Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Soil Physicochemical Analysis
2.3. DNA Extraction, PCR and Sequencing
2.4. Bioinformatics and Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
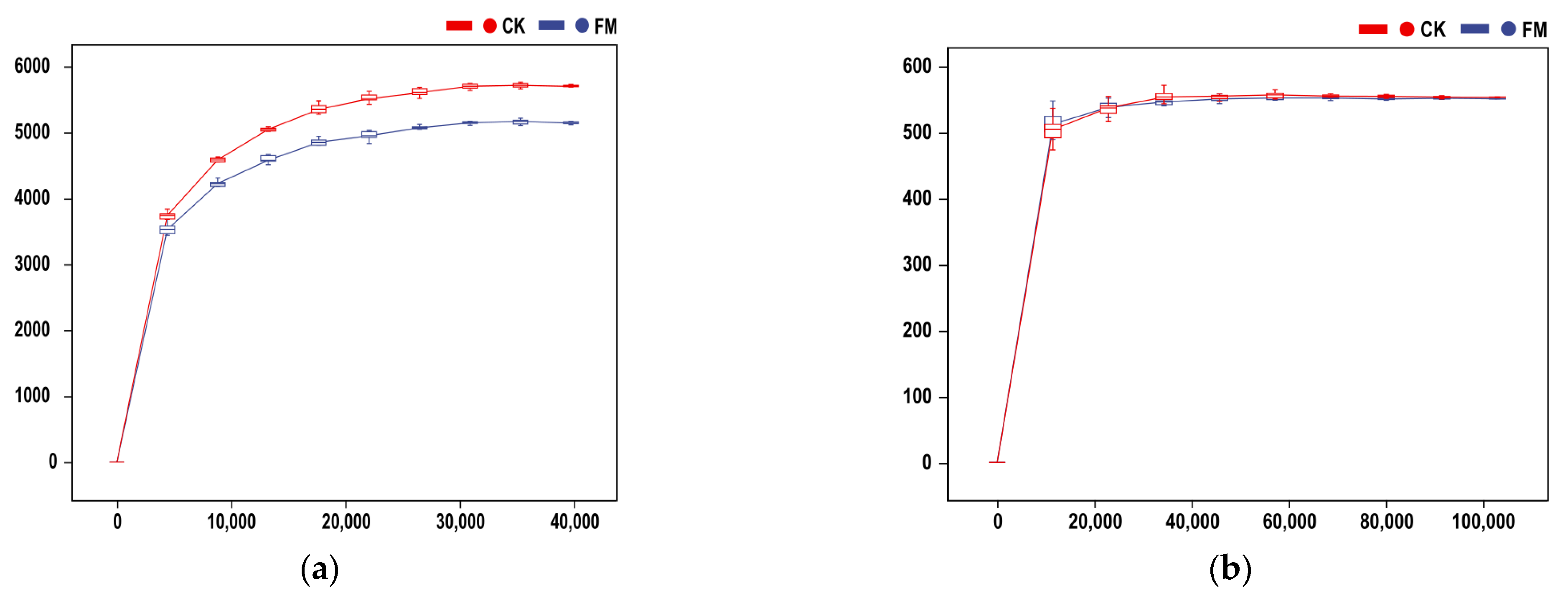
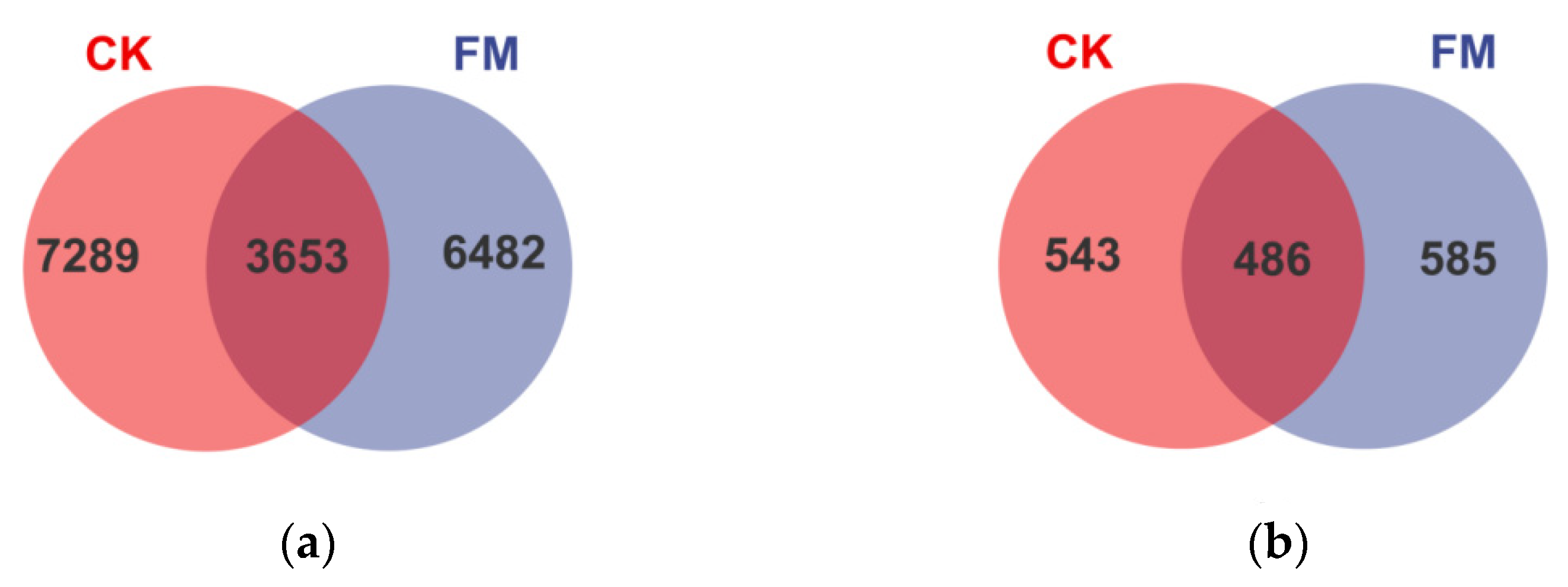
3.2. Illumina MiSeq Sequencing Data
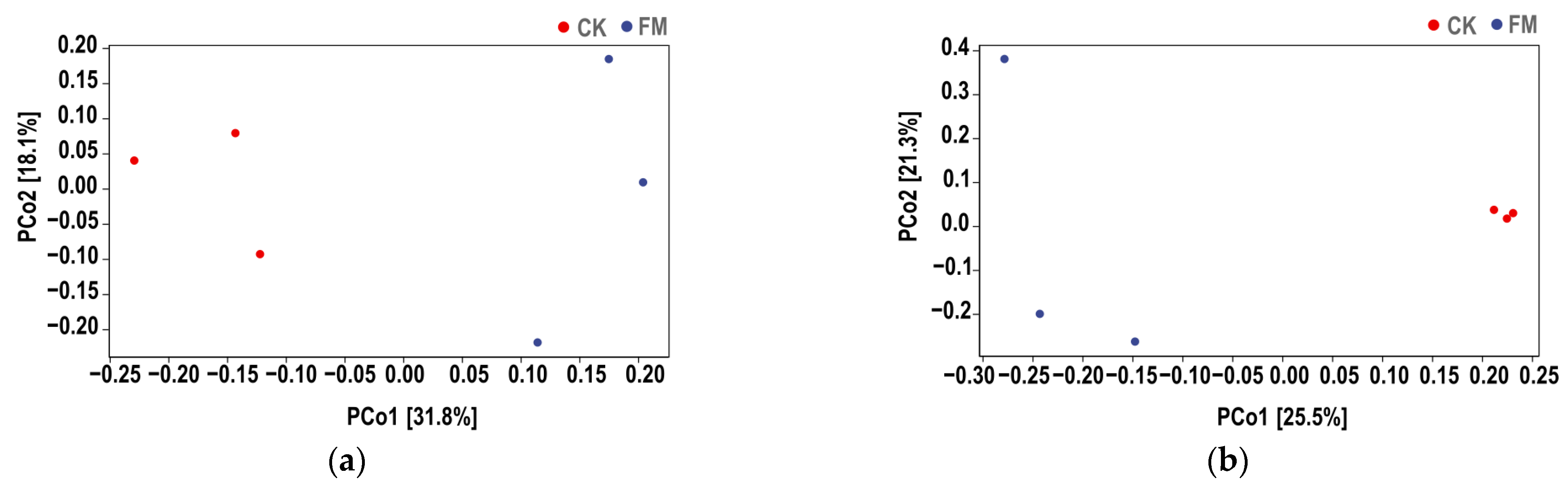
3.3. Soil Bacterial and Fungal Community Diversity
3.4. Composition of the Bacterial and Fungal Communities
3.5. Co-Occurrence Network of the Soil Bacterial and Soil Fungal Communities
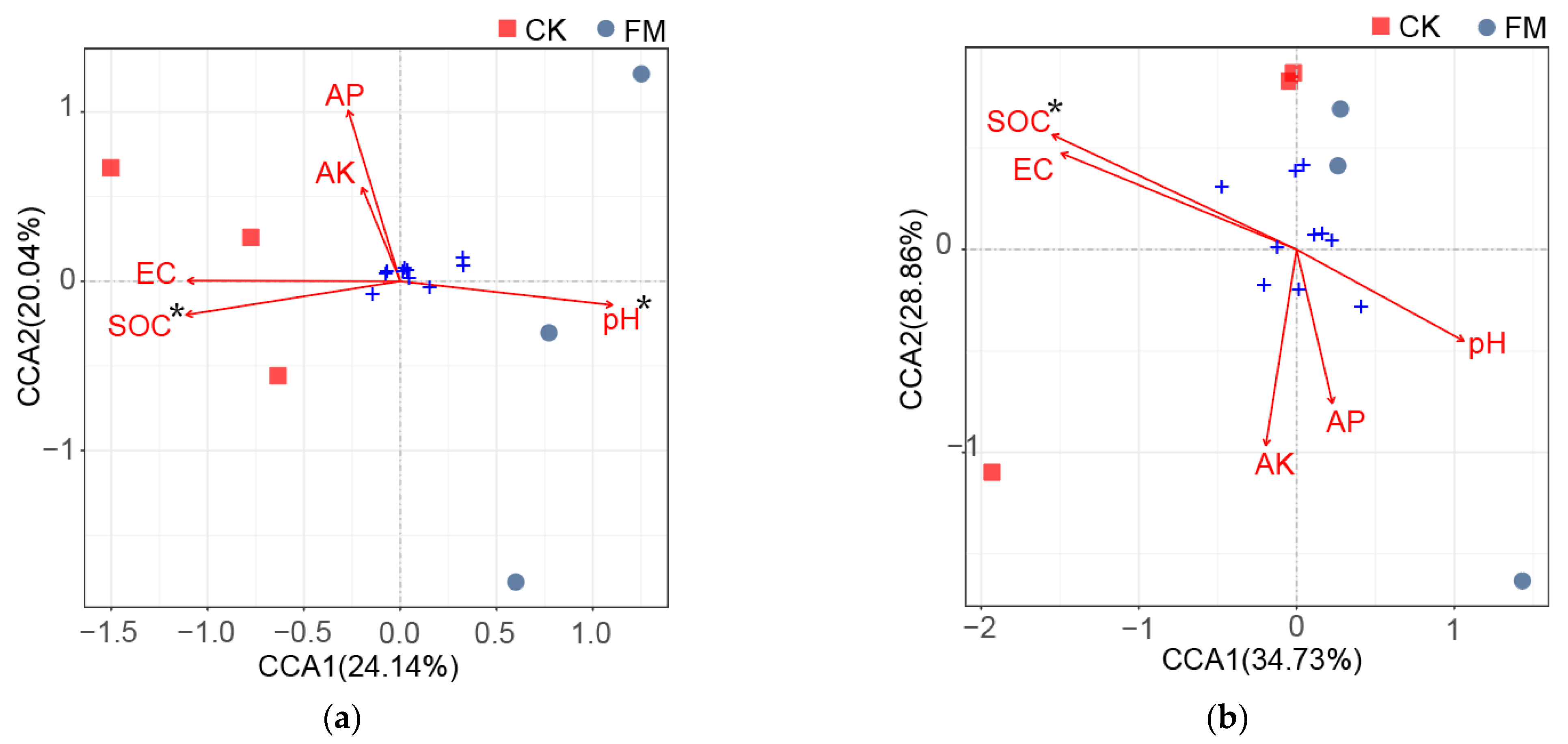
3.6. Soil Physicochemical Properties and Their Relationship with the Soil Microbial Community
3.7. Bacterial Community and Metabolic Prediction during PPC Film Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Sample | Group 1 | Group 2 | Sample Size | Permutations | pseudoF | p Value | q Value |
|---|---|---|---|---|---|---|---|
| Bacteria | all | - | 6 | 999 | 1.792229 | 0.091 | - |
| FM | CK | 6 | 999 | 1.792229 | 0.097 | 0.097 | |
| Fungus | all | - | 6 | 999 | 1.484782 | 0.224 | - |
| FM | CK | 6 | 999 | 1.484782 | 0.205 | 0.205 |
| Bacteria | Fungus | ||||
|---|---|---|---|---|---|
| FM | CK | FM | CK | ||
| Subgroup_6 | 0.0591 | 0.0489 | Tausonia | 0.2061 | 0.2198 |
| KD4-96 | 0.0443 | 0.0479 | Fusarium | 0.0802 | 0.0697 |
| Rubrobacter | 0.0419 | 0.0457 | Talaromyces | 0.0111 | 0.0759 |
| Blastococcus | 0.0380 | 0.0381 | Didymella | 0.0327 | 0.0375 |
| 67-14 | 0.0362 | 0.0364 | Gibberella | 0.0331 | 0.0345 |
| MB-A2-108 | 0.0408 | 0.0270 | Solicoccozyma | 0.0342 | 0.0288 |
| JG30-KF-CM45 | 0.0291 | 0.0261 | Mortierella | 0.0313 | 0.0240 |
| Gaiella | 0.0257 | 0.0240 | Pseudogymnoascus | 0.0331 | 0.0204 |
| Solirubrobacter | 0.0219 | 0.0263 | Laetisaria | 0.0000 | 0.0308 |
| RB41 | 0.0159 | 0.0168 | Myrmecridium | 0.0042 | 0.0218 |
References
- Sun, D.; Li, H.; Wang, E.; He, W.; Hao, W. An overview of the use of plastic-film mulching in China to increase crop yield and water-use efficiency. Natl. Sci. Rev. 2020, 7, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Li, X.; Zhou, M.; Li, H.J.; Zhao, Y. Plastic film mulching on soil water and maize (Zea mays L.) yield in a ridge cultivation system on Loess Plateau of China. Soil Sci. Plant Nutr. 2016, 621, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zong, R.; Han, H.; Li, Q. Grain yield and water-use efficiency of summer maize in response to mulching with different plastic films in the North China Plain. Exp. Agric. 2021, 571, 1–12. [Google Scholar] [CrossRef]
- Gao, H.; Yan, C.; Liu, Q.; Ding, W.; Chen, B. Effects of plastic mulching and plastic residue on agricultural production: A meta-analysis. Sci. Total Environ. 2018, 651, 484–492. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 99, 091001. [Google Scholar] [CrossRef] [Green Version]
- Thomas, Z.M.; Arno, S.; Frederick, N.T.; Rebekka, B.; Dagmar, W. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 47, 9024. [Google Scholar]
- Rillig, M.C.; Lehmann, A.; Machado, A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 2233, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Dick, R.P.; Myrold, D.D.; Kerle, E.A. Microbial Biomass and Soil Enzyme Activities in Compacted and Rehabilitated Skid Trail Soils. Soil Sci. Soc. Am. J. 1988, 52, 512–516. [Google Scholar] [CrossRef]
- Chen, X.; Guo, S.; Jingkuan, W.; Jian, Z. Effect of mulching cultivation with plastic film on soil microbial population and biological activity. Chin. J. Appl. Ecol. 1998, 4, 435–439. [Google Scholar]
- Jiang, X.J.; Liu, W.; Wang, E.; Zhou, T.; Xin, P. Residual plastic mulch fragments effects on soil physical properties and water flow behavior in the Minqin Oasis, northwestern China—ScienceDirect. Soil Tillage Res. 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, C.; Yan, C.; Mao, L.; Liu, Q. Effects of agricultural plastic film residues on transportation and distribution of water and nitrate in soil. Chemosphere 2020, 242, 125131. [Google Scholar]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G. Performance and environmental impact of biodegradable polymers as agricultural mulching films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Analysis on the Development and Application of Biodegradable Polymers. IOP Conf. Ser. Earth Environ. Sci. 2021, 6471, 012156. [Google Scholar] [CrossRef]
- Sreejata, B.; Lluis, M.C.; Pelacho, A.M.; De Bruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar]
- Bandopadhyay, S.; Sintim, H.Y.; Debruyn, J.M. Effects of biodegradable plastic film mulching on soil microbial communities in two agroecosystems. PeerJ 2020, 8, 9015. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T. Synthesis and properties of CO 2 -based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Liang, X.; Guo, X.; Feng, J. A Novel One-Pot Synthesis of Poly(Propylene Carbonate) Containing Cross-Linked Networks by Copolymerization of Carbon Dioxide, Propylene Oxide, Maleic Anhydride, and Furfuryl Glycidyl Ether. Polymers 2019, 115, 881. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, H.; Inoue, S. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. Part A Polym. Chem. 2004, 4222, 5561–5573. [Google Scholar] [CrossRef]
- Xiaoting, Y.; Jinggui, W.; Jianming, L. Study on degradation characteristics of PPC plastic film and ordinary plastic film in semi-arid soil. Environ. Sci. Technol. 2020, 4311, 9. [Google Scholar]
- Xinyuan, B.; Jianmin, Y.; Weiwei, Y.; Yang, G.; Huijie, L. Experiment of PPC biodegradable water seepage mulch covering sorghum. Shanxi Agric. Sci. 2020, 48, 4. [Google Scholar]
- Bai, Y.; Mueller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Tian, L.; Nasir, F.; Chang, J.; Tian, C. Impacts of replanting American ginseng on fungal assembly and abundance in response to disease outbreaks. Arch. Microbiol. 2021, 203, 2157–2170. [Google Scholar] [CrossRef]
- Luo, S.; Tian, L.; Chang, C.; Wang, S.; Zhang, J. Grass and maize vegetation systems restore saline—Sodic soils in the Songnen Plain of northeast China. Land Degrad. Dev. 2018, 29, 1107–1119. [Google Scholar] [CrossRef]
- Shi, S.; Lei, T.; Nasir, F.; Bahadur, A.; Tian, C. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2018, 135, 16–24. [Google Scholar] [CrossRef]
- Tian, L.; Shi, S.; Sun, Y.; Tran, L.S.P.; Tian, C. The compositions of rhizosphere microbiomes of wild and cultivated soybeans changed following the hybridization of their F1 and F2 generations—ScienceDirect. Eur. J. Soil Biol. 2020, 101, 103249. [Google Scholar] [CrossRef]
- Shah, Z.; Gulzar, M.; Hasan, F.; Shah, A.A. Degradation of polyester polyurethane by an indigenously developed consortium of Pseudomonas and Bacillus species isolated from soil. Polym. Degrad. Stab. 2016, 134, 349–356. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.; Ma, Q.; Li, S.; Dai, C. Performance and microbial community analysis of bioaugmented activated sludge system for indigo production from indole. Appl. Biochem. Biotechnol. 2019, 1874, 1437–1447. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Qin, W.; Hu, C.; Oenema, O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: A meta-analysis. Sci. Rep. 2015, 5, 16210. [Google Scholar] [CrossRef]
- Mola, I.D.; Ventorino, V.; Cozzolino, E.; Ottaiano, L.; Mori, M. Biodegradable mulching vs traditional polyethylene film for sustainable solarization: Chemical properties and microbial community response to soil management. Appl. Soil Ecol. 2021, 163, 103921. [Google Scholar] [CrossRef]
- Moon, J.Y.; Song, J.K.; Shin, J.H.; Cho, Y.C.; Bae, J.W. Effect of Biodegradable Mulch Film on Soil Microbial Community. Korean J. Soil Sci. Fertil. 2016, 49, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xi, Y.; Shi, X.Y.; Zhong, Y.J.; Guo, C.L. Effect of plastic film mulching and film residues on phthalate esters concentrations in soil and plants, and its risk assessment. Environ. Pollut. 2021, 286, 117546. [Google Scholar] [CrossRef]
- Anwar, M.S.; Kapri, A.; Chaudhry, V.; Mishra, A.; Ansari, M.W. Response of indigenously developed bacterial consortia in progressive degradation of polyvinyl chloride. Protoplasma 2016, 2534, 1023–1032. [Google Scholar] [CrossRef]
- Di, Z.J.C.C.F.; Hao, S.J.L.K.L. Effects of different planting patterns on cotton yield and soil water-salt under brackish water irrigation before sowing. Trans. Chin. Soc. Agric. Mach. 2013. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 2006, 952–953, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Xiukang, W.; Zhanbin, L.; Yingying, X. Effects of mulching and nitrogen on soil temperature, water content, nitrate-N content and maize yield in the Loess Plateau of China. Agric. Water Manag. 2015, 161, 53–64. [Google Scholar] [CrossRef]
- Li, F.-M.; Song, Q.-H.; Jjemba, P.K.; Shi, Y.-C. Dynamics of soil microbial biomass C and soil fertility in cropland mulched with plastic film in a semiarid agro-ecosystem. Soil Biol. Biochem. 2004, 3611, 1893–1902. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Sintim, H.Y.; Debruyn, J.M. Structural and Functional Responses of Soil Microbial Communities to Biodegradable Plastic Film Mulching in Two Agroecosystems. BioRxiv 2019, 650317. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fertil. Soils 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Menno, V.D.V.; Kempenaar, M.; Van Driel, M.; Raaijmakers, J.M.; Mendes, R. Impact of soil heat on reassembly of bacterial communities in the rhizosphere microbiome and plant disease suppression. Ecol. Lett. 2016, 194, 375–382. [Google Scholar]
- Fu, X.; Wang, J.; Xie, M.; Zhao, F.; Doughty, R. Increasing temperature can modify the effect of straw mulching on soil C fractions, soil respiration, and microbial community composition. PLoS ONE 2020, 158, e0237245. [Google Scholar] [CrossRef]
- Goh, Y.K.; Zoqratt, M.Z.H.M.; Goh, Y.K.; Ayub, Q.; Ting, A.S.Y. Discovering naturally-occurring microbiota in disease suppressive soil: Potential role of biological elements in suppressing Ganoderma boninense. Biol. Control 2022, 165, 104787. [Google Scholar] [CrossRef]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S. A novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri–structural and functional insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Wallace, P.W.; Haernvall, K.; Ribitsch, D.; Zitzenbacher, S.; Schittmayer, M. PpEst is a novel PBAT degrading polyesterase identified by proteomic screening of Pseudomonas pseudoalcaligenes. Appl. Microbiol. Biotechnol. 2017, 1016, 2291–2303. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.-S.; Zingarelli, S.; Nadeau, L.J.; Biffinger, J.C.; Drake, C.A. Carbon catabolite repression and Impranil polyurethane degradation in Pseudomonas protegens strain Pf-5. Appl. Environ. Microbiol. 2016, 8220, 6080–6090. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Roager, L.; Sonnenschein, E.C. Bacterial candidates for colonization and degradation of marine plastic debris. Environ. Sci. Technol. 2019, 5320, 11636–11643. [Google Scholar] [CrossRef]
- Huang, H.; Liu, P.; Shi, Y.; Wu, X.; Gao, S. Remarkable characteristics and distinct community of biofilms on the photoaged polyethylene films in riverine microcosms. Environ. Pollut. 2022, 292, 118485. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamada, K.; Iijima, T.; Iriguchi, T.; Kido, Y. Complete degradation of the endocrine-disrupting chemical phthalic acid by Flavobacterium sp. J. Health Sci. 2006, 526, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Kido, Y.; Tanaka, T.; Yamada, K.; Hachiyanagi, H.; Baba, H. Complete degradation of the endocrine-disrupting chemical dimethyl phthalate ester by Flavobacterium sp. J. Health Sci. 2007, 536, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Sharuddin, S.S.N.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Potential bifunctional rhizobacteria from crude oil sludge for hydrocarbon degradation and biosurfactant production. Process Saf. Environ. Prot. 2021, 155, 108–121. [Google Scholar] [CrossRef]

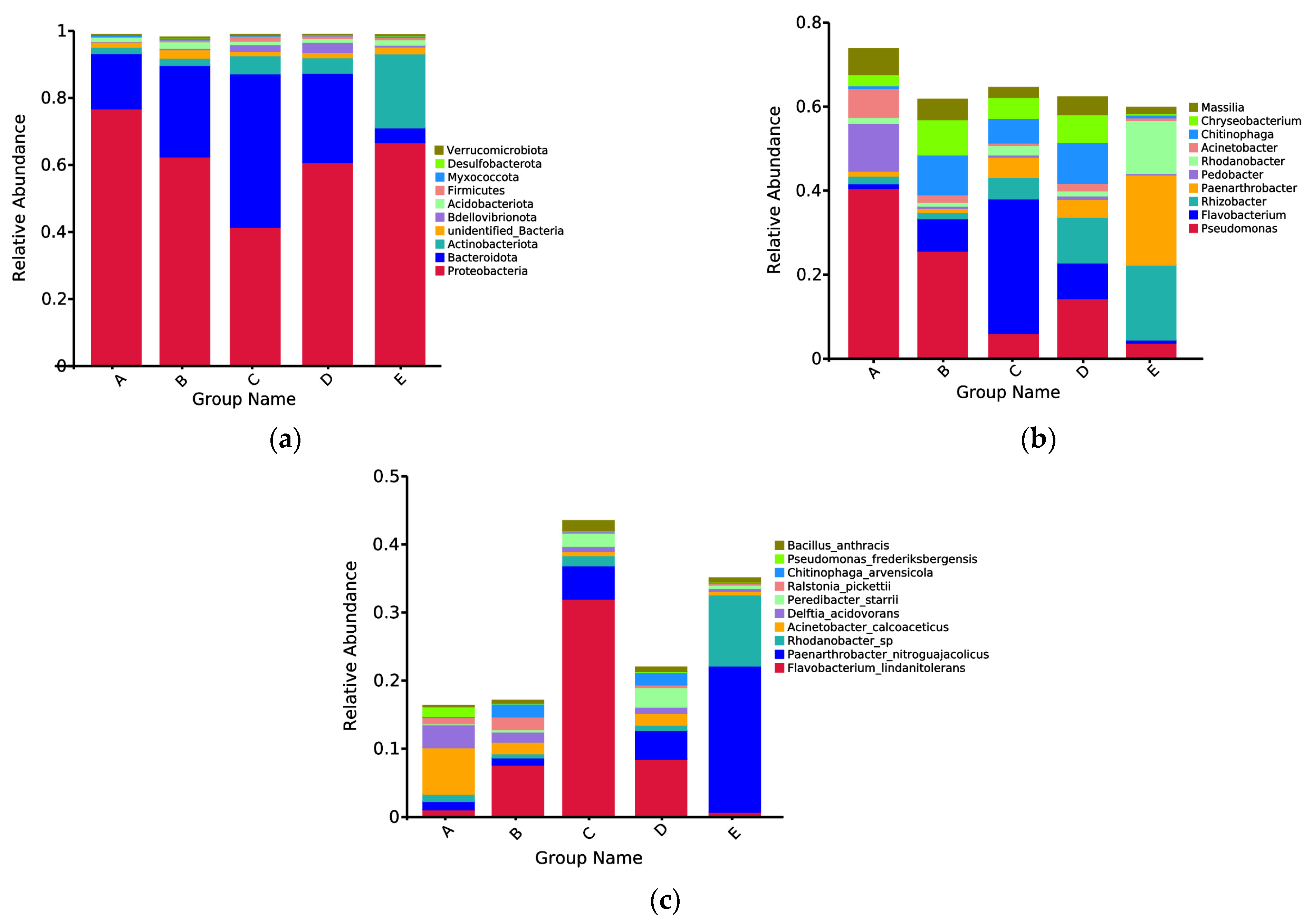
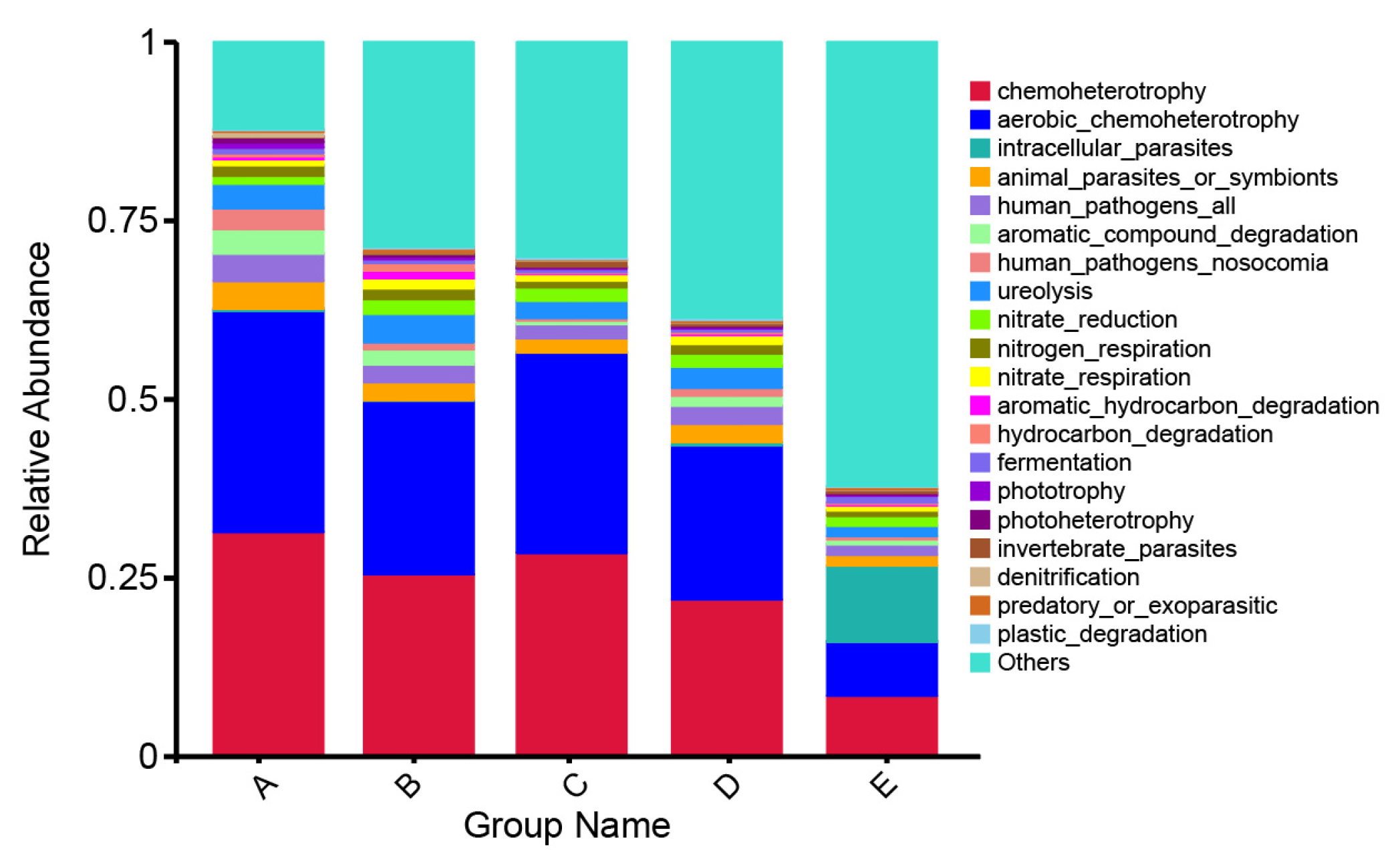
| Treat | pH | EC (μs/cm) | SOC (g/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|
| FM | 7.75 ± 0.11 a | 45.93 ± 5.85 b | 18.93 ± 1.15 b | 10.94 ± 4.78 a | 157.67 ± 23.54 a |
| CK | 7.15 ± 0.07 b | 70.80 ± 4.07 a | 24. 61 ± 1.51 a | 14.05 ± 2.72 a | 171.0 ± 20.42 a |
| Sample | Bacteria | Fungus | ||
|---|---|---|---|---|
| Chao1 | Shannon | Chao1 | Shannon | |
| FM | 5149.6 ± 1140.5 a | 10.88 ± 0.24 a | 551.3 ± 58.7 a | 5.85 ± 0.36 a |
| CK | 5701.9 ± 152.9 a | 11.04 ± 0.02 a | 553.7 ± 58.2 a | 5.75 ± 0.18 a |
| Sample | Bacteria | Fungus | ||||||
|---|---|---|---|---|---|---|---|---|
| Nodes | Edges | Positive | Negative | Nodes | Edges | Positive | Negative | |
| FM | 571 | 25,974 | 60.30% | 39.70% | 233 | 4752 | 63.44% | 36.56% |
| CK | 563 | 27,250 | 55.52% | 44.48% | 211 | 4506 | 62.16% | 37.84% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Zhang, J.; Yao, Z.; Luo, S.; Tian, L.; Tian, C.; Sun, Y. Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community. Agriculture 2022, 12, 406. https://doi.org/10.3390/agriculture12030406
Liang J, Zhang J, Yao Z, Luo S, Tian L, Tian C, Sun Y. Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community. Agriculture. 2022; 12(3):406. https://doi.org/10.3390/agriculture12030406
Chicago/Turabian StyleLiang, Jing, Jiafan Zhang, Zongmu Yao, Shouyang Luo, Lei Tian, Chunjie Tian, and Yu Sun. 2022. "Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community" Agriculture 12, no. 3: 406. https://doi.org/10.3390/agriculture12030406
APA StyleLiang, J., Zhang, J., Yao, Z., Luo, S., Tian, L., Tian, C., & Sun, Y. (2022). Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community. Agriculture, 12(3), 406. https://doi.org/10.3390/agriculture12030406






