deficit compared to plants grown under optimal conditions.
5. Conclusions
Changes in the botanical composition in the sward of all mixtures during the study period were influenced by the competitiveness of individual plants and weather conditions. In the tested mixtures on temporary grasslands, D. glomerata was very competitive in relation to other species and maintained the best over the three years of use, whereas L. perenne and T. pratense showed little competitiveness and a small share in the sward. F. braunii had a high share in the sward of all mixtures in the first year of use, and in the grass mixture in the second year. Our findings showed a slight effect of silicon application on changes in the botanical composition of the tested mixtures.
The chemical composition of plants was varied, depending on the year and species, as well as silicon application. We noted the beneficial effect of silicon fertilization, especially of multicomponent Herbagreen, on the CP content and on the reduction of CF, NDF and ADF in plants. The nutritional value of mixtures under silicon fertilization varied over the years and depended on the botanical composition. In low rainfall conditions, a large share of F. braunii and an approximately 20% contribution of T. pratense resulted in a higher content of CP. These results may be used in the fertilization practices of temporary grassland for enhancing nutritional value, especially crude protein content, and the reduction of fibre in the sward. There is a need to continue experiments that allow us to better understand the interactions between silicon application and plant response, especially in grass–legume mixture swards.
Author Contributions
Conceptualization, B.B.-J., G.M. and M.J.; methodology, B.B.-J., G.M. and M.J.; software, G.M.; formal analysis. B.B.-J. and G.M.; investigation, B.B.-J., G.M., M.J. and B.W.; writing—original draft preparation, B.B.-J., G.M. and M.J.; writing—review and editing, B.B.-J. and G.M.; visualization, B.B.-J. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mălinas, A.; Rotar, I.; Vidican, R.; Iuga, V.; Păcurar, F.; Mălinas, C.; Moldovan, C. Designing a sustainable temporary grassland system by monitoring nitrogen use efficiency. Agronomy 2020, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Dalton, S.J.; Bettany, A.J.E.; Timms, E.; Morris, P. Co-transformed, diploid Lolium perenne (perennial ryegrass), Lolium multiflorum (Italian ryegrass) and Lolium temulentum (darnel) plants produced by microprojectile bombardment. Plant Cell Rep. 1999, 18, 721–726. [Google Scholar] [CrossRef]
- Søegaard, K.; Gierus, M.; Hopkins, A.; Halling, M. Temporary grassland-challenges in the future. In Permanent and Temporary Grassland: Plant, Environment and Economy, Proceedings of the 14th Symposium of the European Grassland Federation, Ghent, Belgium, 3–5 September 2007; Belgian Society for Grassland and Forage Crops: Genth, Belgium, 2007; pp. 27–38. [Google Scholar]
- Kipling, P.; Virkajärvi, R.P.; Breitsameter, L.; Curnel, Y.; De Swaef, T.; Gustavsson, A.M.; Hennart, S.; Höglind, M.; Järvenranta, K.; Minet, J.; et al. Key challenges and priorities for modelling European grasslands under climate change. Sci. Total Environ. 2016, 566, 851–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land Dev. 2021, 51, 243–249. [Google Scholar] [CrossRef]
- Bélanger, G.; Castonguay, Y.; Lajeunesse, J. Benefits of mixing timothy with alfalfa for forage yield, nutritive value, and weed suppression in northern environments. Can. J. Plant Sci. 2014, 94, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Vasileva, V.; Naydenova, Y. Nutritive value of forage biomass from mixtures of alfalfa with cocksfoot and tall fescue. J. Glob. Environ. Agric. Soc. Sci. 2017, 5, 121–129. [Google Scholar]
- Gaweł, E. The role of fine-grained legume plants in a farm. Woda-Sr.-Obsz. Wiej. 2011, 11, 73–91. [Google Scholar]
- Olszewska, M.; Grzegorczyk, S.; Bałuch-Małecka, A. The effect of different proportions of Medicago media Pers. in mixtures with Festulolium braunii (K. Richt.) A. Camus on the yield and feed value of green fodder. Agric. Food Sci. 2019, 28, 18–26. [Google Scholar] [CrossRef]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadóttir, Á.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Bijelić, Z.; Tomić, Z.; Ružić-Muslić, D.; Krnjaja, V.; Mandić, V.; Vučković, S.; Nikšić, D. Forage quality and energy content of perennial legume-grass mixtures at three level of N fertilization. Biotechnol. Anim. Husb. 2014, 30, 539–547. [Google Scholar] [CrossRef]
- Bélanger, G.; Tremblay, G.F.; Papadopoulos, Y.A.; Duynisveld, J.; Lajeunesse, J.; Lafrenière, C.; Fillmore, S.A.E. Yield and nutritive value of binary legume-grass mixtures under grazing or frequent cutting. Can. J. Plant Sci. 2018, 98, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, A.; Blevins, R.L.; Frye, W.W.; Grove, J.; Cornelius, P. Sustaining soil quality with legumes in no-tillage systems. Commun. Soil Sci. Plant Anal. 2008, 39, 1680–1699. [Google Scholar] [CrossRef]
- Rutkowska, A.; Pikuła, D. Effect of crop rotation and nitrogen fertilization on the quality and quantity of soil organic matter. In Soil Processes and Current Trends in Quality Assessment; Soriano, M.C.H., Ed.; Intech Open Book: Rijeka, Croatia, 2013; pp. 249–267. [Google Scholar]
- Hajduk, E.; Właśniewski, S.; Szpunar-Krok, E. Influence of legume crops on content of organic carbon in sandy soil. Soil Sci. Ann. 2015, 66, 52–56. [Google Scholar] [CrossRef]
- Hejduk, S.; Kno, P. Effect of provenance and ploidity of red clover varieties on productivity, persistence and growth pattern in mixture with grasses. Plant Soil Environ. 2010, 56, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Vasileva, V.; Kostov, O.; Vasilev, E.; Athar, M. Effect of mineral nitrogen fertilization on growth characteristics of lucerne under induced water deficiency stress. Pak. J. Bot. 2011, 43, 2925–2928. [Google Scholar]
- Shen, Y.; Jiang, H.; Zhai, G.; Cai, Q. Effects of cutting height on shoot regrowth and forage yield of alfalfa (Medicago sativa L.) in a short-term cultivation system. Grassl. Sci. 2013, 59, 73–79. [Google Scholar] [CrossRef]
- Sheldrick, R.D. Sward establishment and renovation. In Grass Its Production and Utilisation; Hopkins, A., Ed.; Blackwell Science: Oxford, UK, 2000; pp. 13–30. [Google Scholar]
- Janicka, M.; Borawska-Jarmułowicz, B.; Mastalerczuk, G. Development and growth of grass cultivars in pure stands and in meadow mixtures. Grassl. Sci. Eur. 2012, 17, 130–132. [Google Scholar]
- Heshmati, S.; Tonn, B.; Isselstein, J. White clover population effects on the productivity and yield stability of mixtures with perennial ryegrass and chicory. Field Crops Res. 2020, 252, 107802. [Google Scholar] [CrossRef]
- Borawska-Jarmułowicz, B. The influence of 12-year utilisation on stability of species and cultivars of grasses in meadow mixtures with different earliness. Ann. UMCS Sec. E Agric. 2004, 59, 1397–1406. [Google Scholar]
- Østrem, L.; Volden, B.; Larsen, A. Morphology, dry matter yield and phenological characters at different maturity stages of Festulolium compared with other grass species. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 63, 531–542. [Google Scholar]
- Borawska-Jarmułowicz, B. The response of Dactylis glomerata used in meadow mixture on the curse of weather conditions in the long term. Grassl. Sci. Pol. 2005, 8, 27–33. [Google Scholar]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Janicka, M. Evaluation of biological characteristics and yield of selected varieties of Dactylis glomerata, Festuca pratensis and Phleum pratense in pure stands and mixtures. Grassl. Sci. Pol. 2016, 19, 35–50. [Google Scholar]
- Przybysz, A.; Wrochna, M.; Słowiński, A.; Gawrońska, H. Stimulatory effect of Asahi SL on selected plant species. Acta Sci. Pol. Hortorum Cultus 2010, 9, 53–64. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 72, 1212–1226. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Guo, Z.G.; Liu, H.X.; Tian, F.P.; Zhang, Z.H.; Wang, S.M. Effect of silicon on the morphology of shoots and roots of alfalfa (Medicago sativa). Aust. J. Exp. Agric. 2006, 46, 1161–1166. [Google Scholar] [CrossRef]
- Yan, G.C.; Nikolic, M.; Ye, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Sun, W.C.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Kalaji, H.M.; Dąbrowski, P.; Paderewski, J. Gas-exchange parameters and morphological features of festulolium (Festulolium braunii K. Richert A. Camus) in response to nitrogen dosage. Photosynthetica 2017, 55, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Sacała, E. Role of silicon in plant resistance to water stress. J. Elementol. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Sakr, N. Silicon control of bacterial and viral diseases in plants. J. Plant Prot. Res. 2016, 56, 4. [Google Scholar] [CrossRef]
- Mir, S.H.; Rashid, I.; Hussain, B.; Reshi, Z.A.; Assad, R.; Sofi, I.A. Silicon supplementation of rescue grass reduces herbivory by a grasshopper. Front. Plant Sci. 2019, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Cuong, T.X.; Ullah, H.; Datta, A.; Hanh, T.C. Effects of silicon-based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Xuebin, Q.; Zhao, Z.; Du, Z.; Imtiaz, M.; Mehmood, F.; Hongfei, L.; Hussain, B.; Ashraf, M.N. Alleviatory effects of silicon on the morphology, physiology, and antioxidative mechanisms of wheat (Triticum aestivum L.) roots under cadmium stress in acidic nutrient solutions. Sci. Rep. 2021, 11, 1958. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Higgs, D.; Murillo-Amador, B.; Aydemir, S.; Girgin, A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2008, 62, 10–16. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chiatanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Hameed, M. Improving drought tolerance potential in wheat (Triticum aestivum L.) through exogenous silicon supply. Pak. J. Bot. 2015, 47, 1641–1648. [Google Scholar]
- Sierra, M.J.; Schmid, T.; Guirado, M.; Escolano, O.; Millán, R. How management practices affect silicon uptake by Hordeum vulgare grown in a highly calcareous soil. Soil Use Manag. 2021, 37, 1–13. [Google Scholar] [CrossRef]
- Shridevi; Hebsur, N.S. Effect of silicon fertilization on growth and yield of soybean (Glycine max (L.) Merrill) in Vertisol. J. Farm Sci. 2019, 33, 1572–1575. [Google Scholar]
- Liang, Y.; Nikolic, M.; Belanger, R.; Gong, H.; Song, A. Silicon in Agriculture. From Theory to Practice; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2015. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Ajay; Kundu, S.; Subba Rao, A. A Case for Silicon Fertilization to Improve Crop Yieldsin Tropical Soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Sienkiewicz-Cholewa, U.; Zajączkowska, A. The role and yield-forming effect of silicon application based on the example of global research. Prog. Plant Prot. 2020, 60, 313–319. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kucińska, K. The effect of silicon foliar fertilization in sugar beet—Beta vulgaris (L.) ssp. vulgaris conv. crassa (Alef.) prov. altissima (Döll). Turk. J. Field Crops 2015, 20, 115–119. [Google Scholar] [CrossRef]
- Artyszak, A.; Kondracka, M.; Gozdowski, D.; Siuda, A.; Litwińczuk-Bis, M. Impact of foliar application of various forms of silicon on the chemical composition of sugar beet plants. Sugar Tech. 2021, 23, 546–559. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Jakubowska, M.; Krzymińska, J. Effect of different forms of silicon on growth of spring wheat cultivated in organic farming system. Silicon 2021, 13, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Radkowski, A.; Sosin-Bzducha, E.; Radkowska, I. Effects of silicon foliar fertilization of meadow plants on nutritional value of silage fed to dairy cows. J. Elem. 2017, 22, 1311–1322. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Dąbrowski, P.; Szara, E.; Perzanowska, A.; Wróbel, B. Can the application the silicon improve the productivity and nutritional value of grass–clover sward in conditions of rainfall shortage in organic management? Agronomy 2020, 10, 1007. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources (WRBSR) 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No 106; FAO: Rome, Italy, 2015.
- Staniak, M. Yielding and fodder value of mixtures of Festulolium braunii (Richt.) A. Camus with di- and tetraploid varieties of red clover. Fragm. Agron. 2009, 26, 105–115. [Google Scholar]
- Staniak, M. Yields and fodder value of Festulolium braunii variety Felopa depending on the first time harvest. I. Yields and selected yield components. Pamiet. Pulawski 2004, 137, 117–131. [Google Scholar]
- Abberton, M.T.; Marshall, A.H. Progress in breeding perennial clovers for temperate agriculture. Centenary review. J. Agric. Sci. 2005, 143, 117–135. [Google Scholar] [CrossRef]
- Staniak, M.; Bojarszczuk, J.; Księżak, J. Changes in yield and gas exchange parameters in Festulolium and alfalfa grown in pure sowing and in mixture under drought stress. Acta Agric. Scand. Sect. B. Soil Plant Sci. 2018, 68, 255–263. [Google Scholar] [CrossRef]
- Jelinowska, A.; Staniak, M. The interaction of plants in single-species and mixed sowing on the example of mixtures of alfalfa with grasses. Post. Nauk Rol. 2007, 5, 37–49. [Google Scholar]
- Sullivan, M.L.; Hatfield, R.D. Polyphenol oxidase and o-diphenols inhibit postharvest proteolysis in red clover and alfalfa. Crop Sci. 2006, 46, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Serajchi, M.; Schellenberg, M.P.; Mischkolz, J.M.; Lamb, E.G. Mixtures of native perennial forage species produce higher yields than monocultures in a long-term study. Can. J. Plant Sci. 2017, 98, 633–647. [Google Scholar] [CrossRef]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Peoples, M.B. Legumes root nitrogen in cropping system nitrogen cycling. Graine Legume 2001, 33, 8–9. [Google Scholar]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Staniak, M.; Kocoń, A. Forage grasses under drought stress in conditions of Poland. Acta Physiol. Plant 2015, 37, 116. [Google Scholar] [CrossRef] [Green Version]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; Baert, J.; De Riek, J.; Staniak, M.; Pecio, Ł. Mild drought stress-induced changes in yield, physiological processes and chemical composition in Festuca, Lolium and Festulolium. J. Agron. Crop Sci. 2017, 203, 103–116. [Google Scholar] [CrossRef]
- Kuchenmeister, K.; Kuchenmeister, F.; Kayser, M.; Wrange-Monning, N.; Isselstein, J. Influence of drought stress on nutritive value of perennial forage legumes. Int. J. Plant Prod. 2013, 7, 1735–8043. [Google Scholar]
- Black, A.D.; Laidlaw, A.S.; Moot, D.J.; Kiely, P.O. Comparative growth and management of white and red clovers. Ir. J. Agric. Food Res. 2009, 48, 149–166. [Google Scholar]
- Staniak, M.; Harasim, E. Changes in nutritive value of alfalfa (Medicago x varia T. Martyn) and Festulolium (Festulolium braunii (K. Richt) A. Camus) under drought stress. J. Agron. Crop Sci. 2018, 204, 456–466. [Google Scholar] [CrossRef]
- INRA. Alimentation des Bovins, Ovins et Caprins: Besoins des Animaux. Valeur des Aliments; Feeding of Cattle, Sheep and Goats. Animal Needs, Feed Value; Editions Quae; Tables INRA: Paris, France, 2007. [Google Scholar]
- Bruinenberg, M.H.; Struik, P.C.; Valk, H. Digestibility and plant characteristic of forages in semi-natural grasslands. Grassl. Sci. Eur. 2001, 6, 154–157. [Google Scholar]
- Miller, L.A.; Moorby, J.M.; Davies, D.R.; Humphreys, M.O.; Scollan, N.D.; MacRae, J.C.; Theodorou, M.K. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne L.): Milk production from late-lactation dairy cows. Grass Forage Sci. 2001, 56, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Harris, L.; Moorby, J.; Humphreys, M.; Theodorou, M.; MacRae, J.; Scollan, N. Rumen metabolism and nitrogen flow to the small intestine in steers offered Lolium perenne containing different levels of water-soluble carbohydrate. Anim. Sci. 2002, 74, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, M.; Kobyliński, A. The relative feed value of mixtures Festulolium braunii (K. Richt.) A. Camus with Medicago media Pers. depending on the varying participation on alfalfa in sowing. Acta Agrophysica 2016, 23, 481–490. [Google Scholar]
- Ciepiela, G.A. Content of structural and ninstructural carbohydrates and lignin in Dactylis glomerata L. and Festulolium braunii (K. Richt.) A. Camus supplied by bistimulator Kelpak SL and nitrogen. Nauka Przyr. Technol. 2014, 8, 2. [Google Scholar]
- Purwin, C.; Stanek, M.; Lipiński, K.; Wierzbowska, J.; Nogalska, A.; Fijałkowska, M. Effect of a harvest time and cultivar on the chemical composition and in vitro ruminal dry matter degradability of perennial ryegrass (Lolium perenne L.). J. Elem. 2016, 21, 811–822. [Google Scholar]
- Thorvaldsson, G.; Tremblay, G.F.; Kunelius, H.T. The effects of growth temperature on digestibility and fibre concentration of seven temperate grass species. Acta Agric. Scand. Sect. B. Soil Plant Sci. 2007, 57, 322–328. [Google Scholar] [CrossRef]
- Kurdali, F.; Al-Chammaa, M.; Mouasess, A. Growth and nitrogen fixation in silicon and/or potassium fed chickpeas grown under drought and well-watered conditions. J. Stress Physiol. Biochem. 2013, 9, 386–406. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).