Use of Symbiotic Fungi to Reduce the Phytotoxic Effect of DCD Nitrification Inhibitors in Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Preparation and Dicyandiamide Treatment
2.3. Plant Growth Conditions
2.4. Seed Inoculation with Fungi
2.5. SEM and Plant Growth Measurements
2.6. Root Colonization
2.7. Total Chlorophyll and Carotenoids Contents
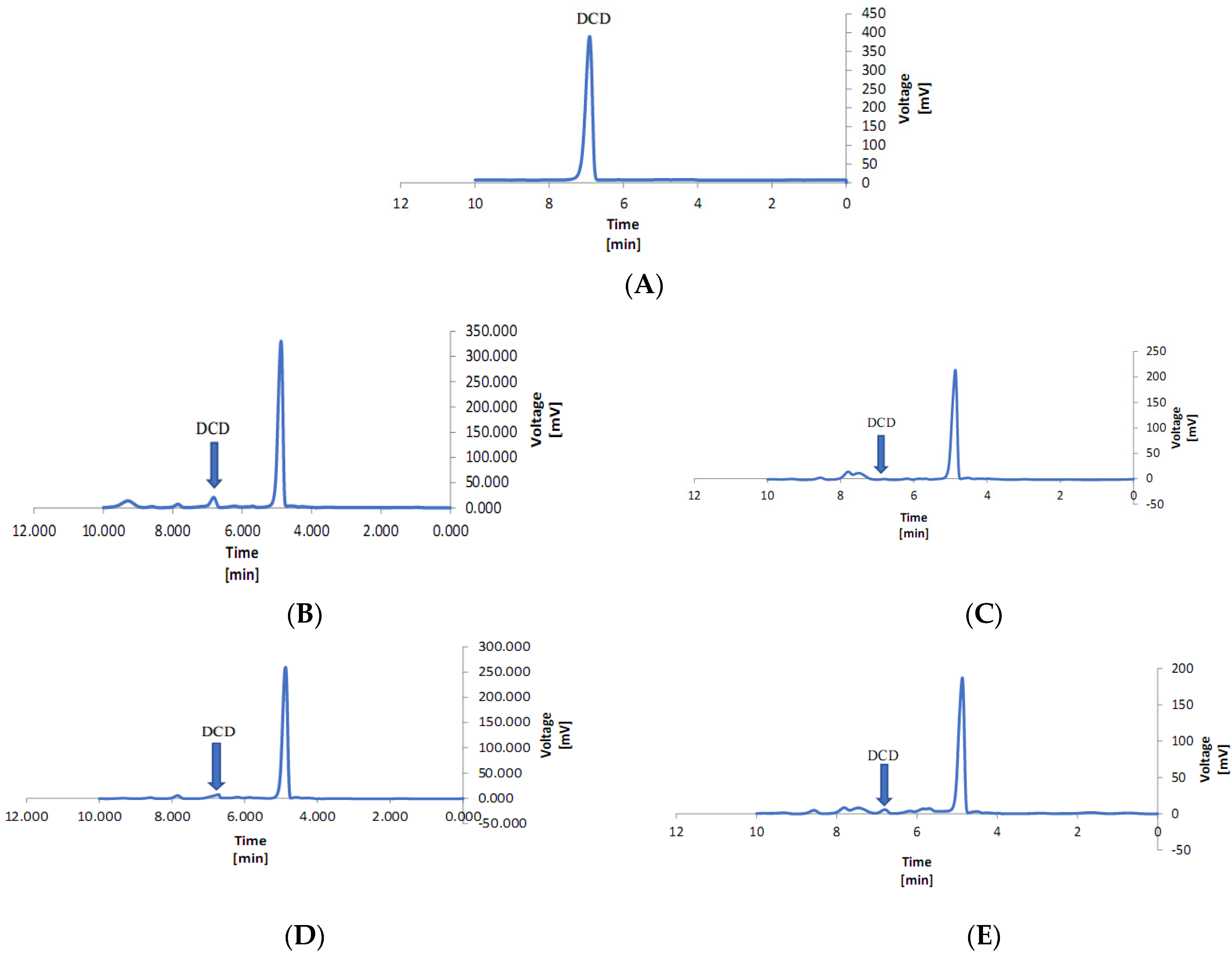
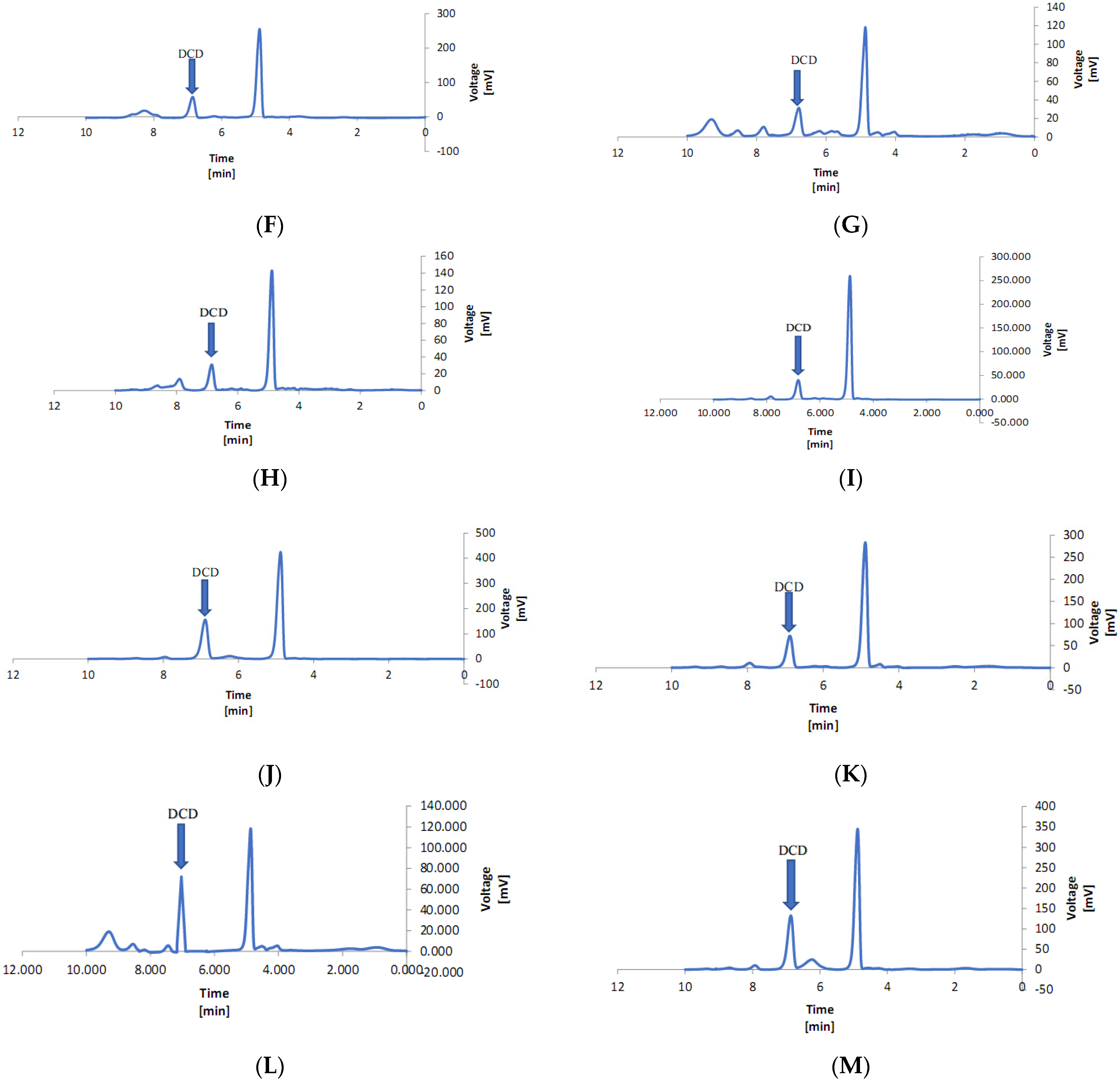
2.8. Measurement of DCD in Lettuce Leaves
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of DCD on Root Colonization by P. indica, G. etonicatum, and G. mosseae
3.2. Root Fresh Weight and Root Length
3.3. The Effect of DCD on Shoot Development
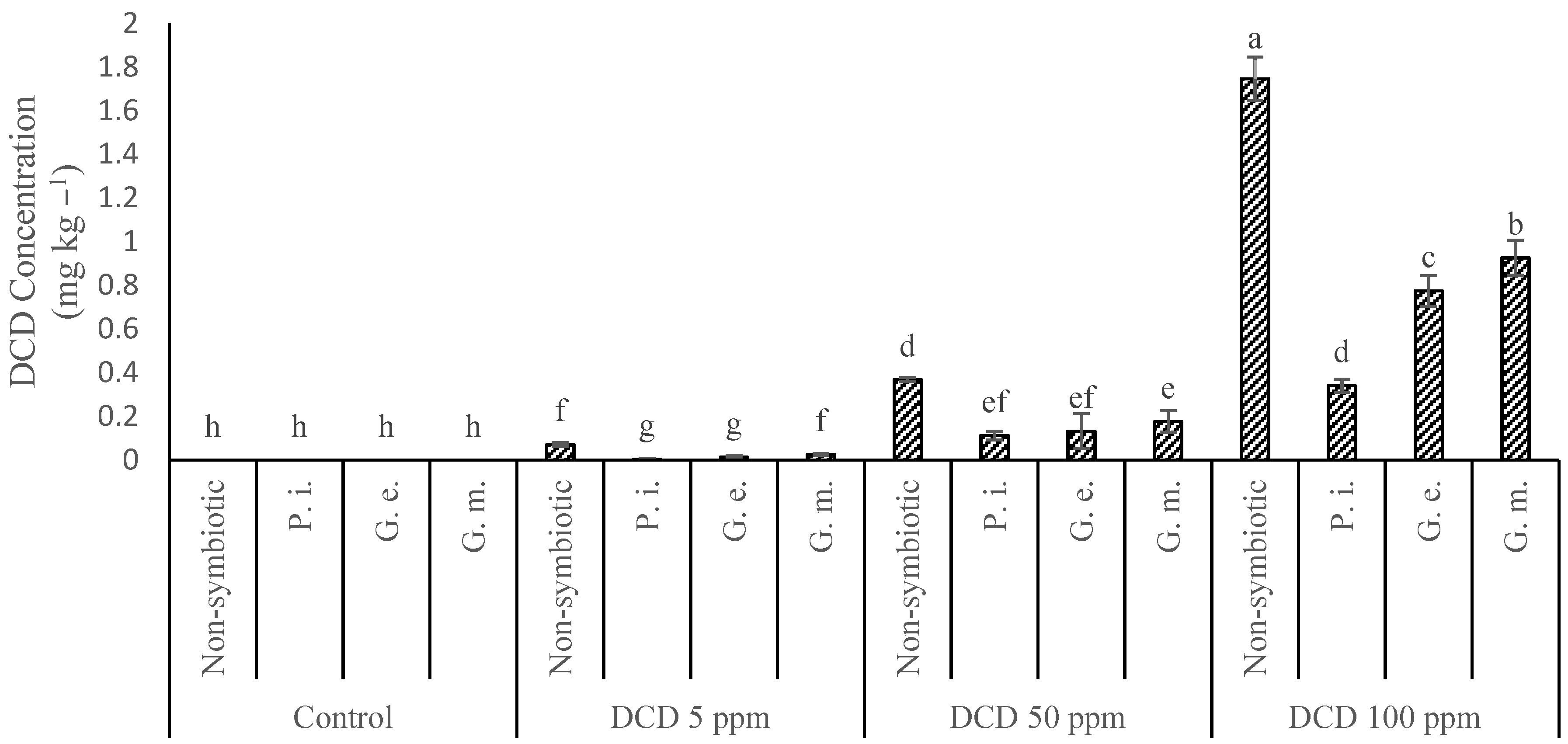
3.4. Dicyandiamide in the Leaves
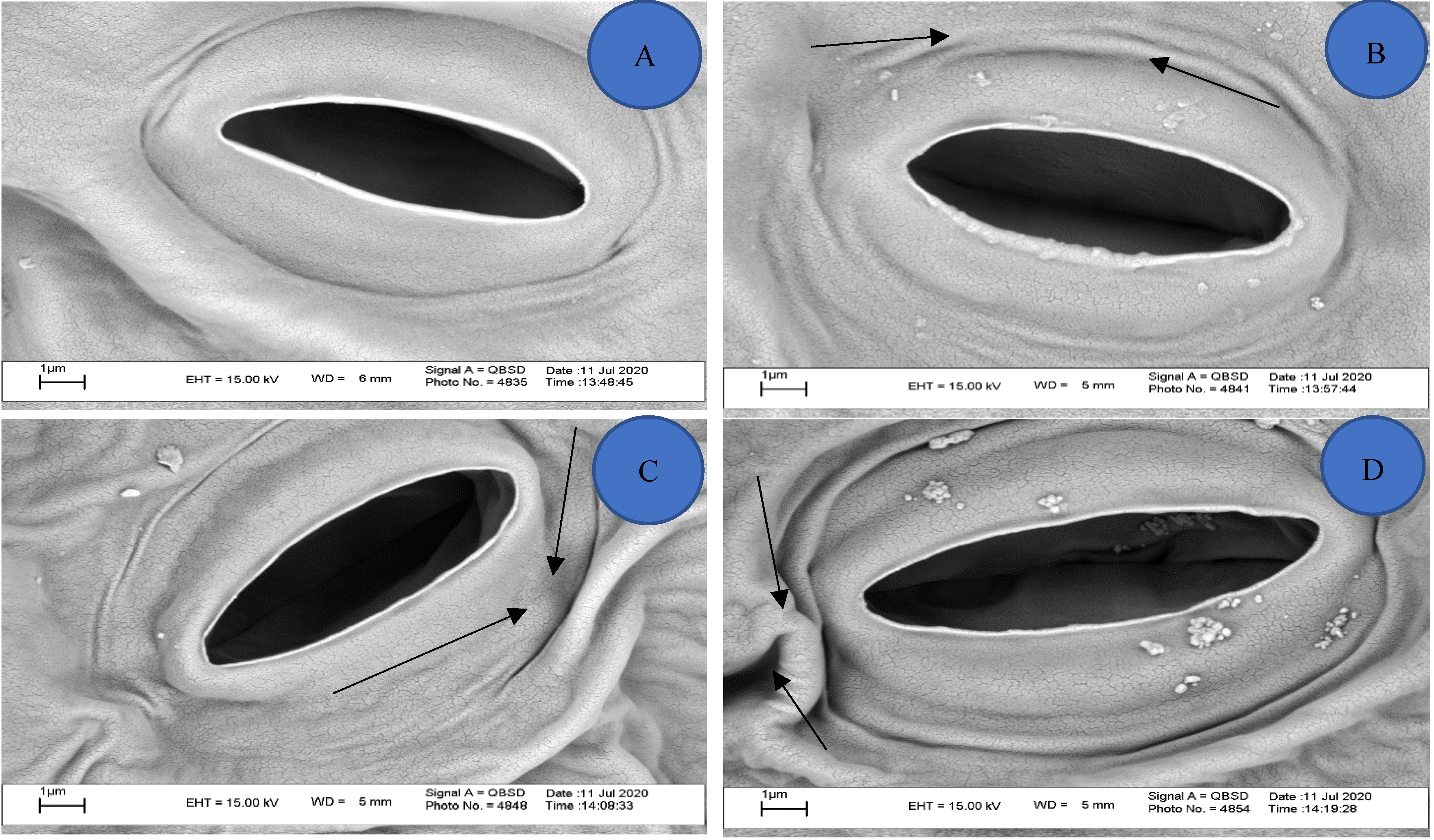
3.5. Effect of Dicyandiamide on Stomatal Behavior
3.6. Stomata Area in the Leaves
3.7. Effect of Dicyandiamide on Appearance of Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bock, E.; Wagner, M. Oxidation of Inorganic Nitrogen Compounds as an Energy Source. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 457–495. [Google Scholar]
- Li, J.H.; Yu, X.Z.; Wu, S.C.; Wang, X.R.; Wang, S.H.; Tam, N.F.Y.; Wong, M.H. Responses of Bioaugmented Ryegrass to Pah Soil Contamination. Int. J. Phytoremed. 2011, 13, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3,4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Science 2016, 6, 22075. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.M.; Lasa, B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A.H. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. An introduction. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Lal, K.; Kumar, L.; Kumar, A.; Kumar, A. Oxazolone–1,2,3-Triazole Hybrids: Design, Synthesis and Antimicrobial Evaluation. Curr. Top. Med. Chem. 2018, 18, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Omozele, A.B. Cr (VI) removal by indigenous Klebsiella species PB6 isolated from contaminated soil under the influence of various factors. Curr. Res. Bacteriol. 2018, 8, 62. [Google Scholar] [CrossRef]
- Varma, A.; Sativa, S.; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant growth-promoting root endophyte. Appl. Environ. Microbiol. 1998, 65, 2741–2744. [Google Scholar] [CrossRef]
- Vadassery, J.; Tripathi, S.; Prasad, R.; Varma, A.; Oelmüller, R. Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J. Plant Physiol. 2009, 166, 1263–1274. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Q.; Ling, W.; Zhu, X. Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2011, 185, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Maftoun, M.; Sheibany, B. Comparative phytotoxicity of several nitrification inhibitors to soybean plants. J. Agric. Food Chem. 1979, 27, 1365–1368. [Google Scholar] [CrossRef]
- Maftoun, M.; Banihashemi, Z. Effects of added sulfur, aluminium sulfate and ferrous sulfate on CO2 evolution, microbial population and nitrification in alfalfa-and straw-amended soil. Agrochimica 1981, 25, 318–326. [Google Scholar]
- Hill, T.W.; Käfer, E. Improved protocols for Aspergillus minimal medium: Trace element and minimal medium salt stock solutions. Fungal Genet. Newsl. 2001, 48, 20–21. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots andstaining parasitic and vesicular-arbuscular mycorrhizal fungi for rapidassessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Arnon, A. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Kim, D.G.; Giltrap, D.; Saggar, S.; Palmada, T.; Berben, P.; Drysdale, D. Fate of the nitrification inhibitor dicyandiamide (DCD) sprayed on a grazed pasture: Effect of rate and time of application. Soil Res. 2012, 50, 337–347. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Coppola, L.; Comitini, F.; Casucci, C.; Milanovic, V.; Monaci, E.; Marinozzi, M.; Taccari, M.; Ciani, M.; Vischetti, C. Fungicides degradation in an organic biomixture: Impact on microbial diversity. New Biotechnol. 2011, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Maienza, A.; Bååth, E.; Stazi, S.R.; Benedetti, A.; Grego, S.; Dell’Abate, M.T. Microbial dynamics after adding bovine manure effluent together with a nitrification inhibitor (3,4 DMPP) in a microcosm experiment. Biol. Fertil. Soils 2014, 50, 869–877. [Google Scholar] [CrossRef]
- Florio, A.; Maienza, A.; Dell’Abate, M.T.; Stazi, S.R.; Benedetti, A. Changes in the activity and abundance of the soil microbial community in response to the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP). J. Soils Sediments 2016, 16, 2687–2697. [Google Scholar] [CrossRef]
- Xiuzhen, S.; Hang-Wei, H.; Zhu-Barker, X.; Hayden, H.; Wang, J.; Suter, H.; Chen, D.; Ji-Zheng, H. Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3,4-dimethylpyrazole phosphate. Environ. Microbiol. 2017, 19, 4851–4865. [Google Scholar]
- Sigler, W.V.; Turco, R.F. The impact of chlorothalonil application on soil bacterial and fungal populations as assessed by denaturing gradient gel electrophoresis. Appl. Soil Ecol. 2002, 21, 107–118. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R. Arbuscular mycorrhizal symbiosis modules antioxidant response in salt stressed Trigonella foenum-graecum plants. Mycorrhiza 2014, 24, 197–208. [Google Scholar] [CrossRef]
- Sharma, P.; Kharkwal, A.C.; Abdin, M.Z.; Varma, A. Piriformospora indica-mediated salinity tolerance in Aloe vera plantlets. Symbiosis 2016, 72, 103–115. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, J.; Zou, P.; Lin, H.; Sun, W.; Yin, J.; Fu, J. Effects of combined application of organic and inorganic fertilizers plus nitrification inhibitor DMPP on nitrogen runoff loss in vegetable soils. Environ. Sci. Pollut. Res. 2015, 22, 472–481. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- García, I.V.; Mendoza, R.E. Relationships among soil properties, plant nutrition and arbuscular mycorrhizal fungi–plant symbioses in a temperate grassland along hydrologic, saline and sodic gradients. FEMS Microbiol. Ecol. 2008, 63, 359–371. [Google Scholar] [CrossRef]
- Ray, P.; Abraham, P.E.; Guo, Y.; Giannone, R.J.; Engle, N.L.; Yang, Z.K.; Craven, K.D. Scavenging organic nitrogen and remodelling lipid metabolism are key survival strategies adopted by the endophytic fungi, Serendipita vermifera and Serendipita bescii to alleviate nitrogen and phosphorous starvation in vitro. Environ. Microbiol. Rep. 2019, 11, 548–557. [Google Scholar] [CrossRef]
- Teutscherova, N.; Vazquez, E.; Arango, J.; Arevalo, A.; Benito, M.; Pulleman, M. Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application. Geoderma 2018, 338, 493–501. [Google Scholar] [CrossRef]
- Marsden, K.A.; Scowen, M.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. Plant acquisition and metabolism of the synthetic nitrification inhibitor dicyandiamide and naturally- occurring guanidine from agricultural soils. Plant Soil 2015, 395, 201–214. [Google Scholar] [CrossRef]
- Pal, P.; McMillan, A.M.S.; Saggar, S. Pathways of dicyandiamide uptake in pasture plants: A laboratory study. Biol. Fertil. Soils 2016, 52, 539–546. [Google Scholar] [CrossRef]
- Vilsmeier, K. Fate of ammonium-N in pot studies as affected by DCD addition. Fert. Res. 1991, 29, 187–189. [Google Scholar] [CrossRef]
- Padash, A.; Shahabivand, S.; Behtash, F.; Aghaee, A. A practicable method for zinc enrichment in lettuce leaves by the endophyte fungus Piriformospora indica under increasing zinc supply. Sci. Hortic. 2016, 213, 367–372. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Elkholy, S.; Fernández, J.A.; Junge, H.; Cheetham, R.D.; Guardiola, J.L.; Weathers, P.J. The effect of Bacillus subtilis FZB24® on flowers quantity and quality of saffron (Crocus sativus L.). Planta Medica 2007, 73, P_607. [Google Scholar] [CrossRef]
- Tanha, S.R.; Ghasemnezhad, A.; Babaeizad, V. A study on the effect of endophyte fungus, Piriformospora indica, on the yield and phytochemical changes of globe artichoke (Cynara scolymus L.) leaves under water stress. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1907–1921. [Google Scholar]
- Rathod, D.P.; Brestic, M.; Shao, H.B. Chlorophyll a fluorescence determines the drought resistance capabilities in two varieties of mycorrhized and non-mycorrhized Glycine max Linn. Afr. J. Microbiol. Res. 2011, 5, 4197–4206. [Google Scholar] [CrossRef]
- Reeves, D.W.; Touchton, J.T. Relative Phytotoxicity of Dicyandiamide and Availability of its Nitrogen to Cotton, Corn, and Grain Sorghum. Soil Sci. Soc. Am. J. 1986, 50, 1353–1357. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Lenoir, I.; Lounes-Hadj Sahraoui, A.; Laruelle, F.; Dalpe, Y.; Fontaine, J. Arbuscular mycorrhizal wheat inoculation promotes alkane and polycyclic aromatic hydrocarbon biodegradation: Microcosm experiment on aged-contaminated soil. Environ. Pollut. 2016, 213, 549–560. [Google Scholar]
- Sainz, M.J.; Gonzalez, P.B.; Vilarino, A. Effects of hexachlorocyclohexane on rhizosphere fungal propagules and root colonization by arbuscular mycorrhizal fungi in Plantago lanceolata. Eur. J. Soil Sci. 2006, 57, 83–90. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The Potassium Transporter SlHAK10 Is Involved in Mycorrhizal Potassium Uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Binet, P.; Portal, J.M.; Leyval, C. Application of GC–MS to the study of anthracene disappearance in the rhizosphere of ryegrass. Org. Geochem. 2001, 32, 217–222. [Google Scholar] [CrossRef]
- Aranda, E.; Scervino, J.M.; Godoy, P.; Reina, R.; Ocampo, J.A.; Wittich, R.M.; García-Romera, I. Role of arbuscular mycorrhizal fungus Rhizophagus custos in the dissipation of PAHs under root-organ culture conditions. Environ. Pollut. 2019, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, J.; Xiang, X. Impact of four plant species and arbuscular mycorrhizal (AM) fungi on polycyclic aromatic hydrocarbon (PAH) dissipation in spiked soil. Polish J. Environ. Stud. 2013, 22, 1239–1245. [Google Scholar]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 16, 277–289. [Google Scholar] [CrossRef]
- Fatma, M.; Khan, M.I.R.; Masood, A.; Khan, N.A. Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu. Rev. Res. Biol. 2013, 3, 267–295. [Google Scholar]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Vahabi, K.; Dorcheh, S.K.; Monajembashi, S.; Westermann, M.; Reichelt, M.; Falkenberg, D.; Hemmerich, P.; Sherameti, I.; Oelmüller, R. Stress promotes Arabidopsis-Piriformospora indica interaction. Plant Signal. Behav. 2016, 11, 1136763. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; McMillan, A.; Saggar, S. Routes of Dicyandiamide Uptake in Pasture Plants: A Preliminary Laboratory Study. Int. Grassl. Congr. Proc. 2020, 28, 1–9. [Google Scholar]
- Macadam, X.M.B.; Del Prado, A.; Merino, P.; Estavillo, J.M.; Pinto, M.; González-Murua, C. Dicyandiamide and 3,4-dimethyl pyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover. J. Plant Physiol. 2003, 160, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
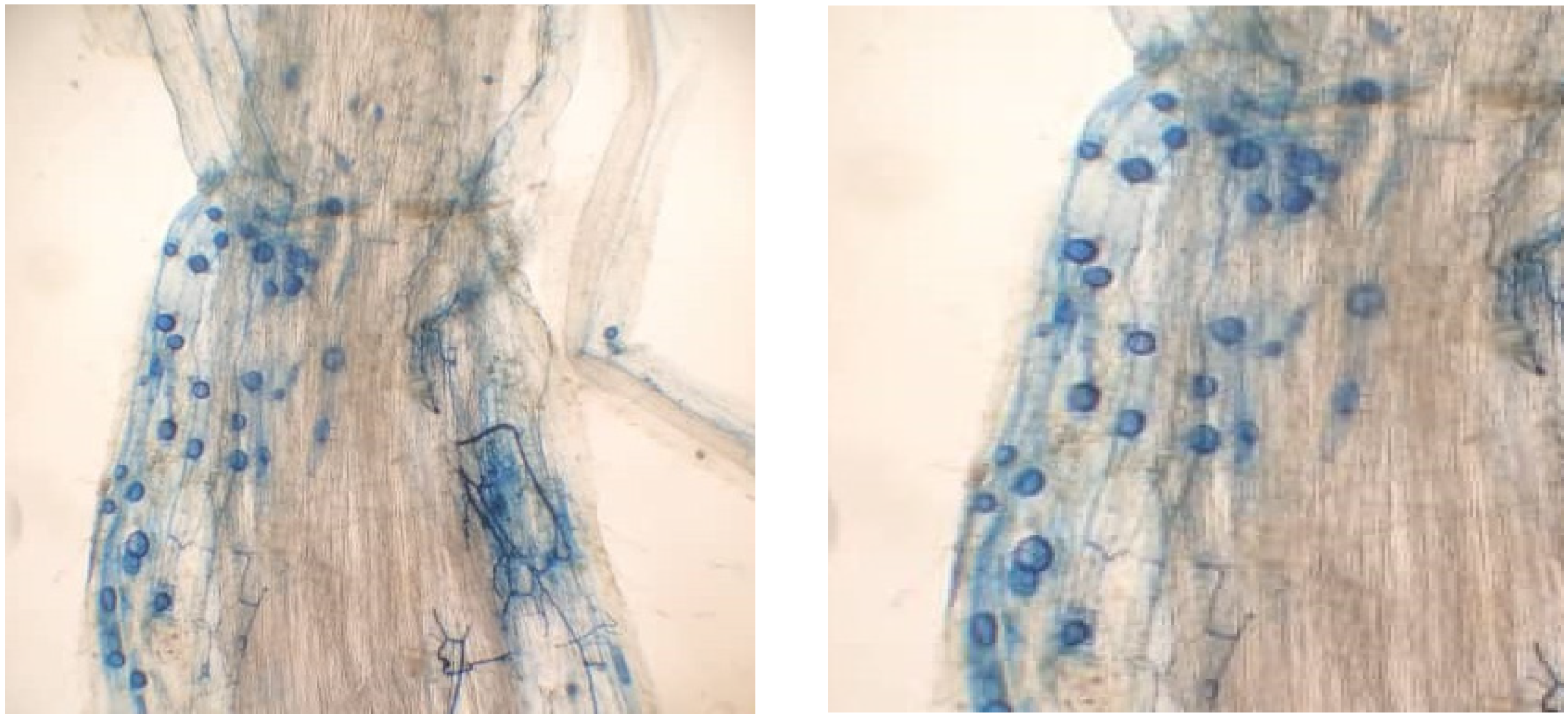
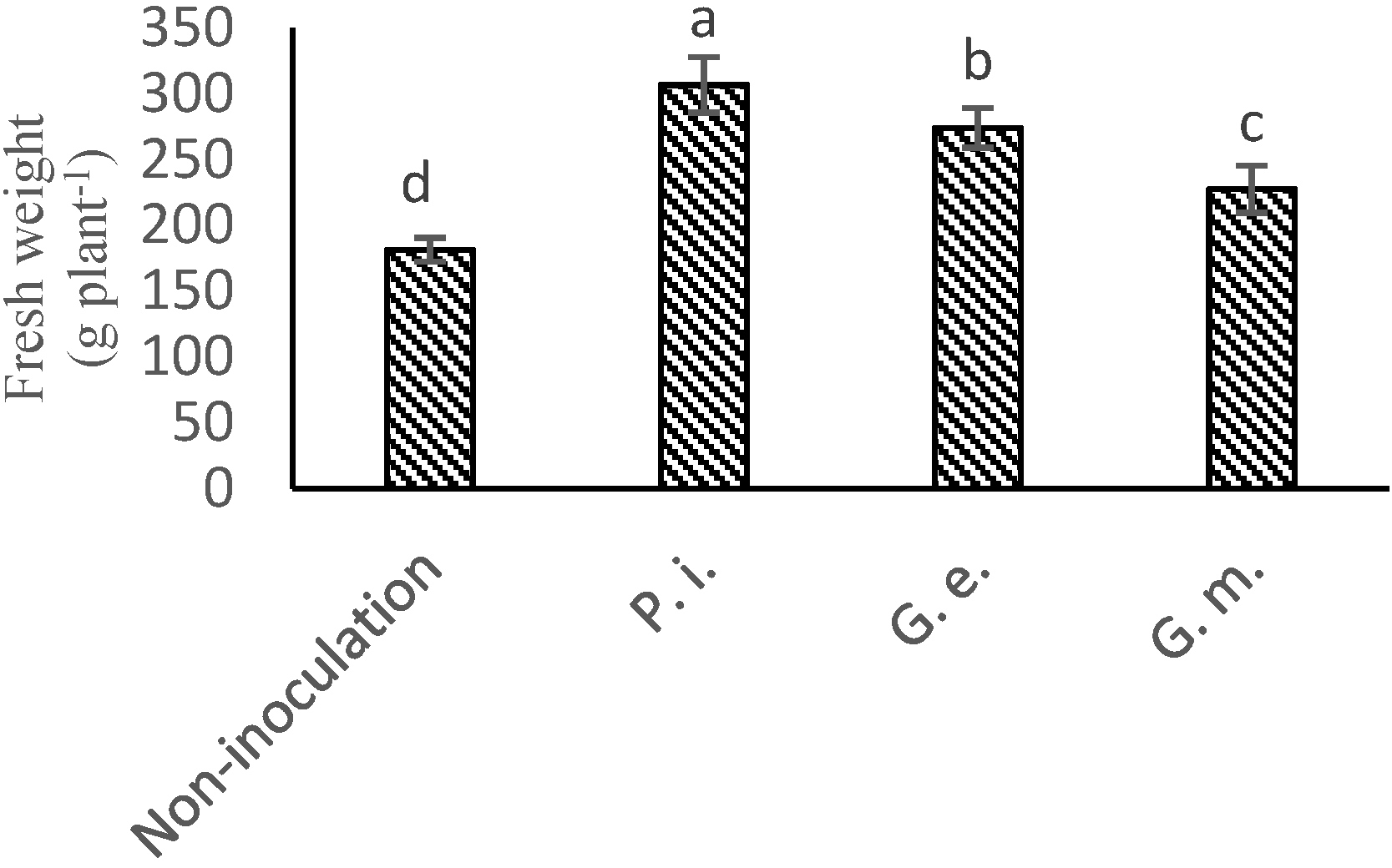

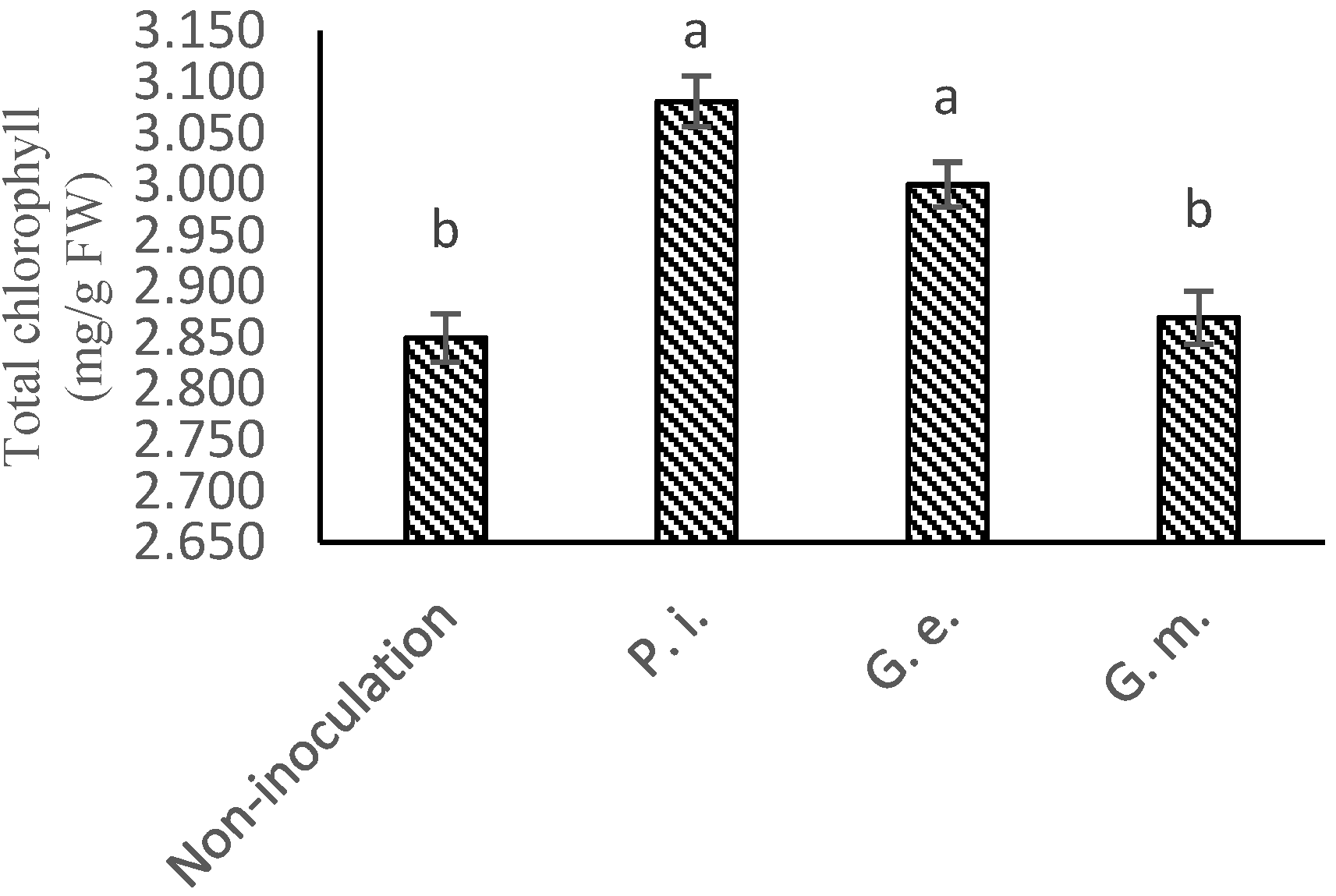
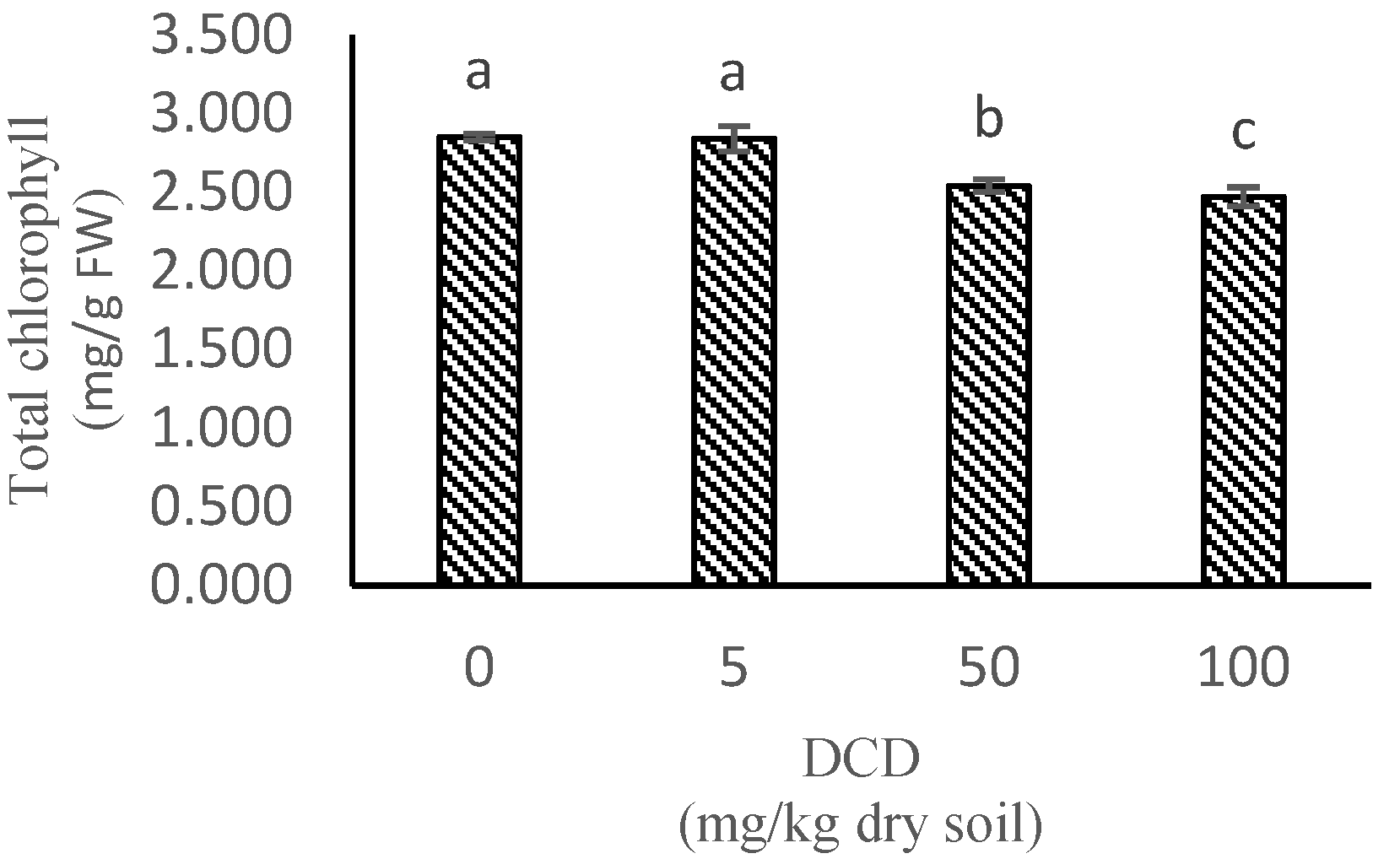


| Treatment | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| DCD (mg kg−1 Dry Soil) | Symbiotic Fungi | Root Colonization (%) | Shoot Dry Weight (g plant−1) | Shoot Height (cm) | Leaf Number (No plant−1) | Root Fresh Weight (g plant−1) | Root Length (cm) |
| Control | 0 | ND | 14.1 de ± 1.2 | 29.8 defg ± 0.2 | 31.7 efg ± 0.2 | 43.4 e ± 1.7 | 41.5 hi ± 2.3 |
| + P.i | 82.2 a | 20.6 ab ± 2.1 | 37.1 a ± 0.8 | 38.5 a ± 0.3 | 55.9 ab ± 1 | 50.6 def ± 3.1 | |
| + G.e | 76.2 ab | 15.6 cd ± 0.5 | 33.1 bc ± 0.4 | 34.5 bcd ± 0.7 | 51.0 bcd ± 2.4 | 53.2 cd ± 1.2 | |
| + G. m | 77.4 ab | 13.7 de ± 1.3 | 30.7 de ± 1.1 | 33.4 cd ± 0.3 | 48.7 cde ± 1.5 | 51.5 def ± 3.2 | |
| 5 | 0 | ND | 13.7 de ± 2.3 | 30.1 def ± 0.9 | 31.9 ef ± 0.4 | 49.5 cde ± 1.7 | 46.1 fgh ± 1.6 |
| + P.i | 67.4 bc | 21.8 a ± 3.4 | 34.3 b ± 0.2 | 35.7 abc ± 0.5 | 58.7 a ± 2.6 | 66.5 a ± 2.1 | |
| + G.e | 66.2 bc | 17.7 bcd ± 1.5 | 31.5 cd ± 0.6 | 37.0 ab ± 0.7 | 55.5 ab ± 1.3 | 60.2 b ± 4.5 | |
| + G. m | 68.1 bc | 16.7 bcd ± 2 | 30.0 def ± 0.7 | 32.9 def ± 0.8 | 47.5 cde ± 2.2 | 52.8 cde ± 1.6 | |
| 50 | 0 | ND | 10.9 ef ± 1.35 | 28.0 g ± 0.3 | 29.1 gh ± 0.1 | 38.7 f ± 1.8 | 39.2 i ± 2.4 |
| + P.i | 60.0 cd | 15.7 cd ± 3.1 | 29.9 def ± 1.3 | 31.0 efgh ± 0 | 51.2 bcd ± 1.3 | 57.2 bc ± 1.6 | |
| + G.e | 52.7 e | 15.3 cd ± 1.4 | 30.3 de ± 0.8 | 31.7 efg ± 0.5 | 51.5 bc ± 1.3 | 55.2 bcd ± 2.2 | |
| + G. m | 64.4 cd | 11.3 ef ± 1.8 | 29.1 efg ± 0.6 | 31.9 ef ± 0.6 | 46.0 cde ± 2.5 | 47.8 efg ± 33 | |
| 100 | 0 | ND | 5.7 g ± 0.71 | 25.2 h ± 1.1 | 20.5 i ± 0.2 | 34.0 f ± 3.1 | 29.5 j ± 4.1 |
| + P.i | 43.2 ef | 13.8 de ± 1.6 | 29.7 defg ± 0.6 | 29.2 gh ± 0.3 | 48.2 cde ± 1.2 | 50.5 def ± 3.7 | |
| + G.e | 45.8 e | 13.7 de ± 0.8 | 29.5 efg ± 0.4 | 30.1 fgh ± 0.7 | 46.2 cde ± 1.3 | 51.0 def ± 2.3 | |
| + G. m | 32.3 ef | 8.3 fg ± 1.02 | 28.2 fg ± 0.7 | 28.4 h ± 0.6 | 45.4 de ± 2.1 | 43.5 ghi ± 1.1 | |
| Inhibitor | ** | ** | ** | ** | ** | ** | |
| fungi | ** | ** | ** | ** | ** | ** | |
| In. × Fu. | ** | * | ** | ** | * | * | |
| - | + P. i. | + G. e. | G. m. | |
|---|---|---|---|---|
| Control | 78.6 b ± 3.4 | 86.3 a ± 5.3 | 79.3 b ± 2.6 | 78.8 b ± 3.6 |
| DCD 5 mg kg−1 | 78.7 b ± 5.6 | 86.1 a ± 4.6 | 79 b ± 3.1 | 79.2 b ± 2.6 |
| DCD 50 mg kg−1 | 75.1 c ± 2.4 | 75.4 c ± 3.6 | 74.8 c ± 2 | 75.3 c ± 3.2 |
| DCD 100 mg kg−1 | 64.3 f ± 4.1 | 71.3 d ± 2.6 | 68.6 e ± 1.2 | 67.1 e ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padash, A.; Azarmi, R.; Ashraf Soltani Toularoud, A.; Esmailpour, B.; Cruz, C. Use of Symbiotic Fungi to Reduce the Phytotoxic Effect of DCD Nitrification Inhibitors in Lettuce. Agriculture 2022, 12, 251. https://doi.org/10.3390/agriculture12020251
Padash A, Azarmi R, Ashraf Soltani Toularoud A, Esmailpour B, Cruz C. Use of Symbiotic Fungi to Reduce the Phytotoxic Effect of DCD Nitrification Inhibitors in Lettuce. Agriculture. 2022; 12(2):251. https://doi.org/10.3390/agriculture12020251
Chicago/Turabian StylePadash, Akbar, Rasoul Azarmi, Ali Ashraf Soltani Toularoud, Behrooz Esmailpour, and Cristina Cruz. 2022. "Use of Symbiotic Fungi to Reduce the Phytotoxic Effect of DCD Nitrification Inhibitors in Lettuce" Agriculture 12, no. 2: 251. https://doi.org/10.3390/agriculture12020251
APA StylePadash, A., Azarmi, R., Ashraf Soltani Toularoud, A., Esmailpour, B., & Cruz, C. (2022). Use of Symbiotic Fungi to Reduce the Phytotoxic Effect of DCD Nitrification Inhibitors in Lettuce. Agriculture, 12(2), 251. https://doi.org/10.3390/agriculture12020251








