Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pollination Procedure, Sample Storage and Seed Germination
2.3. Experimental Procedure
2.3.1. Experiment 1
2.3.2. Experiment 2
2.3.3. Experiment 3
2.4. Histological Observations
2.5. Ploidy Level Analysis by Flow Cytometry
2.6. Genetic Analysis with Simple Sequence Repeat (SSR) and Single Nucleotide Polymorphism (SNP) Markers
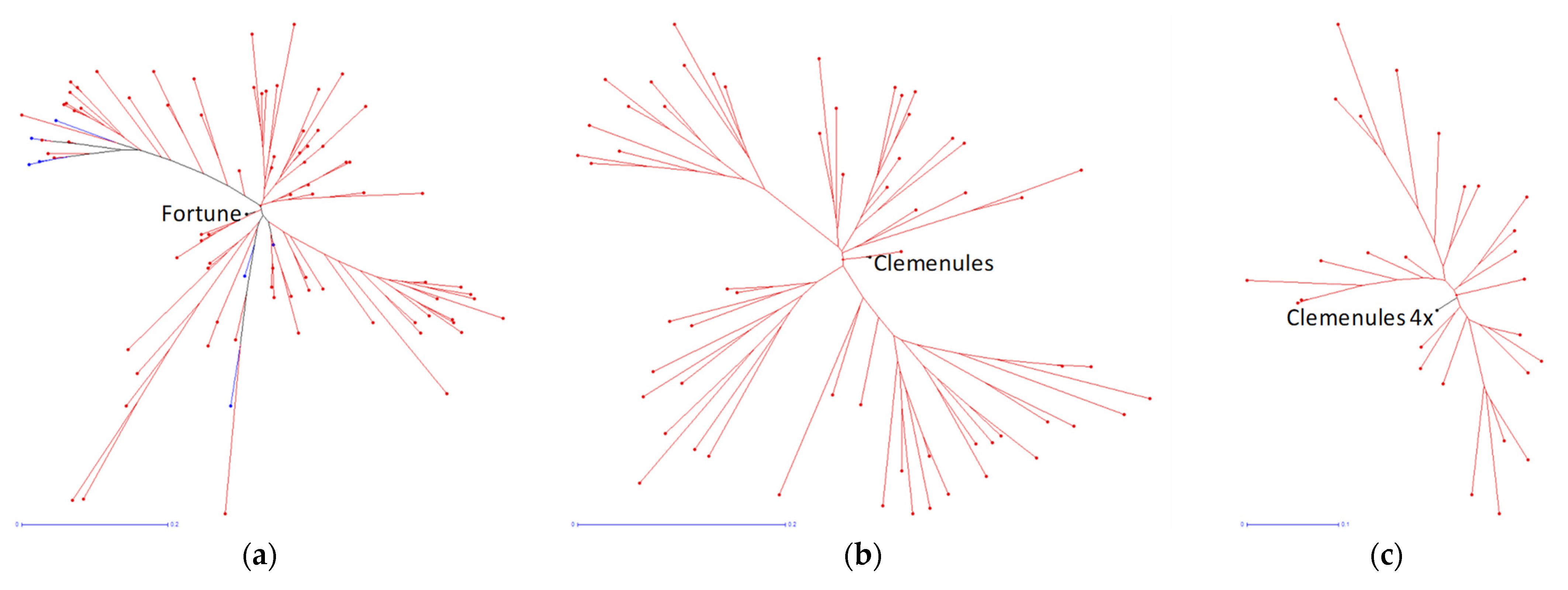
2.7. Population Diversity Analysis
3. Results
3.1. SI Breakdown by Temperature Stress (Experiment 1)
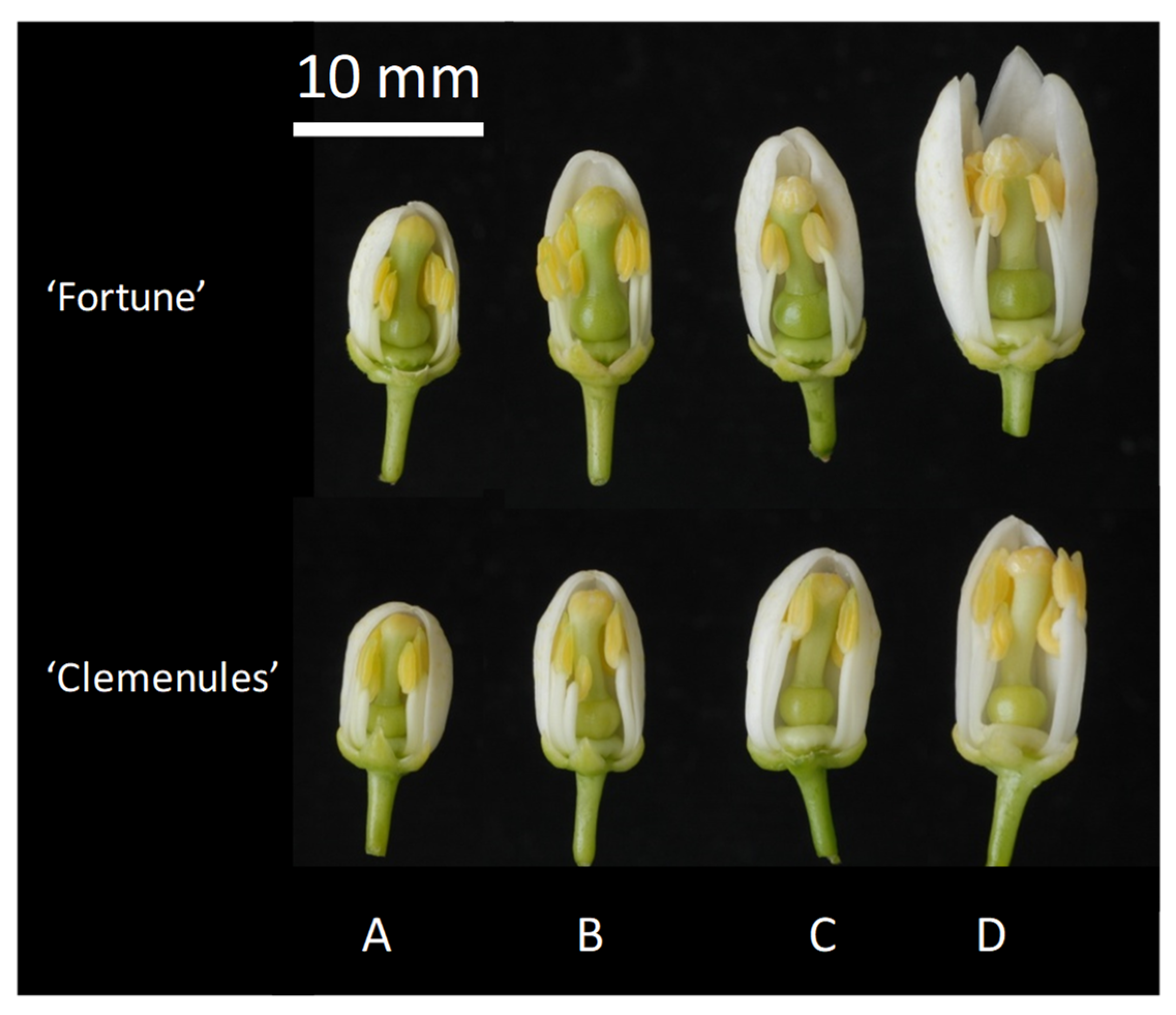
3.2. SI Breakdown by Bud Pollination (Experiment 2)
3.3. SI Breakdown by Polyploidization (Experiment 3)
3.4. Ploidy Level Analysis by Flow Cytometry
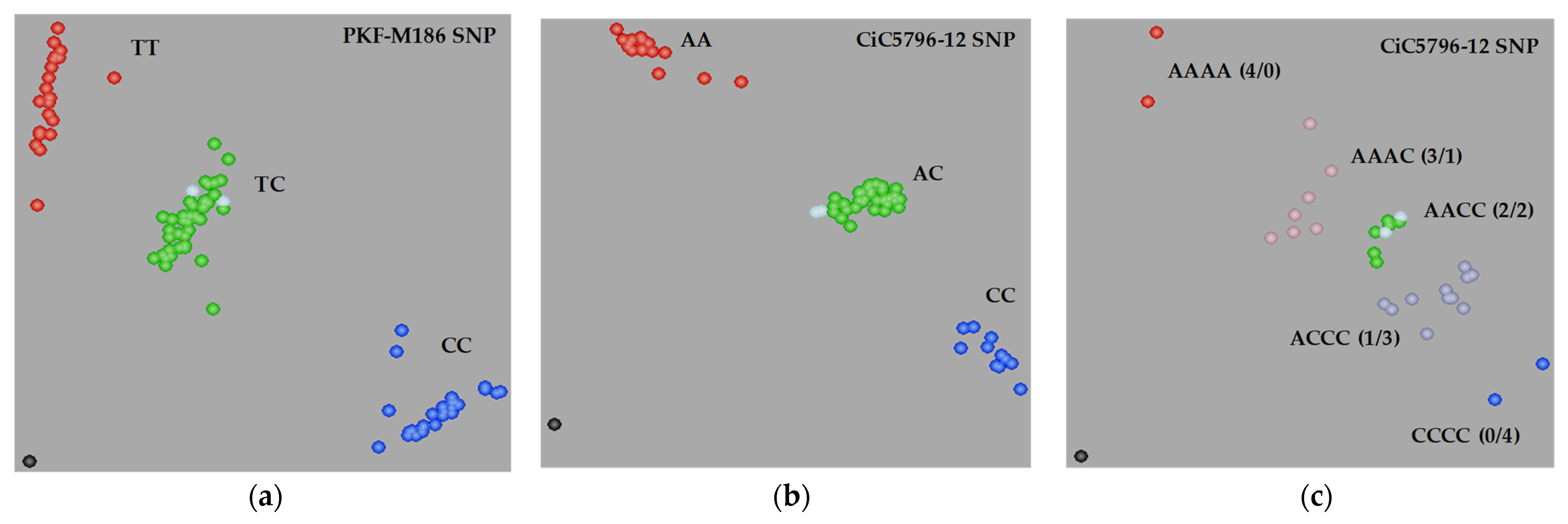
3.5. Genetic Analysis with the SSR and SNP Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franklin-Tong, V.E. Self-Incompatibility in Flowering Plants: Evolution, Diversity, and Mechanisms; Franklin-Tong, V.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-68485-5. [Google Scholar]
- Allen, A.M.; Hiscock, S.J. Evolution and phylogeny of self-incompatibility systems in angiosperms. In Self-Incompatibility in Flowering Plants: Evolution, Diversity, and Mechanisms; Franklin-Tong, V.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–101. ISBN 978-3-540-68486-2. [Google Scholar]
- Barrett, S.C.H. The evolution of plant reproductive systems: How often are transitions irreversible? Proc. R. Soc. B Biol. Sci. 2013, 280, 20130913. [Google Scholar] [CrossRef]
- Gibbs, P.E. Late-acting self-incompatibility—The pariah breeding system in flowering plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef]
- Raduski, A.R.; Haney, E.B.; Igić, B. The expression of self-incompatibility in angiosperms is bimodal. Evolution 2011, 66, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D.; Vekemans, X.; Castric, V.; Glémin, S. Plant self-incompatibility systems: A molecular evolutionary perspective. New Phytol. 2005, 168, 61–69. [Google Scholar] [CrossRef]
- Takayama, S.; Isogai, A. Self-incompatibility in plants. Annu. Rev. Plant Biol. 2005, 56, 467–489. [Google Scholar] [CrossRef]
- McClure, B. S-RNase and SLF determine S-haplotype–specific pollen recognition and rejection. Plant Cell 2004, 16, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishio, T. Commonalities and differences between Brassica and Arabidopsis self-incompatibility. Hortic. Res. 2014, 1, 14054. [Google Scholar] [CrossRef]
- Sehgal, N.; Singh, S. Progress on deciphering the molecular aspects of cell-to-cell communication in Brassica self-incompatibility response. 3 Biotech 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, C.; Alston, F.H.; Van Arkel, G.; Brown, L.M.; Chevreau, E.; Dunemann, F.; Evans, K.M.; Gardiner, S.; Guilford, P.; Van Heusden, A.W. Aligning male and female linkage maps of apple (Malus pumila Mill.) using multi-allelic markers. Theor. Appl. Genet. 1998, 97, 60–73. [Google Scholar] [CrossRef]
- Claessen, H.; Keulemans, W.; Van de Poel, B.; De Storme, N. Finding a compatible partner: Self-incompatibility in european pear (Pyrus communis); molecular control, genetic determination, and impact on fertilization and fruit set. Front. Plant Sci. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.; Baraket, G.; Perez, V.; Ben Mustapha, S.; Salhi-Hannachi, A.; Hormaza, J.I. Analysis of self-incompatibility and genetic diversity in diploid and hexaploid plum genotypes. Front. Plant Sci. 2019, 10, 896. [Google Scholar] [CrossRef]
- Brancher, T.L.; Hawerroth, M.C.; Kvitschal, M.V.; Manenti, D.C.; Guidolin, A.F. Self-incompatibility alleles in important genotypes for apple breeding in Brazil. Crop Breed. Appl. Biotechnol. 2020, 20, e28652041. [Google Scholar] [CrossRef]
- Knight, R.; Rogers, H.H. Sterility in Theobroma cacao L. Nature 1953, 172, 164. [Google Scholar] [CrossRef]
- Alagna, F.; Caceres, M.E.; Pandolfi, S.; Collani, S.; Mousavi, S.; Mariotti, R.; Cultrera, N.G.M.; Baldoni, L.; Barcaccia, G. The paradox of self-fertile varieties in the context of self-incompatible genotypes in olive. Front. Plant Sci. 2019, 10, 725. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, M.; Wang, N.; Xu, Q.; Deng, X.; Chai, L. Reproduction in woody perennial citrus: An update on nucellar embryony and self-incompatibility. Plant Reprod. 2018, 31, 43–57. [Google Scholar] [CrossRef]
- Goldberg, E.E.; Kohn, J.R.; Lande, R.; Robertson, K.A.; Smith, S.A.; Igić, B. Species selection maintains self-incompatibility. Science 2010, 330, 493–495. [Google Scholar] [CrossRef]
- Surridge, C. Self-incompatibility: Avoiding inbreeding in Arabidopsis. Nat. Plants 2015, 1, 15198. [Google Scholar] [CrossRef]
- Lin, Z.; Eaves, D.J.; Sanchez-Moran, E.; Franklin, F.C.; Franklin-Tong, V.E. The Papaver rhoeas S determinants confer self-incompatibility to Arabidopsis thaliana in planta. Science 2015, 350, 684–687. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Perez, V.; Salhi Hannachi, A.; Hormaza, J.I. Self-compatibility in peach [Prunus persica (L.) Batsch]: Patterns of diversity surrounding the S-locus and analysis of SFB alleles. Hortic. Res. 2020, 7, 170. [Google Scholar] [CrossRef]
- de Nettancourt, D. Incompatibility in angiosperms. Sex. Plant Reprod. 1997, 10, 185–199. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Good, S.V. Self-sterility in flowering plants: Preventing self-fertilization increases family diversification rates. Ann. Bot. 2012, 110, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Igic, B.; Bohs, L.; Kohn, J.R. Ancient polymorphism reveals unidirectional breeding system shifts. Proc. Natl. Acad. Sci. USA 2006, 103, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Morita, M.; Iwano, M. Self/non-self recognition mechanisms in sexual reproduction: New insight into the self-incompatibility system shared by flowering plants and hermaphroditic animals. Biochem. Biophys. Res. Commun. 2014, 450, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Mendez, A.; McClure, B. Plant reproduction: Self-incompatibility to go. Curr. Biol. 2016, 26, R115–R117. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P.; Ahmed, D.; Costantino, G.; Evrard, J.C.; Cardi, C.; Mournet, P.; Perdereau, A.; Froelicher, Y. Segregation distortion for male parents in high density genetic maps from reciprocal crosses between two self-incompatible cultivars confirms a gametophytic system for self-incompatibility in citrus. Agriculture 2021, 11, 379. [Google Scholar] [CrossRef]
- Vilanova, S.; Badenes, M.L.; Burgos, L.; Martínez-Calvo, J.; Llácer, G.; Romero, C. Self-compatibility of two apricot selections is associated with two pollen-part mutations of different nature. Plant Physiol. 2006, 142, 629–641. [Google Scholar] [CrossRef]
- Soost, R.K. Incompatibility alleles in genus citrus. Proc. Am. Soc. Hort. Sci. 1965, 87, 176–180. [Google Scholar]
- Soost, R.K. The incompatibility gene system. Proc. Int. Soc. Citric. 1969, 1, 189–190. [Google Scholar]
- Vardi, A.; Neumann, H.; Frydman-Shani, A.; Yaniv, Y.; Spiegel-Roy, P. Tentative model on the inheritance of juvenility, self-incompatibility and parthenocarpy. Acta Hortic. 2000, 535, 199–206. [Google Scholar] [CrossRef]
- Caruso, M.; Merelo, P.; Distefano, G.; La Malfa, S.; Lo Piero, A.R.; Tadeo, F.R.; Talon, M.; Gentile, A. Comparative transcriptome analysis of stylar canal cells identifies novel candidate genes implicated in the self-incompatibility response of Citrus clementina. BMC Plant Biol. 2012, 12, 20. [Google Scholar] [CrossRef]
- Honsho, C.; Ushijima, K.; Anraku, M.; Ishimura, S.; Yu, Q.; Gmitter, F.G.; Tetsumura, T. Association of T2/S-RNase with self-incompatibility of japanese citrus accessions examined by transcriptomic, phylogenetic, and genetic approaches. Front. Plant Sci. 2021, 12, 638321. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Cao, Z.; Zhu, A.; Liu, Y.; Tao, M.; Yang, H.; Xu, Q.; Wang, S.; Liu, J.; Li, Y.; et al. Evolution of self-compatibility by a mutant Sm -RNase in citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef]
- Wilms, H.J.; Van Went, J.L.; Cresti, M.; Ciampolini, F. Structural aspects of female sterility in Citrus limon. Acta Bot. Neerl. 1983, 32, 87–96. [Google Scholar] [CrossRef]
- Osawa, I. Cytological and experimental studies in Citrus. J. Coll. Agric. Tokyo Univ. 1912, 4, 83–116. [Google Scholar]
- Wong, C.Y. The influence of pollination on seed development in certain varieties of citrus. Soc. Hortic. Sci. 1939, 37, 161–164. [Google Scholar]
- Yamasaki, A.; Kitajima, A.; Ohara, N.; Tanaka, M.; Hasegawa, K. Characteristics of arrested seeds in Mukaku kishu-type seedless citrus. J. Jpn. Soc. Hortic. Sci. 2009, 78, 61–67. [Google Scholar] [CrossRef]
- Yamasaki, A.; Kitajima, A.; Ohara, N.; Tanaka, M.; Hasegawa, K. Histological study of expression of seedlessness in Citrus kinokuni ‘Mukaku Kishu’ and its progenies. J. Am. Soc. Hortic. Sci. 2007, 132, 869–875. [Google Scholar] [CrossRef]
- Goto, S.; Yoshioka, T.; Ohta, S.; Kita, M.; Hamada, H.; Shimizu, T. Segregation and heritability of male sterility in populations derived from progeny of satsuma mandarin. PLoS ONE 2016, 11, e0162408. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Effects of gamma-irradiation mutagenesis for induction of seedlessness, on the quality of mandarin fruit. Food Nutr. Sci. 2014, 5, 943–952. [Google Scholar] [CrossRef]
- Bermejo, A.; Pardo, J.; Cano, A. Influence of gamma irradiation on seedless citrus production: Pollen germination and fruit quality. Food Nutr. Sci. 2011, 2, 169–180. [Google Scholar] [CrossRef]
- Ollitrault, P.; Froelicher, Y.; Dambier, D.; Luro, F.; Yamamoto, M. Seedlessness and ploidy manipulation. In Citrus Genetics, Breeding and Biotechnology; Khan, I., Ed.; CABI: Wallingfor, UK, 2007; pp. 197–218. ISBN 9780851990194. [Google Scholar]
- Ollitrault, P.; Dambier, D.; Francois, L.; Froelicher, Y. Ploidy manipulation for breeding seedless triploid citrus. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 30, pp. 323–352. ISBN 9780470380130. [Google Scholar]
- Navarro, L.; Aleza, P.; Cuenca, J.; Juárez, J.; Pina, J.A.; Ortega, C.; Navarro, A.; Ortega, V. The mandarin triploid breeding program in Spain. Acta Hortic. 2015, 1065, 389–396. [Google Scholar] [CrossRef]
- Montalt, R.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Parthenocarpy and self-incompatibility in mandarins. Agronomy 2021, 11, 2023. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kubo, T.; Tominaga, S. Self- and cross-incompatibility of various citrus accessions. J. Jpn. Soc. Hortic. Sci. 2006, 75, 372–378. [Google Scholar] [CrossRef]
- Kim, J.-H.; Handayani, E.; Wakana, A.; Sato, M.; Miyamoto, M.; Miyazaki, R.; Zhou, X.; Sakai, K.; Mizunoe, Y.; Shigyo, M.; et al. Distribution and evolution of Citrus accessions with S3 and/or S11 alleles for self-incompatibility with an emphasis on sweet orange [Citrus sinensis (L.) Osbeck; SfS3 or SfS3sm]. Genet. Resour. Crop Evol. 2020, 67, 2101–2117. [Google Scholar] [CrossRef]
- Ngo, B.X.; Wakana, A.; Park, S.M.; Nada, Y.; Fukudome, I. Pollen tube behaviors in self-incompatible and self-compatible citrus cultivars. J. Fac. Agric. Kyushu Univ. 2001, 45, 443–457. [Google Scholar] [CrossRef]
- Distefano, G.; Las Casas, G.; La Malfa, S.; Gentile, A.; Tribulato, E.; Herrero, M. Pollen tube behavior in different mandarin hybrids. J. Am. Soc. Hortic. Sci. 2009, 134, 583–588. [Google Scholar] [CrossRef]
- Distefano, G.; Caruso, M.; la Malfa, S.; Gentile, A.; Tribulato, E. Histological and molecular analysis of pollen-pistil interaction in clementine. Plant Cell Rep. 2009, 28, 1439–1451. [Google Scholar] [CrossRef]
- Eti, S.; Stosser, R. Pollen tube growth and development of ovules in relation to fruit set in mandarines, cv.’Clementine’(Citrus reticulata Blanco). Acta Hortic. 1992, 621–625. [Google Scholar] [CrossRef]
- East, E.M. Genetical aspects of self- and cross-sterility. Am. J. Bot. 1923, 10, 468–473. [Google Scholar] [CrossRef]
- Herrero, M.; Dickinson, H.G. Ultrastructural and physiological differences between buds and mature flowers of Petunia hybrida prior to and following pollination. Planta 1980, 148, 138–145. [Google Scholar] [CrossRef]
- Kim, H.J.; Niimi, Y. Frequency of viable seeds obtained from several Lilium spp. cross-pollinated at different floral stages. J. Jpn. Soc. Hortic. Sci. 2002, 71, 231–235. [Google Scholar] [CrossRef][Green Version]
- Hiscock, S.J.; Dickinson, H.G. Unilateral incompatibility within the brassicaceae: Further evidence for the involvement of the self-incompatibility (S)-locus. Theor. Appl. Genet. 1993, 86, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Ascher, P.D.; Peloquin, S.J. Influence of temperature on incompatible and compatible pollen tube growth in Lilium longiflorum. Can. J. Genet. Cytol. 1966, 8, 661–664. [Google Scholar] [CrossRef]
- Campbell, R.J.; Linskens, H.F. Temperature effects on self incompatibility in Lilium longiflorum. Theor. Appl. Genet. 1984, 68, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.E. Self-compatibility studies with diploid alsike clover, Trifolium hybridum L. III. Response to temperature. Crop Sci. 1968, 8, 269–272. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishimura, K.; Kitashiba, H.; Sakamoto, W.; Nishio, T. High temperature causes breakdown of S haplotype-dependent stigmatic self-incompatibility in self-incompatible Arabidopsis thaliana. J. Exp. Bot. 2019, 70, 5745–5751. [Google Scholar] [CrossRef]
- Lewis, D. Competition and dominance of incompatibility alleles in diploid pollen. Heredity 1947, 1, 85–108. [Google Scholar] [CrossRef]
- Distefano, G.; Hedhly, A.; Las Casas, G.; La Malfa, S.; Herrero, M.; Gentile, A. Male-female interaction and temperature variation affect pollen performance in citrus. Sci. Hortic. (Amst.) 2012, 140, 1–7. [Google Scholar] [CrossRef]
- Distefano, G.; Gentile, A.; Hedhly, A.; La Malfa, S. Temperatures during flower bud development affect pollen germination, self-incompatibility reaction and early fruit development of clementine (Citrus clementina Hort. ex Tan.). Plant Biol. 2018, 20, 191–198. [Google Scholar] [CrossRef]
- Montalt, R.; Cuenca, J.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Influence of temperature on the progamic phase in Citrus. Environ. Exp. Bot. 2019, 166, 103806. [Google Scholar] [CrossRef]
- Aloisi, I.; Distefano, G.; Antognoni, F.; Potente, G.; Parrotta, L.; Faleri, C.; Gentile, A.; Bennici, S.; Mareri, L.; Cai, G.; et al. Temperature-dependent compatible and incompatible pollen-style interactions in Citrus clementina Hort. ex Tan. show different transglutaminase features and polyamine pattern. Front. Plant Sci. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Wakana, A.; Ngo, B.X.; Fukudome, I.; Kajiwara, K. Estimation of the degree of self-incompatibility reaction during flower bud development and production of self-fertilized seeds by bud pollination in self-incompatible Citrus cultivars. J. Fac. Agric. Kyushu Univ. 2004, 49, 307–320. [Google Scholar] [CrossRef]
- Aleza, P.; Froelicher, Y.; Schwarz, S.; Agustí, M.; Hernández, M.; Juárez, J.; Luro, F.; Morillon, R.; Navarro, L.; Ollitrault, P. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment. Ann. Bot. 2011, 108, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Oda, K.; Nakamura, N. Seed development in self-pollination of 4X Hyuganatsu and reciprocal crosses between 2X and 4X Hyuganatsu, and overcoming the self-incompatibility of 2X Hyuganatsu using pollen of 4X Hyuganatsu. J. Jpn. Soc. Hortic. Sci. 1990, 59, 23–28. [Google Scholar] [CrossRef][Green Version]
- Aleza, P.; Juárez, J.; Ollitrault, P.; Navarro, L. Production of tetraploid plants of non apomictic citrus genotypes. Plant Cell Rep. 2009, 28, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D. Plant Microtechniques; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Linskens, F.H.; Esser, K. Über eine spezifische anfarbung der pollenschlauche im griffel und die zahl der kallospefropfen nach slbstdung und femddung. Naturwissenschaften 1957, 44, 16. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Kasahara, R.D. Mechanics of pollen tube elongation: A perspective. Front. Plant Sci. 2020, 11, 589712. [Google Scholar] [CrossRef]
- Ollitrault, P.; Terol, J.; Chen, C.; Federici, C.T.; Lotfy, S.; Hippolyte, I.; Ollitrault, F.; Bérard, A.; Chauveau, A.; Cuenca, J.; et al. A reference genetic map of C. clementina hort. ex Tan.; citrus evolution inferences from comparative mapping. BMC Genom. 2012, 13, 593. [Google Scholar] [CrossRef]
- Cuenca, J.; Froelicher, Y.; Aleza, P.; Juárez, J.; Navarro, L.; Ollitrault, P. Multilocus half-tetrad analysis and centromere mapping in citrus: Evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv “Fortune”. Heredity 2011, 107, 462–470. [Google Scholar] [CrossRef]
- Kijas, J.M.H.; Thomas, M.R.; Fowler, J.C.S.; Roose, M.L. Integration of trinucleotide microsatellites into a linkage map of Citrus. Theor. Appl. Genet. 1997, 94, 701–706. [Google Scholar] [CrossRef]
- Froelicher, Y.; Dambier, D.; Bassene, J.B.; Constantino, G.; Lotfy, S.; Didout, C.; Beaumont, V.; Brottier, P.; Risterucci, A.M.; Luro, F.; et al. Characterization of microsatellite markers in mandarin orange (Citrus reticulata Blanco). Mol. Ecol. Resour. 2008, 8, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lor, A.; Curk, F.; Snoussi-Trifa, H.; Morillon, R.; Ancillo, G.; Luro, F.; Navarro, L.; Ollitrault, P. A nuclear phylogenetic analysis: SNPs, indels and SSRs deliver new insights into the relationships in the ‘true citrus fruit trees’ group (Citrinae, Rutaceae) and the origin of cultivated species. Ann. Bot. 2013, 111, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P.; Terol, J.; Garcia-Lor, A.; Bérard, A.; Chauveau, A.; Froelicher, Y.; Belzile, C.; Morillon, R.; Navarro, L.; Brunel, D.; et al. SNP mining in C. clementina BAC end sequences; transferability in the Citrus genus (Rutaceae), phylogenetic inferences and perspectives for genetic mapping. BMC Genom. 2012, 13, 13. [Google Scholar] [CrossRef]
- Cuppen, E. Genotyping by allele-specific amplification (KASPar). CSH Protoc. 2007, 2007, pdb.prot4841. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. Available online: http://darwin.cirad.fr/2006 (accessed on 28 December 2021).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Esselink, G.D.; Nybom, H.; Vosman, B. Assignment of allelic configuration in polyploids using the MAC-PR (microsatellite DNA allele counting—Peak ratios) method. Theor. Appl. Genet. 2004, 109, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, J.; Aleza, P.; Navarro, L.; Ollitrault, P. Assignment of SNP allelic configuration in polyploids using competitive allele-specific PCR: Application to citrus triploid progeny. Ann. Bot. 2013, 111, 731–742. [Google Scholar] [CrossRef][Green Version]
- Aleza, P.; Cuenca, J.; Juárez, J.; Ollitrault, P.; Navarro, L. Differences in the genetic structure of citrus triploid hybrids recovered from 2x x 2x and 4x x 2x sexual hybridizations. Acta Hortic. 2015, 1065, 487–493. [Google Scholar] [CrossRef]
- Muller, H.J. A new mode of segregation in Gregory’s tetraploid primulas. Am. Nat. 1914, 48, 508–512. [Google Scholar] [CrossRef]
- Kawano, S.; Li, Y.; Yahata, M.; Kunitake, H. Effect of temperature on self-incompatibility in Citrus pistil and mature pollen culture systems. Acta Hortic. 2016, 1135, 117–122. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cabin, R.; Evans, A.; Jennings, D.; Marshall, D.; Mitchell, R.; Sher, A. Using bud pollinations to avoid self-incompatibility: Implications from studies of three mustards. Can. J. Bot. 1996, 74, 285–289. [Google Scholar] [CrossRef]
- Yasuda, S. Physiological research on self-incompatibility in Petunia violacea. Bull. Imp. Coll. Agric. For. Marioka Nippon. 1934, 20, 1–82. [Google Scholar]
- Sykes, S.R. The effect on Citrus fruit of excluding pollinating insects at flowering and implications for breeding new seedless cultivars. J. Hortic. Sci. Biotechnol. 2008, 83, 713–718. [Google Scholar] [CrossRef]
- Golz, J.F.; Clarke, A.E.; Newbigin, E. Mutational approaches to the study of self-incompatibility: Revisiting the pollen-part mutants. Ann. Bot. 2000, 85, 95–103. [Google Scholar] [CrossRef][Green Version]
- Crane, M.B.; Lewis, D. Genetical studies in pears. III Incompatibility and sterility. J. Genet. 1942, 43, 31–43. [Google Scholar] [CrossRef]
- Adachi, Y.; Komori, S.; Hoshikawa, Y.; Tanaka, N.; Abe, K.; Bessho, H.; Watanabe, M.; Suzuki, A. Characteristics of fruiting and pollen tube growth of apple autotetraploid cultivars showing self-compatibility. J. Jpn. Soc. Hortic. Sci. 2009, 78, 402–409. [Google Scholar] [CrossRef][Green Version]
- Zuo, J.-F.; Niu, Y.; Cheng, P.; Feng, J.-Y.; Han, S.-F.; Zhang, Y.-H.; Shu, G.; Wang, Y.; Zhang, Y.-M. Effect of marker segregation distortion on high density linkage map construction and QTL mapping in Soybean (Glycine max L.). Heredity 2019, 123, 579–592. [Google Scholar] [CrossRef]
- Phytozome 13. Available online: https://phytozome-next.jgi.doe.gov/ (accessed on 28 December 2021).


| Experiment | Genotype | Temp. | Flower Stage | PTG (%) | PTG (No.) | Seed Production (No. per Fruit) |
|---|---|---|---|---|---|---|
| Exp. 1 Temperature stress | ‘Fortune’ | 10 °C | Anthesis | 80 | <5 | 1.4 ± 0.8 |
| ‘Fortune’ | 30 °C | 0 | 0 | - | ||
| Exp. 2 Bud Pollination | ‘Fortune’ | FC | Bud (14.7 ± 1.0) (D) | 0 | 0 | 0 |
| ‘Fortune’ | FC | Bud (12.5 ± 0.7) (C) | 60 | <5 | - | |
| ‘Fortune’ | FC | Bud (9.9 ± 0.9) (A-B) | 100 | >10 | 22.3 ± 2.5 | |
| ‘Clemenules’ | FC | Bud (14.9 ± 0.8) (D) | 0 | 0 | 0 | |
| ‘Clemenules’ | FC | Bud (12.1 ± 0.5) (C) | 40 | <5 | - | |
| ‘Clemenules’ | FC | Bud (9.4 ± 0.9) (A-B) | 100 | >10 | 17.5 ± 7.7 | |
| Exp. 3 Polyploidization | ‘Clemenules’ 4x | FC | Anthesis | 100 | 5–10 | 5 ± 1.4 |
| Marker | aa | AA | Aa | Chi-Squared | p-Value |
|---|---|---|---|---|---|
| SOS1-M50 | 18 | 26 | 36 | 1.455 | 0.228 |
| mCrCIR05A05 | 17 | 16 | 47 | 0.030 | 0.862 |
| JK-TAA41 | 16 | 18 | 43 | 0.118 | 0.732 |
| PKF-M186 | 22 | 21 | 37 | 0.023 | 0.879 |
| mCrCIR06B07 | 17 | 17 | 43 | 0.000 | 1.000 |
| Ci07C07 | 17 | 15 | 48 | 0.125 | 0.724 |
| NADK2-M285 | 31 | 9 | 40 | 12.100 | 0.001 |
| LAPX-M238 | 17 | 26 | 36 | 1.884 | 0.170 |
| MDH-MP69 | 18 | 24 | 33 | 0.857 | 0.355 |
| FLS-M400 | 18 | 14 | 48 | 0.500 | 0.480 |
| HYB-M62 | 10 | 17 | 53 | 1.815 | 0.178 |
| Marker | aa | AA | Aa | Chi-Squared | p-Value |
|---|---|---|---|---|---|
| CiC2110-01 | 15 | 16 | 30 | 0.032 | 0.857 |
| CiC5950-02 | 11 | 21 | 29 | 3.125 | 0.077 |
| CiC6278-01 | 10 | 17 | 32 | 1.815 | 0.178 |
| CiC3712-01 | 13 | 21 | 27 | 1.882 | 0.170 |
| JK-TAA41 | 22 | 28 | 14 | 1.778 | 0.182 |
| mCrCIR06B07 | 14 | 31 | 19 | 0.758 | 0.384 |
| CiC5796-12 | 16 | 11 | 34 | 0.926 | 0.336 |
| CiC1380-05 | 18 | 11 | 32 | 1.690 | 0.194 |
| Ci07C07 | 14 | 30 | 20 | 1.059 | 0.303 |
| CiC5164-03 | 17 | 14 | 29 | 0.290 | 0.590 |
| CiC1749-05 | 15 | 12 | 34 | 0.333 | 0.564 |
| CiC5087-01 | 13 | 18 | 30 | 0.806 | 0.369 |
| Marker | aaaa | aaaA | AAaa | AAAa | AAAA | Chi-Squared | p-Value |
|---|---|---|---|---|---|---|---|
| CiC2110-01 | 0 | 7 | 11 | 9 | 2 | 4.483 | 0.345 |
| CiC5950-02 | 2 | 5 | 16 | 3 | 0 | 5.115 | 0.276 |
| CiC6278-01 | 1 | 3 | 12 | 9 | 1 | 3.423 | 0.490 |
| CiC3712-01 | 0 | 7 | 11 | 8 | 3 | 8.052 | 0.090 |
| JK-TAA41 | 1 | 5 | 16 | 3 | 1 | 2.346 | 0.672 |
| mCrCIR06B07 | 1 | 5 | 13 | 5 | 2 | 2.577 | 0.631 |
| CiC5796-12 | 2 | 7 | 7 | 11 | 2 | 10.690 | 0.030 |
| CiC1380-05 | 3 | 10 | 13 | 2 | 1 | 11.207 | 0.024 |
| Ci07C07 | 1 | 4 | 17 | 5 | 1 | 1.804 | 0.772 |
| CiC5164-03 | 0 | 5 | 10 | 9 | 1 | 3.520 | 0.475 |
| CiC1749-05 | 8 | 7 | 12 | 2 | 0 | 68.603 | 0.000 |
| CiC5087-01 | 0 | 2 | 18 | 8 | 1 | 5.138 | 0.273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalt, R.; Prósper, L.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization. Agriculture 2022, 12, 273. https://doi.org/10.3390/agriculture12020273
Montalt R, Prósper L, Vives MC, Navarro L, Ollitrault P, Aleza P. Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization. Agriculture. 2022; 12(2):273. https://doi.org/10.3390/agriculture12020273
Chicago/Turabian StyleMontalt, Rafael, Laura Prósper, María Carmen Vives, Luis Navarro, Patrick Ollitrault, and Pablo Aleza. 2022. "Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization" Agriculture 12, no. 2: 273. https://doi.org/10.3390/agriculture12020273
APA StyleMontalt, R., Prósper, L., Vives, M. C., Navarro, L., Ollitrault, P., & Aleza, P. (2022). Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization. Agriculture, 12(2), 273. https://doi.org/10.3390/agriculture12020273






