Germination and the Biochemical Response of Pumpkin Seeds to Different Concentrations of Humic Acid under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Design
2.2. Biochemical Tests
2.3. Measurement of Antioxidant Enzymes
2.4. Measurement of the Glyoxylate Cycle Enzymes
2.5. Measurement of Seed Reserve Utilization
2.6. Measurement of Lipid Content
2.7. Measurement of the Total Protein Content
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Germination
3.2. Antioxidant Enzymes
3.3. Glyoxylate Enzyme Activity
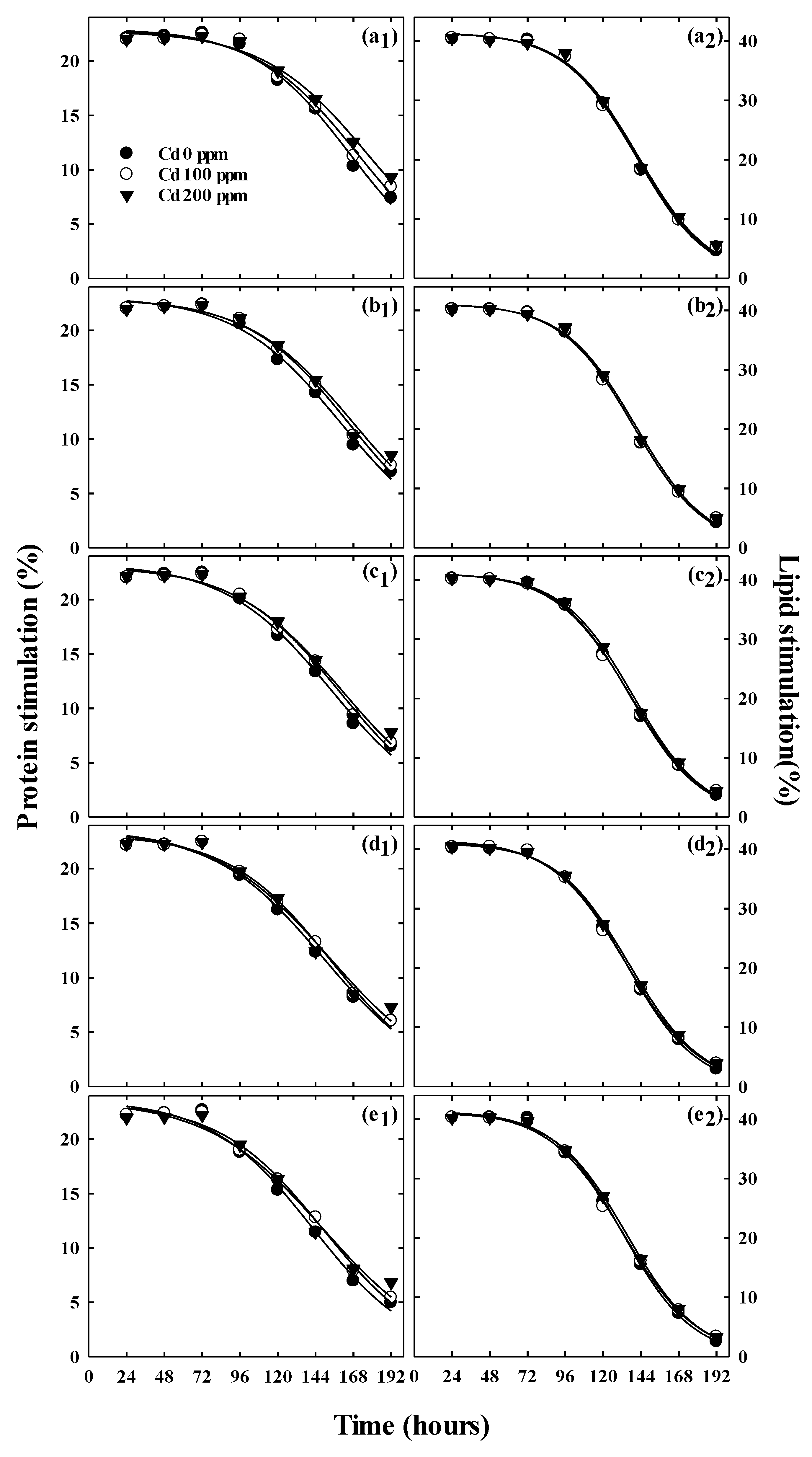
3.4. Utilization of the Seed Reserves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imran, M.A.; Sajid, Z.A.; Chaudhry, M.N. Arsenic (As) toxicity to germination and vegetative growth of sunflower (Helianthus annuus L.). Pol. J. Environ. Stud. 2015, 24, 1993–2002. [Google Scholar] [CrossRef]
- Khan, M.U.; Shahbaz, N.; Waheed, S.; Mahmood, A.; Shinwari, Z.K.; Malik, R.N. Comparative health risk surveillance of heavy metals via dietary foodstuff consumption in different land-use types of Pakistan. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 168–186. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Jones, D.L. Effect of composts, lime and diammonium phosphate on the phytoavailability of heavy metals in a copper mine tailing soil. Pedosphere 2009, 19, 631–641. [Google Scholar] [CrossRef]
- Gupta, S.K.; Scott, C.; Mitra, A. Advances in Land Resource Management for 21st Century; Soil Conservation Society of India: New Delhi, India, 2011; p. 446. [Google Scholar]
- Salazar, M.J.; Rodriguez, J.H.; Nieto, G.L.; Pignata, M.L. Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine max (L.) Merrill]. J. Hazard. Mater. 2012, 233, 244–253. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, X.; Mu, Y.; Cheng, Y.; Ma, Q.; Nian, H.; Yang, C. Metal pollution (Cd, Pb, Zn, and As) in agricultural soils and soybean, glycine max, in southern China. Bull. Environ. Contam. Toxicol. 2014, 92, 427–432. [Google Scholar] [CrossRef]
- Rastgoo, L.; Alemzadeh, A.; Tale, A.M.; Tazangi, S.E.; Eslamzadeh, T. Effects of copper, nickel and zinc on biochemical parameters and metal accumulation in gouan, ‘Aeluropus littoralis’. Plant Knowl. J. 2014, 3, 42–49. [Google Scholar]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Tito, G.A.; Chaves, L.H.G.; Fernandes, J.D.; Monteiro, D.R.; de Vasconcelos, A.C.F. Effect of copper, zinc, cadmium and chromium in the growth of crambe. Agric. Sci. 2014, 5, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Aria, A.; Abbaspour, H.; Sar, S.S.; Sekineh, S.S.; Mohsen, D.G. Antioxidant enzymes functions of Vetiveria zizianoides during the absorption of cadmium in soil. Electron. J. Biol. 2017, 11, 3–4. [Google Scholar]
- Chaab, A.; Moezzi, A.; Olamabbas Sayyad, G.; Chorom, M. Alleviation of cadmium toxicity to maize by the application of humic acid and compost. Life Sci. J. 2016, 13, 56–63. [Google Scholar]
- Menon, P.; Joshi, N.; Joshi, A. Effect of heavy metals on seed germination of Trigonella foenum-graceum L. Int. J. Life-Sci. Sci. Res. 2016, 2, 488–493. [Google Scholar] [CrossRef]
- Macêdo, L.D.S.; Morril, W.B.B. Origem e comportamento dos metais fitotóxicos: Revisão da literatura. Tecnol. Ciênc. Agropecu. 2008, 2, 29–38. [Google Scholar]
- Pizzeghello, D.; Francioso, O.; Ertani, A.; Muscolo, A.; Nardi, S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 2013, 129, 70–75. [Google Scholar] [CrossRef]
- Angelova, V.R.; Akova, V.I.; Artinova, N.S.; Ivanov, K.I. The effect of organic amendments on soil chemical characteristics. Bulg. J. Agric. Sci. 2013, 19, 958–971. [Google Scholar]
- Topcuoglu, B. The influence of humic acids on the metal bioavailability and phytoextraction efficiency in long-term sludge applied soil. In Proceedings of the Conference on International Research on Food Security, Natural Resource Management and Rural Development, Gottingen, Germany, 19–21 September 2012. [Google Scholar]
- Zhang, R.; Zhou, L.; Zhang, F.; Ding, Y.; Gao, J.; Chen, J.; Yan, H.; Shao, W. Heavy metal pollution and assessment in the tidal flat sediments of Haizhou Bay, China. Mar. Pollut. Bull. 2013, 74, 403–412. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Kang, S.-H.; Imdad, A.; Lee, S.C.; Ji, M.G.; Jeong, S.Y.; Shin, G.-I.; Kim, M.G.; Jeon, J.-R.; Kim, W.-Y. Humic acid enhances heat stress tolerance via transcriptional activation of heat-shock proteins in Arabidopsis. Sci. Rep. 2020, 10, 15042–15053. [Google Scholar] [CrossRef]
- Samavatipour, P.; Abdossi, V.; Salehi, R.; Samava, S.; Moghadam, A.L. Investigation of morphological, phytochemical, and enzymatic characteristics of Anethum graveolens L. using selenium in combination with humic acid and fulvic acid. J. Appl. Biol. Biotechnol. 2019, 7, 69–74. [Google Scholar]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–275. [Google Scholar]
- Gales, D.C.; Jităreanu, G. The influence of humic fertilizer on soybean yield and economic efficiency in Moldavian Plateau. Bull. USAMV Ser. Agric. 2015, 72, 1. [Google Scholar]
- Klučáková, M.; Pavlíková, M. Lignitic humic acids as environmentally friendly adsorbent for heavy metals. J. Chem. 2017, 217, 1–5. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zoghalchali, H.B. The effects of humic substances on some physiological properties of Citrus sinensis cv. Thomson navel under lime condition. Int. Res. J. Appl. Basic Sci. 2015, 9, 132–135. [Google Scholar]
- Salman, S.R.; Abou-Hussein, S.D.; Abdel-Mawgoud, A.M.R.; El-Nemr, M.A. Fruit yield and quality of watermelon as affected by hybrids and humic acid application. J. Appl. Sci. Res. 2005, 1, 51–58. [Google Scholar]
- El-Hai, K.M.A.; El-Khateeb, A.Y.; Ghonie, A.A.; Saber, W.I.A. Comparative response of cantaloupe features to amino acids, humic acid and plant oils towards downy mildew disease. J. Biol. Sci. 2019, 19, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Carletti, P.; Masi, A.; Spolaore, B.; De Laureto, P.P.; De Zorzi, M.; Turetta, L.; Ferretti, M.; Nardi, S. Protein expression changes in maize roots in response to humic substances. J. Chem. Ecol. 2008, 34, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Bolandnazar, S.; Tabatabaei, S.J.; Pirdashti, H.; Arzanlou, M.; Ebrahimzadeh, M.A.; Fathi, H. Antioxidant properties of garlic as affected by selenium and humic acid treatments. N. Z. J. Crop Hortic. Sci. 2015, 43, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Konakçi, C.Ö.; Yildiztugay, E.; Elbasan, F.; Yildiztugay, A.; Küçüködük, M. Assessment of antioxdant system and enzyme/nonenzyme regulation related to ascorbate-glutathione cycle in ferulic acid-treated Triticum aestivum L. roots under boron toxicity. Turk. J. Botany 2020, 44, 47–61. [Google Scholar] [CrossRef]
- Sabzevari, S.; Khazaie, H.R.; Kafi, M. Study on the effects of humic acid on germination of four wheat cultivars (Triticun aestivum L.). J. Iran. Field Crop Res. 2010, 8, 473–480. [Google Scholar]
- Sharma, O.P. Plant Taxonomy; Tata McGraw-Hill Education Pvt. Ltd.: New York, NY, USA, 2010; p. 452. [Google Scholar]
- Tartoura, E.A.A.; El-Gamily, E.I.; El-Waraky, Y.B.A.; Kamel, M.K.Y. Effect of phosphorus fertilization and fruit thinning on seed production of summer squash plants 1-vegetative traits and leaves chlorophyll constituent. J. Plant Prod. 2014, 5, 1807–1816. [Google Scholar] [CrossRef] [Green Version]
- Fruhwirth, G.O.; Hermetter, A. Production technology and characteristics of Styrian pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2008, 110, 637–644. [Google Scholar] [CrossRef]
- Danilcenko, H.; Jariene, E.; Gajewski, M.; Cerniauskiene, J.; Kulaitiene, J.; Sawicka, B.; Aleknaviciene, P. Accumulation of elements in some organically grown alternative horticultural crops in Lithuania. Acta Sci. Pol. Hortorum Cultus 2011, 10, 23–31. [Google Scholar]
- Badr, S.E.A.; Shaaban, M.; Elkholy, Y.M.; Helal, M.H.; Hamza, A.S.; Masoud, M.S.; El Safty, M.M. Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita pepo L.) cultivated in Egyptian habitats. Nat. Prod. Res. 2011, 25, 1524–1539. [Google Scholar] [CrossRef]
- Jariene, E.; Danilcenko, H.; Jeznach, M. Heavy metal contamination of plant raw material intended for food. Fresenius Environ. Bull. 2015, 24, 224–227. [Google Scholar]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Sabra, A.; Adam, L.; Daayf, F.; Renault, S. Salinity-induced changes in caffeic acid derivatives, alkamides and ketones in three Echinacea species. Environ. Exp. Bot. 2012, 77, 234–241. [Google Scholar] [CrossRef]
- Aganchich, B.; Tahi, H.; Wahbi, S.; Elmodaffar, C.; Serraj, R. Growth, water relations and antioxidant defence mechanisms of olive (Olea europaea L.) subjected to Partial Root Drying (PRD) and Regulated Deficit Irrigation (RDI). Plant Biosyst. 2007, 141, 252–264. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Ranaldi, F.; Vanni, P.; Giachett, E. Multisite inhibition of Pinus pinea isocitrate lyase by phosphate. Plant Physiol. 2000, 124, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Cooper, T.G.; Beevers, H. Mitochondria and glyoxysomes from castor bean endosperm: Enzyme constituents and catalytic capacity. J. Biol. Chem. 1969, 244, 3507–3513. [Google Scholar] [CrossRef]
- Sedghi, M.; Tolouie, S. Effect of nano-zinc oxide and drought stress on the activity of hydrolytic enzymes and seed reserves mobilization of soybean (Glycine max. L.) cultivar Katul (DPX). Iran. J. Seed Sci. Res. 2018, 18, 1–17. [Google Scholar]
- Magomya, A.M.; Kubmarawa, D.; Ndahi, J.A.; Yebpella, G.G. Determination of plant proteins via the Kjeldahl method and amino acid analysis: A comparative study. Int. J. Sci. Technol. Res. 2014, 3, 68–72. [Google Scholar]
- Aydinalp, C.; Marinova, S. The effects of heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa). Bulg. J. Agric. Sci. 2009, 15, 347–350. [Google Scholar]
- Shah, S.S.; Fida, M.; Shafi, M.; Bakht, J.; Zhou, W. Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak. J. Bot. 2011, 43, 333–340. [Google Scholar]
- Liu, S.; Yang, C.; Xie, W.; Xia, C.; Fan, P. The effects of cadmium on germination and seedling growth of Suaeda salsa. Procedia Environ. Sci. 2012, 16, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Vijayaragavan, M.; Prabhahar, C.; Sureshkumar, J.; Natarajan, A.; Vijayarengan, P.; Sharavanan, S. Toxic effect of cadmium on seed germination, growth and biochemical contents of cowpea (Vigna unguiculata L.) plants. Int. Multidiscip. Res. J. 2011, 1, 1–6. [Google Scholar]
- Sfaxi-Bousbih, A.; Chaoui, A.; El Ferjani, E. Cadmium impairs mineral and carbohydrate mobilization during the germination of bean seeds. Ecotoxicol. Environ. Saf. 2010, 73, 1123–1129. [Google Scholar] [CrossRef]
- Smiri, M.; Chaoui, A.; Rouhier, N.; Gelhaye, E.; Jacquot, J.-P.; El Ferjani, E. Cadmium affects the glutathione/glutaredoxin system in germinating pea sees. Biol. Trace Elem. Res. 2011, 142, 93–105. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes During Seed Germination. In Advances in Seed Biology; Jimene-Lopez, J.C., Ed.; InTech: Vienna, Austria, 2017; pp. 141–166. [Google Scholar] [CrossRef] [Green Version]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Dau, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sofi, A.; Ebrahimi, M.; Shirmohammadi, E. Effect of humic acid on germination, growth, and photosynthetic pigments of Medicago sativa L. under salt stress. Ecopersia 2018, 6, 21–30. [Google Scholar]
- Khazaie, K.; Zadeh, M.; Khan, M.W.; Bere, P.; Gounari, F.; Dennis, K.; Blatner, N.R.; Owen, J.L.; Klaenhammer, T.R.; Mohamadzadeh, M. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl. Acad. Sci. USA 2012, 109, 10462–10467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, M.; Miri Karbasak, E. Investigation effect of humic acid on germination, seedling growth and photosynthesis pigments of medicinal plant Isabgol (Plantago ovata Forssk). Iran. J. Seed Sci. Res. 2016, 3, 35–46. [Google Scholar]
- Grobelak, A.; Świątek, J.; Murtaś, A.; Jaskulak, M. Cadmium-induced oxidative stress in plants, cadmium toxicity, and tolerance in plants: From physiology to remediation. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–231. [Google Scholar]
- Heidari, M.; Sarani, S. Effects of lead and cadmium on seed germination, seedling growth and antioxidant enzymes activities of mustard (Sinapis arvensis L.). ARPN J. Agric. Biol. Sci. 2011, 6, 44–47. [Google Scholar]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Hatata, M.M.; Abdel, A.A.L.E. Oxidative stress and antioxidant defense mechanisms in response to cadmium treatments. Am. -Eurasian J. Agric. Environ. Sci. 2008, 4, 655–669. [Google Scholar]
- Souguir, D.; Ferjani, E.; Ledoigt, G.; Goupil, P. Sequential effects of cadmium on genotoxicity and lipoperoxidation in Vicia faba roots. Ecotoxicology 2011, 20, 329–336. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef]
- Sethy, S.K.; Ghosh, S. Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; pp. 1–7. [Google Scholar]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Zhang, L. The effect of calcium chloride on growth, photosynthesis, and antioxidant response of Zoya japonica under drought conditions. PLoS ONE 2013, 8, e68214. [Google Scholar]
- Bunluesin, S.; Pokethitiyook, P.; Lanza, G.R.; Tyson, J.F.; Kruatrachue, M.; Xing, B.; Upatham, S. Influences of cadmium and zinc interaction and humic acid on metal accumulation in Ceratophyllum demersum. Water Air Soil Pollut. 2007, 180, 225–235. [Google Scholar] [CrossRef] [Green Version]
- De los Reyes, B.G.; Myers, S.J.; Mc Grath, J.M. Differential induction of glyoxylate cycle enzymes by stress as a marker for seedling vigor in sugar beet (Beta vulgaris L.). Mol. Genet. Genom. 2003, 269, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S.; Graham, S.; Graham, I.A. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem. Soc. Trans. 2005, 33, 380–383. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, Z.; Ying, Q.; Ma, Y.; Zang, Y.; Wang, H.; Yu, Y.; Zhu, Z. Glyoxylate cycle and reactive oxygen species metabolism are involved in the improvement of seed vigor in watermelon by exogenous GA3. Sci. Hortic. 2019, 247, 184–194. [Google Scholar] [CrossRef]
- Sedghi, M.; Nemat, A.; Esmaielpour, B. Effect of seed priming on germination and seedling growth of two medicinal plants under salinity. Emir. J. Food Agric. 2010, 22, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Brito, V.C.; de Almeida, C.P.; Barbosa, R.R.; Carosio, M.G.; Ferreira, A.G.; Fernandez, L.G.; de Castro, R.D.; Hilhorst, H.; Ligterink, W.; Ribeiro, P.R. Overexpression of Ricinus communis L. malate synthase enhances seed tolerance to abiotic stress during germination. Ind. Crop. Prod. 2020, 145, 112110. [Google Scholar] [CrossRef]
- Sidari, M.; Mallamaci, C.; Muscolo, A. Drought, salinity and heat differently affect seed germination of Pinus pinea. J. For. Res. 2008, 13, 326–330. [Google Scholar] [CrossRef]
- Yuenyong, W.; Sirikantaramas, S.; Qu, L.-J.; Buabooch, T. Isocitrate lyase plays important roles in plant salt tolerance. BMC Plant Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-H.; Walker, R.P.; Acheson, R.M.; Técsi, L.I.; Wingler, A.; Lea, P.J.; Leegood, R.C. Are isocitrate lyase and phosphoenolpyruvate carboxykinase involved in gluconeogenesis during senescence of barley leaves and cucumber cotyledons? Plant Cell Physiol. 2000, 41, 960–967. [Google Scholar] [CrossRef] [Green Version]
- Quartacci, M.F.; Cosi, E.; Navari-Izzo, F. Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J. Exp. Bot. 2001, 52, 77–84. [Google Scholar] [PubMed]
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signaling molecule. Physiology 2004, 19, 120–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Fath, A.; Bethke, P.; Beligni, V.; Jones, R. Active oxygen and cell death in cereal aleurone cells. J. Exp. Bot. 2002, 53, 1273–1282. [Google Scholar] [CrossRef]
- Sneideris, L.C.; Gavassi, M.A.; Campos, M.L.; D’Amico-Damiao, V.; Carvalh, R.F. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. An. Acad. Bras. Cienc. 2015, 87, 1847–1852. [Google Scholar] [CrossRef] [Green Version]

| Traits | Cd (mg.L−1) | Humic Acid (mg.L−1) | Models | Estimated Parameter | R2 | RMSE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 | a | b | X0 | ||||||||||
| 0 | 100 | 200 | 300 | 400 | |||||||||
| Germination % | 0 | 84.0 de | 88.0 c | 97.3 a | 92.0 b | 92.0 b | y = a × exp (−0.5 × ((x − x0)/b)2) | - | 94.6 | 522.3 | 261.7 | 0.776 | 3.33 |
| 100 | 84.0 de | 82.7 de | 92.0 b | 90.7 bc | 89.3 bc | y = a × exp (−0.5 × ((x − x0)/b)2) | - | 90.4 | 731.3 | 315.6 | 0.632 | 3.55 | |
| 200 | 81.3 e | 81.3 e | 81.3 e | 85.3 de | 85.3 de | y = y0 + b × x | 80.5 | - | 0.012 | - | 0.750 | 1.26 | |
| Vigor | 0 | 7.16 ef | 7.06 ef | 9.27 c | 10.78 b | 12.42 a | y = y0 + b × x | 6.49 | - | 0.014 | - | 0.942 | 0.64 |
| 100 | 5.52 g | 6.02 fg | 8.99 cd | 8.62 cd | 9.22 c | y = y0 + b × x | 5.67 | - | 0.010 | - | 0.807 | 0.89 | |
| 200 | 3.98 h | 5.28 g | 7.10 de | 7.13 de | 7.60 de | y = y0 + b × x | 4.40 | - | 0.009 | - | 0.878 | 0.61 | |
| SOD U/mg protein/min | 0 | 60.2 f | 62.5 d | 63.3 c | 64.5 b | 65.7 a | y = y0 + b × x | 60.6 | - | 0.013 | - | 0.969 | 0.42 |
| 100 | 56.0 j | 56.8 i | 57.2 h | 58.5 g | 61.3 e | y = y0 + b × x | 55.50 | - | 0.012 | - | 0.879 | 0.83 | |
| 200 | 55.3 k | 57.5 h | 58.1 g | 60.3 f | 63.3 c | y = y0 + b × x | 55.14 | - | 0.019 | - | 0.958 | 0.71 | |
| CAT U/mg protein/min | 0 | 16.3 de | 16.7 d | 17.7 c | 18.2 b | 18.8 a | y = y0 + b × x | 16.24 | - | 0.006 | - | 0.984 | 0.14 |
| 100 | 15.1 g | 15.8 f | 16.7 d | 17.4 c | 18.2 b | y = y0 + b × x | 15.08 | - | 0.008 | - | 0.999 | 0.05 | |
| 200 | 14.6 h | 15.0 g | 16.0 ef | 17.3 c | 18.3 b | y = y0 + b × x | 14.3 | - | 0.010 | - | 0.975 | 0.28 | |
| POX U/mg protein/min | 0 | 79.33 e | 80.57 d | 81.80 c | 82.67 b | 84.40 a | y = y0 + b × x | 79.3 | - | 0.012 | - | 0.991 | 0.21 |
| 100 | 71.57 j | 73.60 i | 76.70 h | 78.33 f | 80.30 d | y = y0 + b × x | 71.66 | - | 0.022 | - | 0.990 | 0.41 | |
| 200 | 64.33 m | 67.33 l | 69.40 k | 73.43 i | 77.43 g | y = y0 + b × x | 63.92 | - | 0.032 | - | 0.985 | 0.71 | |
| ICL U/mg protein/min | 0 | 1.21 d | 1.48 c | 1.63 b | 1.67 b | 1.85 a | y = y0 + b × x | 1.274 | - | 0.001 | - | 0.941 | 0.06 |
| 100 | 1.00 hi | 1.06 fgh | 1.09 e–h | 1.15 def | 1.18 de | y = y0 + b × x | 1.006 | - | 0.0004 | - | 0.987 | 0.01 | |
| 200 | 0.82 j | 0.91 ij | 1.04 gh | 1.13 d–g | 1.18 de | y = y0 + b × x | 0.828 | - | 0.0009 | - | 0.980 | 0.02 | |
| MS U/mg protein/min | 0 | 2.58 e | 2.68 d | 2.82 c | 2.94 b | 3.13 a | y = y0 + b × x | 2.55 | - | 0.001 | - | 0.988 | 0.02 |
| 100 | 1.90 i | 2.12 h | 2.21 h | 2.44 f | 2.54 e | y = y0 + b × x | 1.92 | - | 0.002 | - | 0.981 | 0.04 | |
| 200 | 1.36 k | 1.44 k | 1.61 j | 1.86 i | 2.31 g | y = y0 + b × x | 1.25 | - | 0.002 | - | 0.916 | 0.12 | |
| Parameters | Cd (mg.L−1) | Humic Acid (mg.L−1) | Models | Y0 | b1 | b2 | X0 | R2 | RMSE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | 400 | |||||||||
| a | 0 | 23.0 b | 23.1 ab | 23.3 ab | 23.6 a | 23.6 a | y = y0 + b × x | 22.9 | - | 0.002 | - | 0.975 | 0.05 |
| 100 | 22.9 b | 22.9 b | 23.0 b | 23.2 ab | 23.4 ab | y = y0 + b × x | 22.8 | - | 0.001 | - | 0.947 | 0.05 | |
| 200 | 22.8 b | 22.9 b | 23.2 ab | 23.7 a | 23.6 a | y = y0 + b × x | 22.7 | - | 0.002 | - | 0.888 | 0.15 | |
| b | 0 | −30.6 b | −33.2 ab | −33.7 ab | −34.7 ab | −32.6 ab | y = y0 + a × x + b × x2 | −30.5 | −0.030 | 0.0062 | - | 0.927 | 0.57 |
| 100 | −32.1 ab | −32.2 ab | −32.8 ab | −32.4 ab | −32.9 ab | y = y0 + b × x | −32.1 | - | −0.002 | - | 0.655 | 0.24 | |
| 200 | −33.2 ab | −33.4 ab | −34.4 ab | −36.4 a | −36.4 a | y = y0 + a × x + b × x2 | −32.9 | −0.008 | 0.0040 | - | 0.914 | 0.65 | |
| X0 | 0 | 165.6 bc | 159.7 bcd | 154.0 cd | 149.1 de | 142.2 e | y = y0 + b × x | 165.6 | - | −0.057 | - | 0.998 | 0.50 |
| 100 | 170.7 ab | 165.0 bc | 159.4 cd | 153.6 cd | 148.9 de | y = y0 + b × x | 170.5 | - | −0.055 | - | 0.999 | 0.35 | |
| 200 | 177.2 a | 168.1 bc | 161.1 bcd | 152.8 cd | 148.5 de | y = y0 + b × x | 176.0 | - | −0.073 | - | 0.988 | 1.45 | |
| Parameters | Cd (mg.L−1) | Humic Acid (mg.L−1) | Models | Y0 | a | b1 | X0 | R2 | RMSE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | 400 | |||||||||
| a | 0 | 41.35 a | 41.17 ab | 41.09 b | 41.00 b | 41.36 a | y = y0 + a × exp (−0.5 × ((x − x0)/b)2) | 40.1 | 41.78 | 534.9 | 209.8 | 0.833 | 0.130 |
| 100 | 41.47 a | 41.21 ab | 41.17 ab | 41.32 a | 41.46 a | y = y0 + a × exp (−0.5 × ((x − x0)/b)2) | 38.5 | 41.53 | 108.6 | 181.0 | 0.963 | 0.053 | |
| 200 | 41.39 a | 41.11 ab | 41.06 b | 41.05 b | 41.15 ab | y = y0 + a × exp (−0.5 × ((x − x0)/b)2) | 41.0 | 41.51 | 452.9 | 247.2 | 0.969 | 0.049 | |
| b | 0 | −22.42 b | −22.88 ab | −22.69 ab | −22.11 b | −22.10 b | ns | - | - | - | ns | ns | |
| 100 | −23.21 a | −23.27 a | −23.50 a | −23.59 a | −23.64 a | y = y0 + b × x | −23.20 | - | −0.001 | - | 0.942 | 0.054 | |
| 200 | −23.05 a | −22.95 ab | −22.71 ab | −23.25 a | −22.77 ab | ns | - | - | - | ns | ns | ||
| X0 | 0 | 140.8 a | 139.1 a | 137.0 ab | 135.2 b | 132.7 b | y = y0 + b × x | 140.9 | - | −0.020 | - | 0.996 | 0.239 |
| 100 | 140.6 a | 139.2 a | 137.2 ab | 134.6 b | 132.8 b | y = y0 + b × x | 140.9 | - | −0.020 | - | 0.991 | 0.348 | |
| 200 | 142.0 a | 140.7 a | 139.0 a | 136.6 ab | 135.1 b | y = y0 + b × x | 142.2 | - | −0.018 | - | 0.991 | 0.320 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadi Aghbolaghi, M.; Sedghi, M.; Sharifi, R.S.; Dedicova, B. Germination and the Biochemical Response of Pumpkin Seeds to Different Concentrations of Humic Acid under Cadmium Stress. Agriculture 2022, 12, 374. https://doi.org/10.3390/agriculture12030374
Asadi Aghbolaghi M, Sedghi M, Sharifi RS, Dedicova B. Germination and the Biochemical Response of Pumpkin Seeds to Different Concentrations of Humic Acid under Cadmium Stress. Agriculture. 2022; 12(3):374. https://doi.org/10.3390/agriculture12030374
Chicago/Turabian StyleAsadi Aghbolaghi, Masoumeh, Mohammad Sedghi, Raouf Seyed Sharifi, and Beata Dedicova. 2022. "Germination and the Biochemical Response of Pumpkin Seeds to Different Concentrations of Humic Acid under Cadmium Stress" Agriculture 12, no. 3: 374. https://doi.org/10.3390/agriculture12030374





