Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Contaminated Mine Soil Collection
2.2. Stabilizing Agents
2.3. Stabilizing Experiments
2.4. Analyses of Chemical Fractions
2.5. X-ray Powder Diffraction (XRPD) Analysis
2.6. SEM-EDX Analyses
2.7. Physicochemical Analyses
3. Results and Discussion
3.1. Effectiveness of the Stabilization Treatment
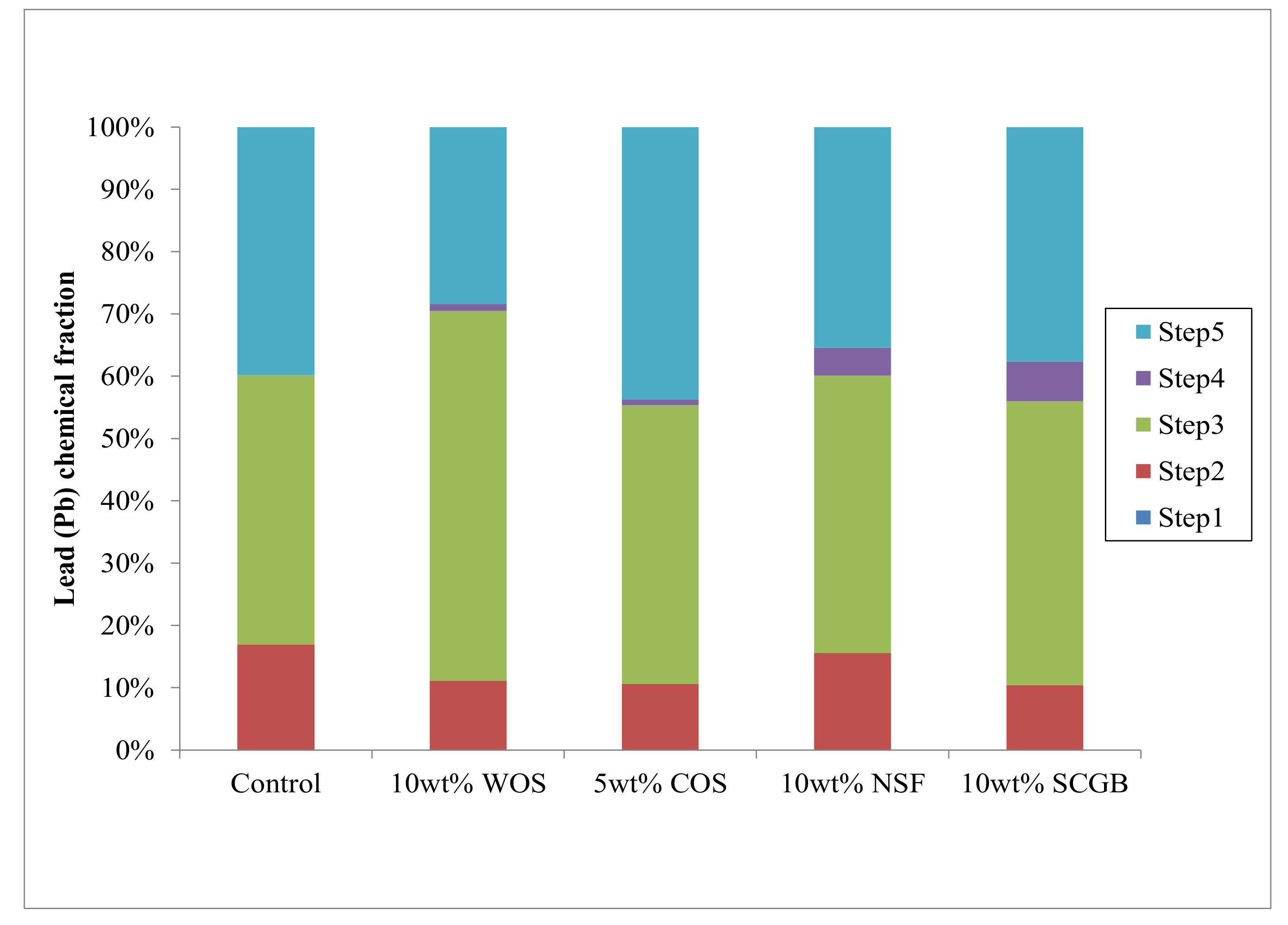
3.2. Sequential Extraction Results
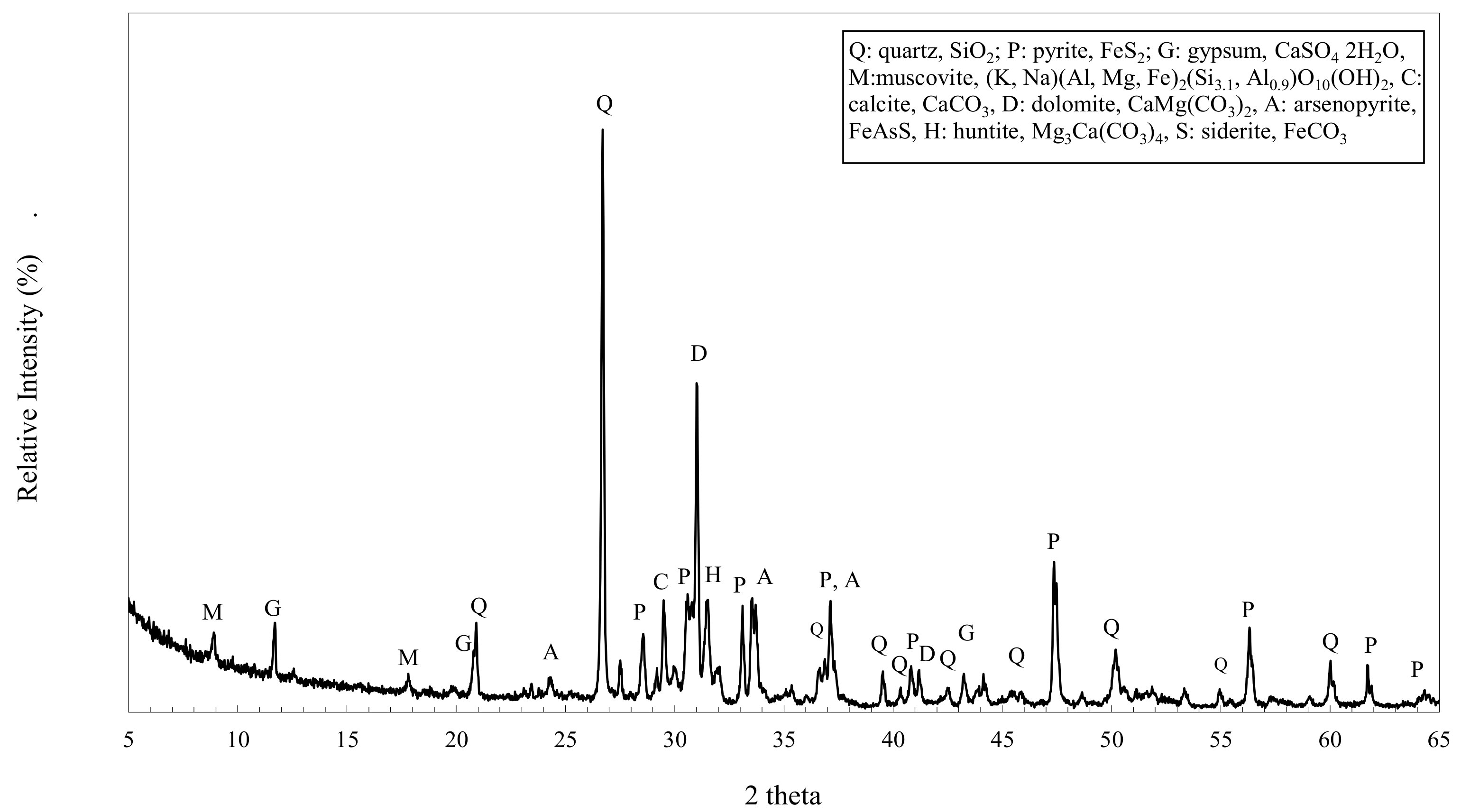
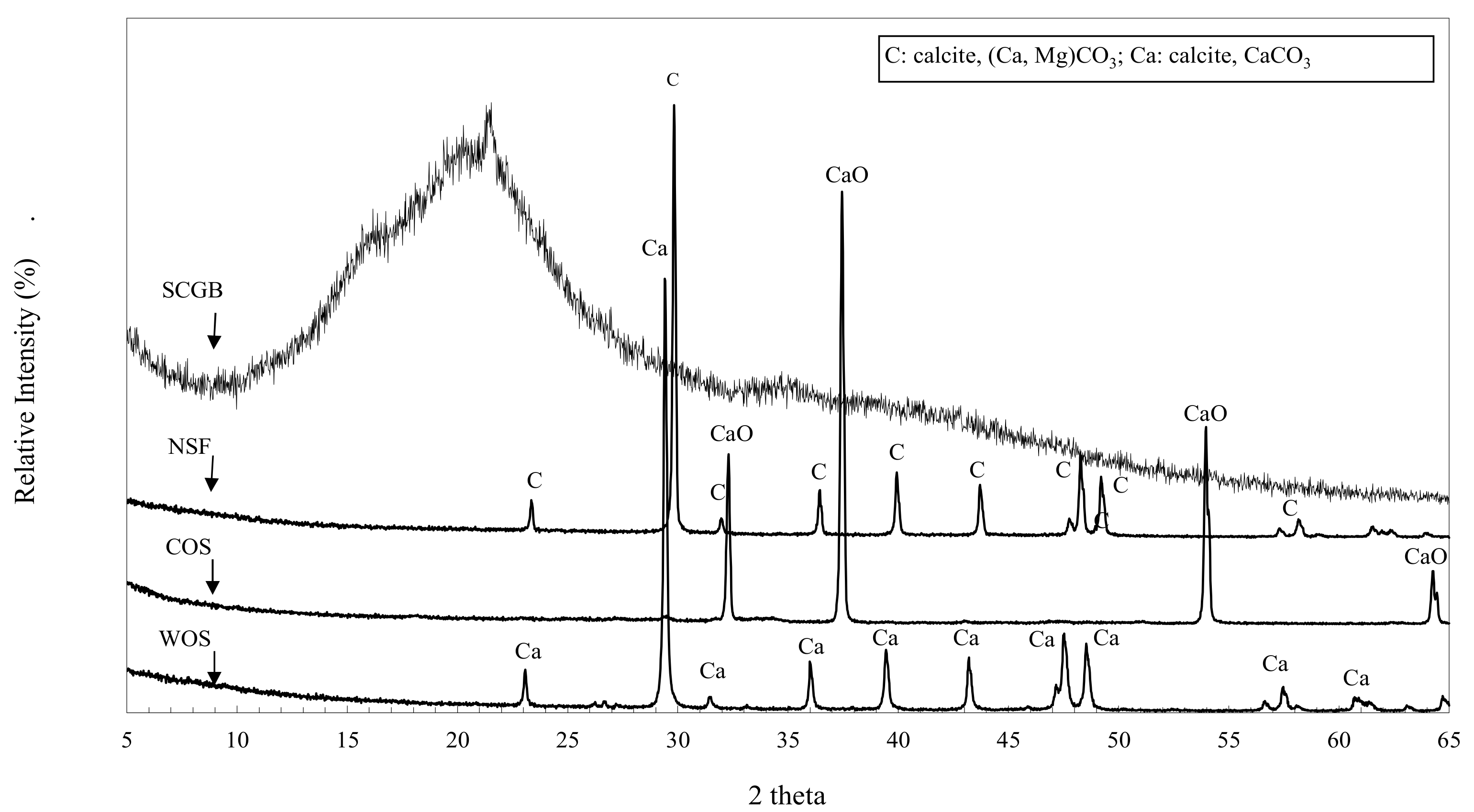
3.3. XRPD Analyses
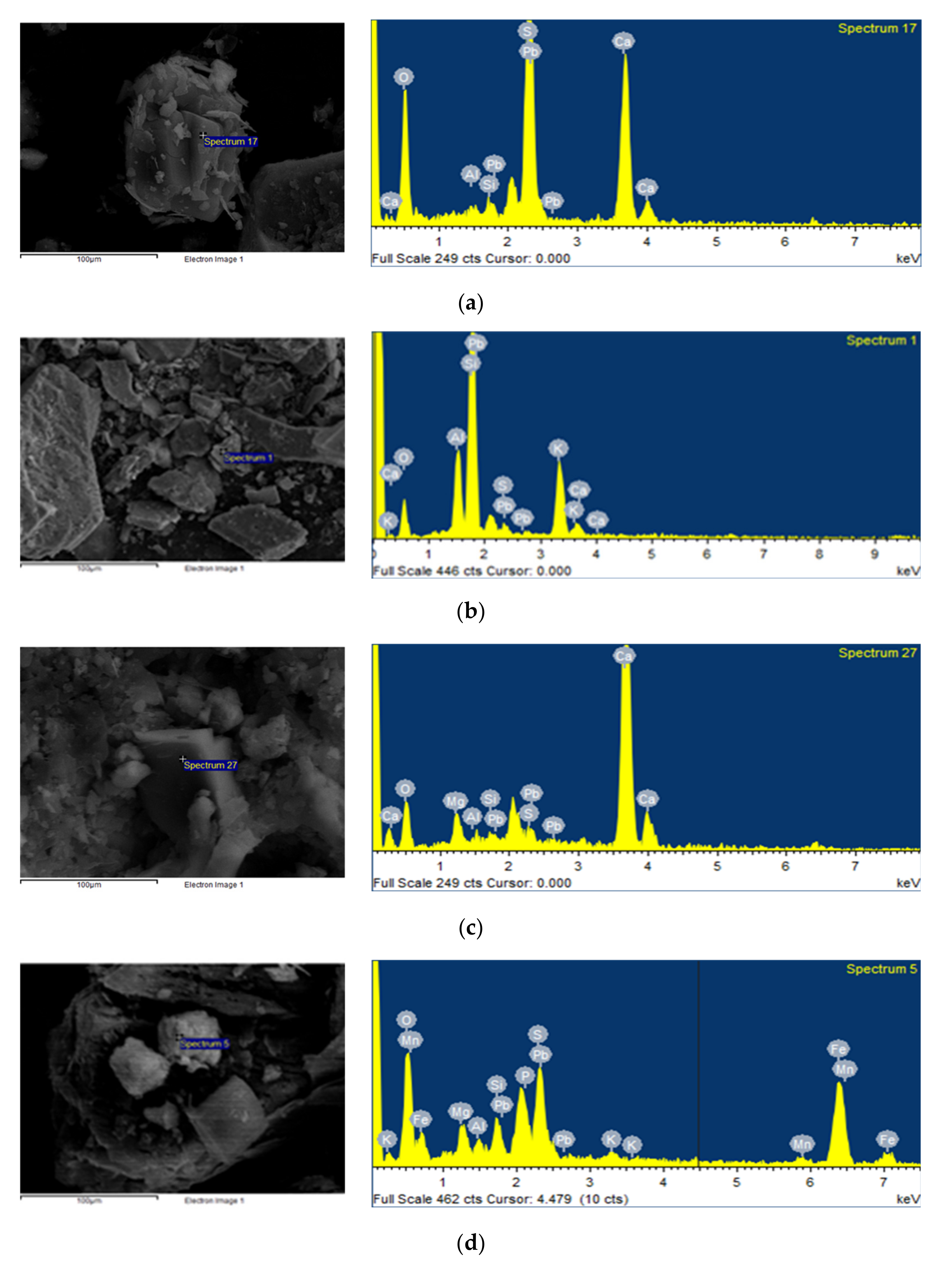
3.4. SEM-EDX Analysess
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.L.; Cross, W.H.; Chian, E.S.K.; Lai, J.S.; Giabbai, M.; Hung, C.H. Stabilization and solidification of lead in contaminated soils. J. Hazard. Mater. 1996, 48, 95–110. [Google Scholar] [CrossRef]
- Long, R.; Zhang, X. Treating lead-contaminated soil by stabilization and solidification. Transp. Res. Record. J. Transp. Res. Board 1998, 1615, 72–78. [Google Scholar] [CrossRef]
- Mine Reclamation Corp. 2013 Yearbook of MIRECO Statistics; MIRECO: Seoul, Korea, 2014; p. 242. (In Korean) [Google Scholar]
- Wang, S.Y.; Vipulanandan, C. Leachability of lead from solidified cement-fly ash binders. Cement Concrete Res. 1996, 26, 895–905. [Google Scholar] [CrossRef]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D.; Menounou, N. Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci. Total Environ. 2004, 330, 171–185. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D. An evaluation of lead leachability from stabilized/solidified soils under modified semi-dynamic leaching conditions. Eng. Geol. 2006, 85, 67–74. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D.; Grubb, D.G. The Effectiveness of Quicklime-Based Stabilization/Solidification on Lead (Pb) Containated Soils. In Environmental Geotechnics (5th ICEG); Thomas, H.R., Ed.; Thomas Telford Publishing: London, UK, 2006; Volume 1, pp. 221–228. [Google Scholar]
- Moon, D.H.; Dermatas, D. Arsenic and lead release from fly ash stabilized/solidified soils under modified semi-dynamic leaching conditions. J. Hazard. Mater. 2007, 141, 388–394. [Google Scholar] [CrossRef]
- Qiao, X.C.; Poon, C.S.; Cheeseman, C.R. Investigation into the stabilization/solidification performance of Portland cement through cement clinker phases. J. Hazard. Mater. 2007, 139, 238–243. [Google Scholar] [CrossRef]
- Moon, D.H.; Wazne, M.; Yoon, I.H.; Grubb, D.G. Assessment of cement kiln dust (CKD) for stabilization/solidification (S/S) of arsenic contaminated soils. J. Hazard. Mater. 2008, 159, 512–518. [Google Scholar] [CrossRef]
- Moon, D.H.; Grubb, D.G.; Reilly, T.L. Stabilization/solidification of selenium-impacted soils using Portland cement and cement kiln dust. J. Hazard. Mater. 2009, 168, 944–951. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.R.; Grubb, D.G.; Park, J.-H. An assessment of Portland cement, cement kiln dust and class C fly ash for the immobilization of Zn in contaminated soils. Environ. Earth Sci. 2010, 61, 1745–1750. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Khim, J.; Grubb, D.G.; Ko, I. Stabilization of Cu-contaminated army firing range soils using waste oyster shells. Environ. Geochem. Health 2011, 33, 159–166. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Koutsospyros, A.; Chang, Y.-Y.; Hyun, S.; Ok, Y.S.; Park, J.-H. Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 2362–2370. [Google Scholar] [CrossRef]
- Moon, D.H.; Hwang, I.; Koutsospyros, A.; Cheong, K.H.; Ok, Y.S.; Ji, W.H.; Park, J.-H. Stabilization of lead (Pb) and zinc (Zn) in contaminated rice paddy soil using starfish: A preliminary study. Chemosphere 2018, 190, 459–467. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, C.O.; Lee, C.H.; Lee, D.K.; Kim, P.J. Dynamics of heavy metals in soil amended with oyster shell meal. Korean J. Environ. Agric. 2005, 24, 358–363. [Google Scholar] [CrossRef][Green Version]
- Moon, D.H.; Park, J.-W.; Chang, Y.-Y.; Ok, Y.S.; Lee, S.S.; Ahmad, M.; Koutsopyros, A.; Park, J.-H.; Baek, K. Immobilization of lead in contaminated firing range soil using biochar. Environ. Sci. Pollut. Res. 2013, 20, 8464–8471. [Google Scholar] [CrossRef]
- Lim, J.E.; Sung, J.K.; Sarkar, B.; Wang, H.; Hashimoto, Y.; Tsang, D.C.W.; Ok, Y.S. Impact of natural and calcined starfish (Asterina pectinifera) on the stabilization of Pb, Zn and As in contaminated agricultural soil. Environ. Geochem. Health 2017, 39, 431–441. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Sorption of deisopropylatrazine on broiler litter biochars. J. Agric. Food Chem. 2010, 58, 12350–12356. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on decomposition of soil organic matter and plants residues as determined by 14C and enzyme activities. Eur. J. Soil Biol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Al-Wabel, M.I.; Lee, S.S. Impact of soybean stover- and pine needle-derived biochars on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef]
- Korea Ministry of Environment. Standard Methods of Soil Sampling and Analysis; Ministry of Environment: Gwacheon, Korea, 2009.
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soil. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- FitzPatrick, E.A. Soils: Their Formation, Classification and Distribution; Longman Science & Technical: London, UK, 1983; p. 353. [Google Scholar]
- MDI. Jade Version 7.1; Material’s Data Inc.: Livermore, CA, USA, 2005. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- ICDD. Powder Diffraction file.PDF-2 Database Release; International Centre for Diffraction Data: Newtown Square, PA, USA, 2002. [Google Scholar]
- Ministry of Environment (MOE). The Korean Standard Test (KST) Methods for Soils; Korean Ministry of Environment: Gwachun, Korea, 2010; p. 225. (In Korean)
- USEPA. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846, 3rd ed.; Method 1311; USEPA: Washington, DC, USA, 1992.
- Ok, Y.S.; Oh, S.E.; Ahmad, M.; Hyun, S.; Kim, K.R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, Y.; Tang, Y. Oyster shell powder for Pb(II) immobilization in both aquatic and sediment environments. Environ. Geochem. Health 2021, 43, 1891–1902. [Google Scholar] [CrossRef]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Gilchrist, J.D. Extraction Metallurgy, 3rd ed.; Pergamon Press: Oxford, UK, 1989; p. 145. [Google Scholar]
- Moon, D.H.; Wazne, M.; Cheong, K.H.; Chang, Y.-Y.; Baek, K.; Ok, Y.S.; Park, J.-H. Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ. Sci. Pollut. Res. 2015, 22, 11162–11169. [Google Scholar] [CrossRef]
- Zhao, X.L.; Masaihiko, S. Amelioration of cadmium polluted paddy soils by porous hydrated calcium silicate. Water Air Soil Pollut. 2007, 183, 309–315. [Google Scholar] [CrossRef]
- Ahmad, M.; Hashimoto, Y.; Moon, D.H.; Lee, S.S.; Ok, Y.S. Immobilization of lead in a Korean military shooting range soil using eggshell waste: An integrated mechanistic approach. J. Hazard. Mater. 2012, 209–210, 392–401. [Google Scholar] [CrossRef] [PubMed]
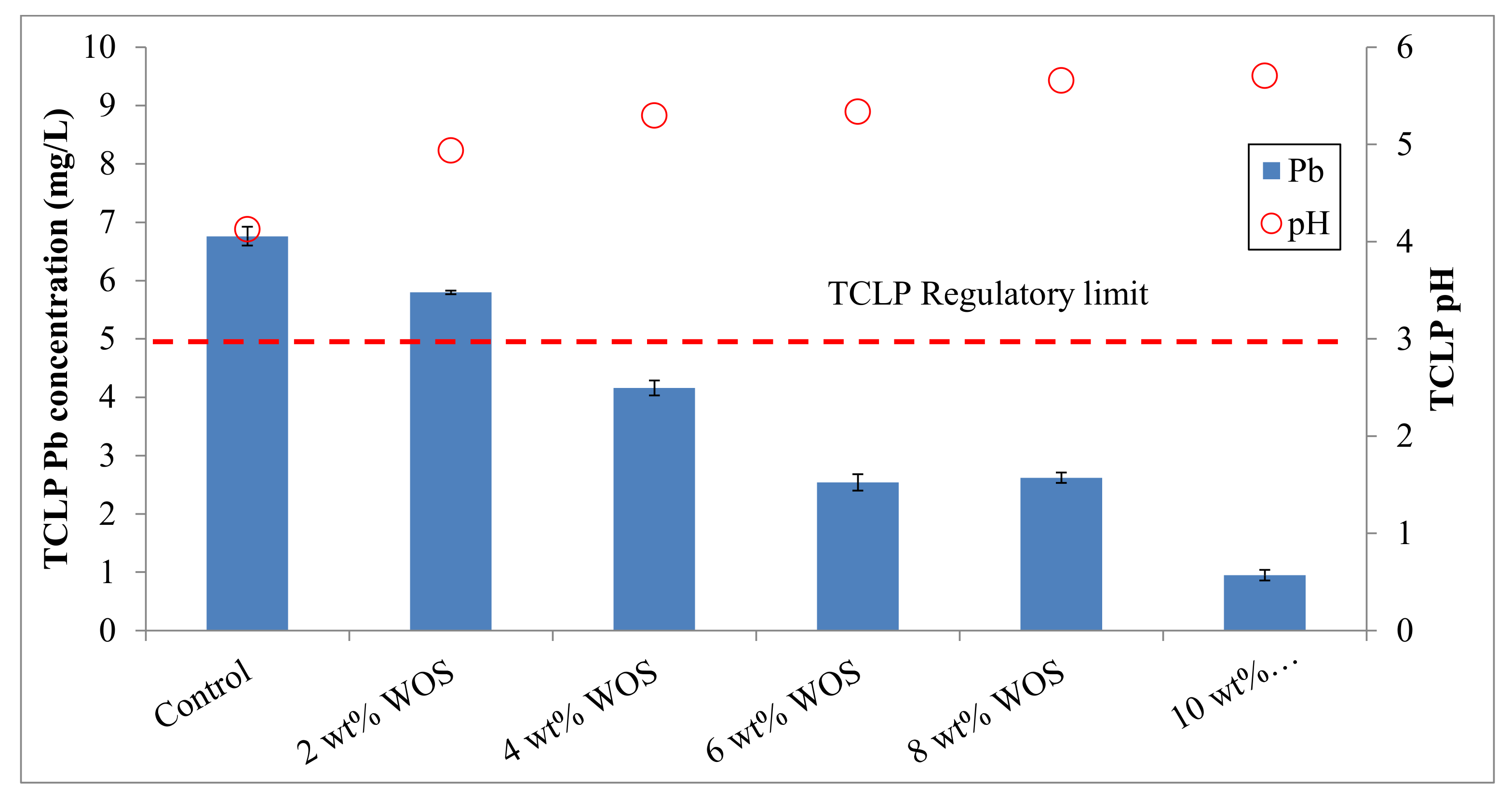
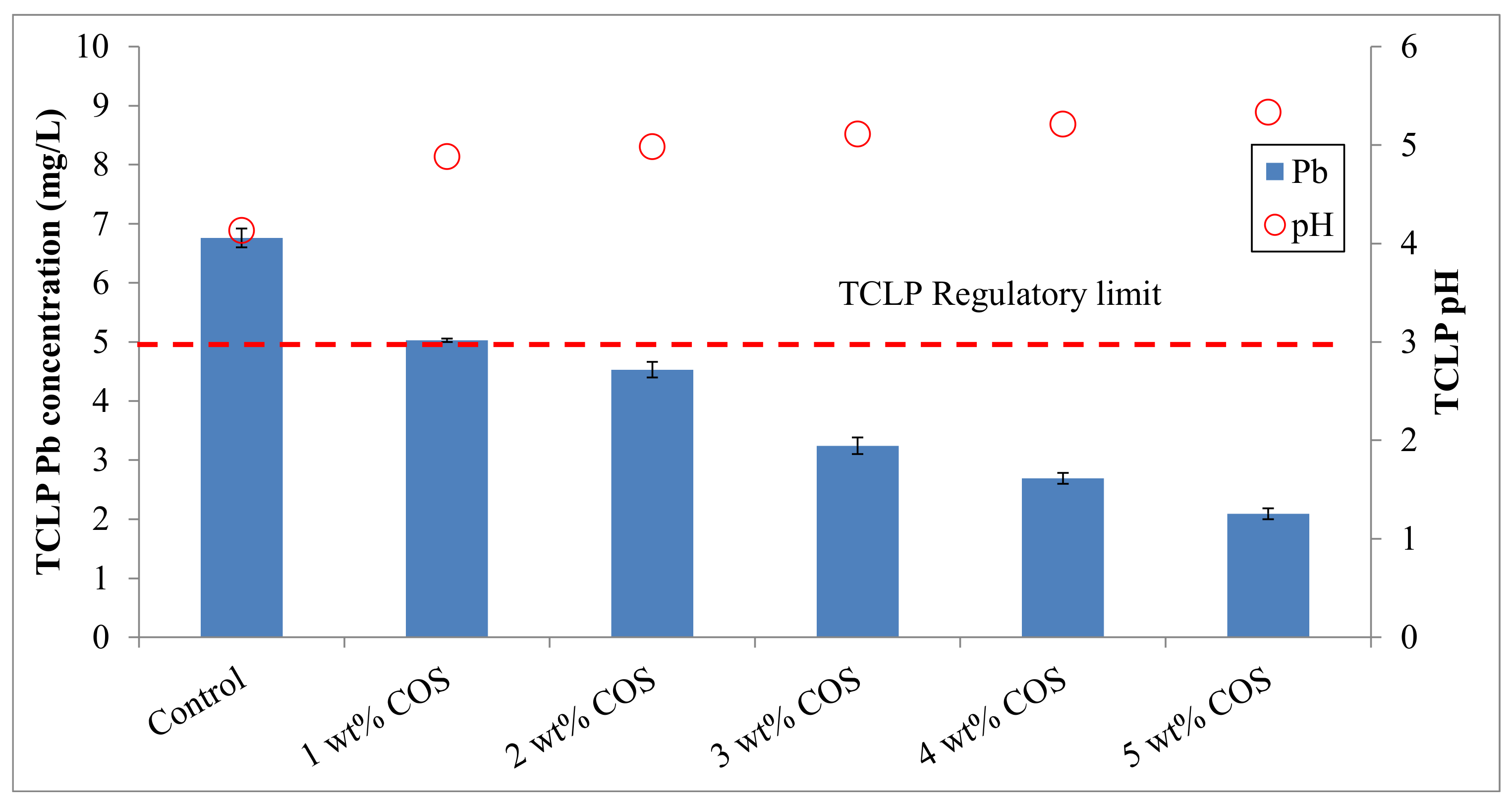
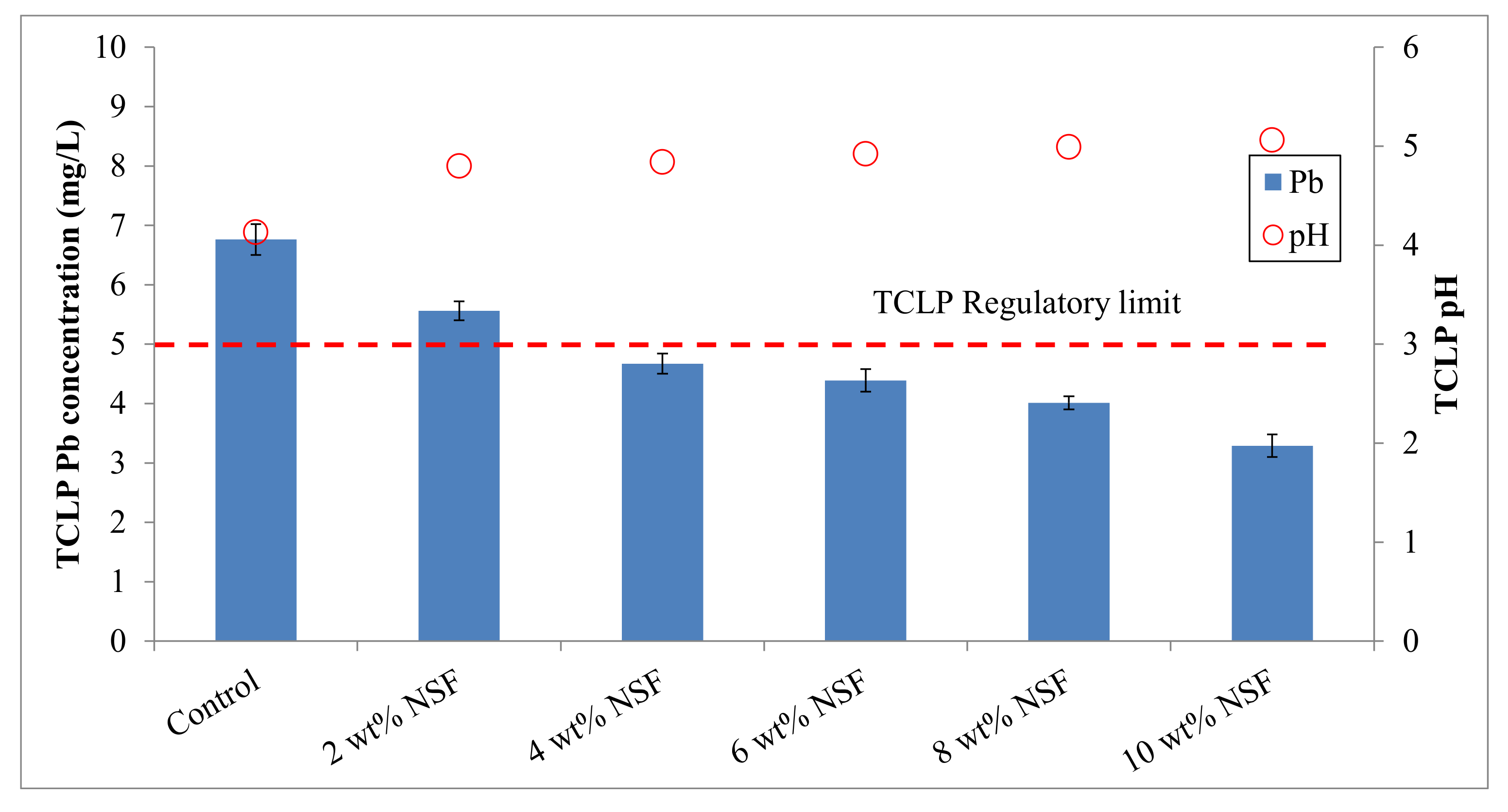
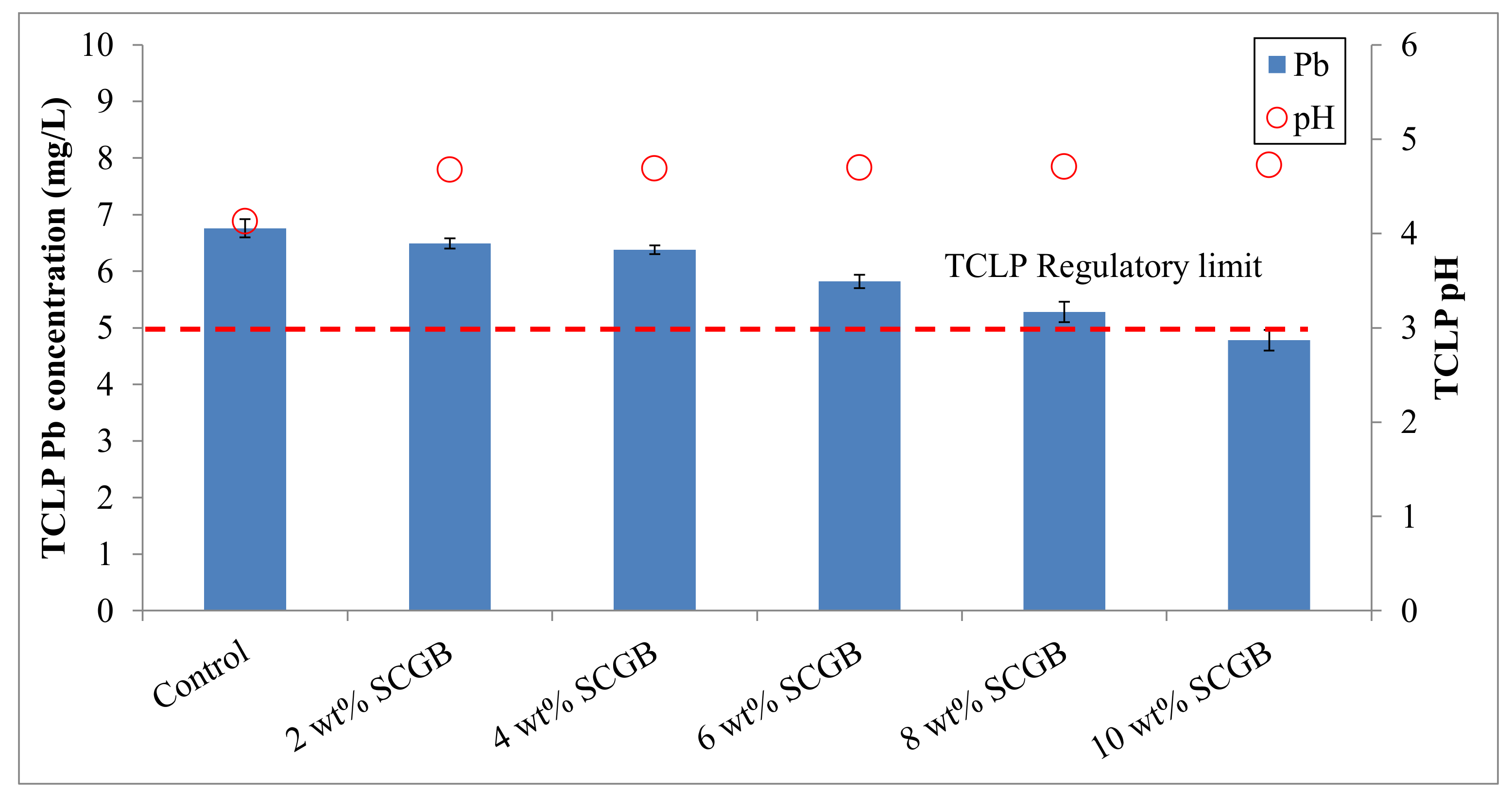
| Soil Properties | Contaminated Mine Soil | Korean Warning Standards 1 |
|---|---|---|
| pH (1:5) | 6.7 | |
| Organic matter content (%) 2 | 5.94 | |
| CEC (cmolc/kg) | 7.59 | |
| EC (dS/m) | 1.86 | |
| Composition (%) 3 | ||
| Sand | 47.2 | |
| Silt | 26.7 | |
| Clay | 26.1 | |
| Texture 4 | Sandy clay loam | |
| Total Pb (mg/kg) | 2800 | 200 |
| Major mineral compositions 5 | ||
| Quartz, Muscovite | ||
| Pyrite, Calcite | ||
| Gypsum, Dolomite | ||
| Arsenopyrite |
| Major Chemical Composition (%) | Contaminated Mine Soil | WOS | COS | NSF | Major Chemical Composition (wt%) | SCGB |
|---|---|---|---|---|---|---|
| SiO2 | 34.8 | 3.51 | 2.59 | 0.31 | C | 46.1 |
| Al2O3 | 8.62 | 1.36 | 0.96 | 0.14 | Mg | 3.83 |
| Na2O | 0.03 | 0.86 | 0.73 | 1.37 | P | 3.68 |
| MgO | 3.72 | 0.71 | 0.86 | 7.24 | K | 33.1 |
| K2O | 1.64 | 0.27 | 0.13 | 0.17 | Ca | 11.9 |
| CaO | 12.3 | 88.07 | 87.69 | 86 | Si | 0.15 |
| Fe2O3 | 22.3 | 0.53 | 0.40 | 0.06 | Fe | 0.53 |
| SO3 | 2.19 | 0.69 | 0.65 | 2.68 | ||
| MnO | 5.76 | 0.04 | 0.04 | 0.02 | ||
| pH (1:5) | 6.7 | 10.5 | 12.4 | 7.28 | 5.93 |
| Sample ID | Contaminated Mine Soil (wt%) | WOS/NSF/SCGB (wt%) | COS (wt%) | L:S Ratio |
|---|---|---|---|---|
| Control | 100 | 0 | 0 | 20:1 |
| 2 wt% WOS/NSF/SCGB | 100 | 2 | 0 | 20:1 |
| 4 wt% WOS/NSF/SCGB | 100 | 4 | 0 | 20:1 |
| 6 wt% WOS/NSF/SCGB | 100 | 6 | 0 | 20:1 |
| 8 wt% WOS/NSF/SCGB | 100 | 8 | 0 | 20:1 |
| 10 wt% WOS/NSF/SCGB | 100 | 10 | 0 | 20:1 |
| 1 wt% COS | 100 | 0 | 1 | 20:1 |
| 2 wt% COS | 100 | 0 | 2 | 20:1 |
| 3 wt% COS | 100 | 0 | 3 | 20:1 |
| 4 wt% COS | 100 | 0 | 4 | 20:1 |
| 5 wt% COS | 100 | 0 | 5 | 20:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.H.; Koutsospyros, A. Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials. Agriculture 2022, 12, 367. https://doi.org/10.3390/agriculture12030367
Moon DH, Koutsospyros A. Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials. Agriculture. 2022; 12(3):367. https://doi.org/10.3390/agriculture12030367
Chicago/Turabian StyleMoon, Deok Hyun, and Agamemnon Koutsospyros. 2022. "Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials" Agriculture 12, no. 3: 367. https://doi.org/10.3390/agriculture12030367
APA StyleMoon, D. H., & Koutsospyros, A. (2022). Stabilization of Lead-Contaminated Mine Soil Using Natural Waste Materials. Agriculture, 12(3), 367. https://doi.org/10.3390/agriculture12030367





