Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Strains
2.1.1. Laboratory Strains
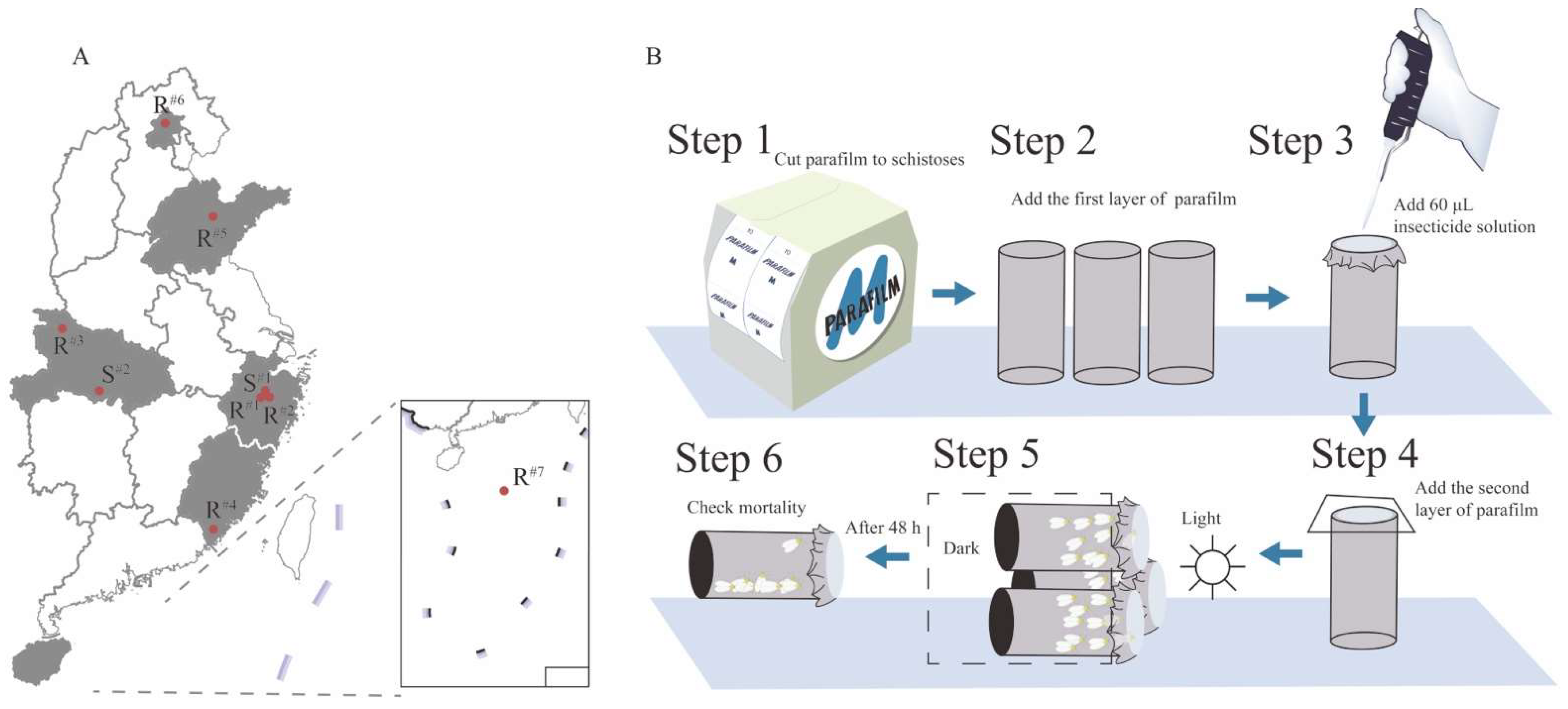
2.1.2. Field Populations
2.2. Bioassays with Imidacloprid and Thiamethoxam
2.3. Molecular Cloning of CYP4G68
2.4. Bioinformatic Analysis of CYP4G68
2.5. Quantitative Real-Time PCR
2.6. Analysis of Spatial-Temporal Specific Expression of CYP4G68
2.7. Functional Validation of CYP4G68 in Laboratory Strains
2.7.1. The Expression of CYP4G68 in Response to Exposure Tests and in Resistant and Susceptible
2.7.2. Functional Validation of CYP4G68 by RNAi
2.8. Functional Validation of CYP4G68 in Field Populations
2.9. Statistical Analysis
3. Results
3.1. Resistance to Imidacloprid and Thiamethoxam in Field B. tabaci MED Populations
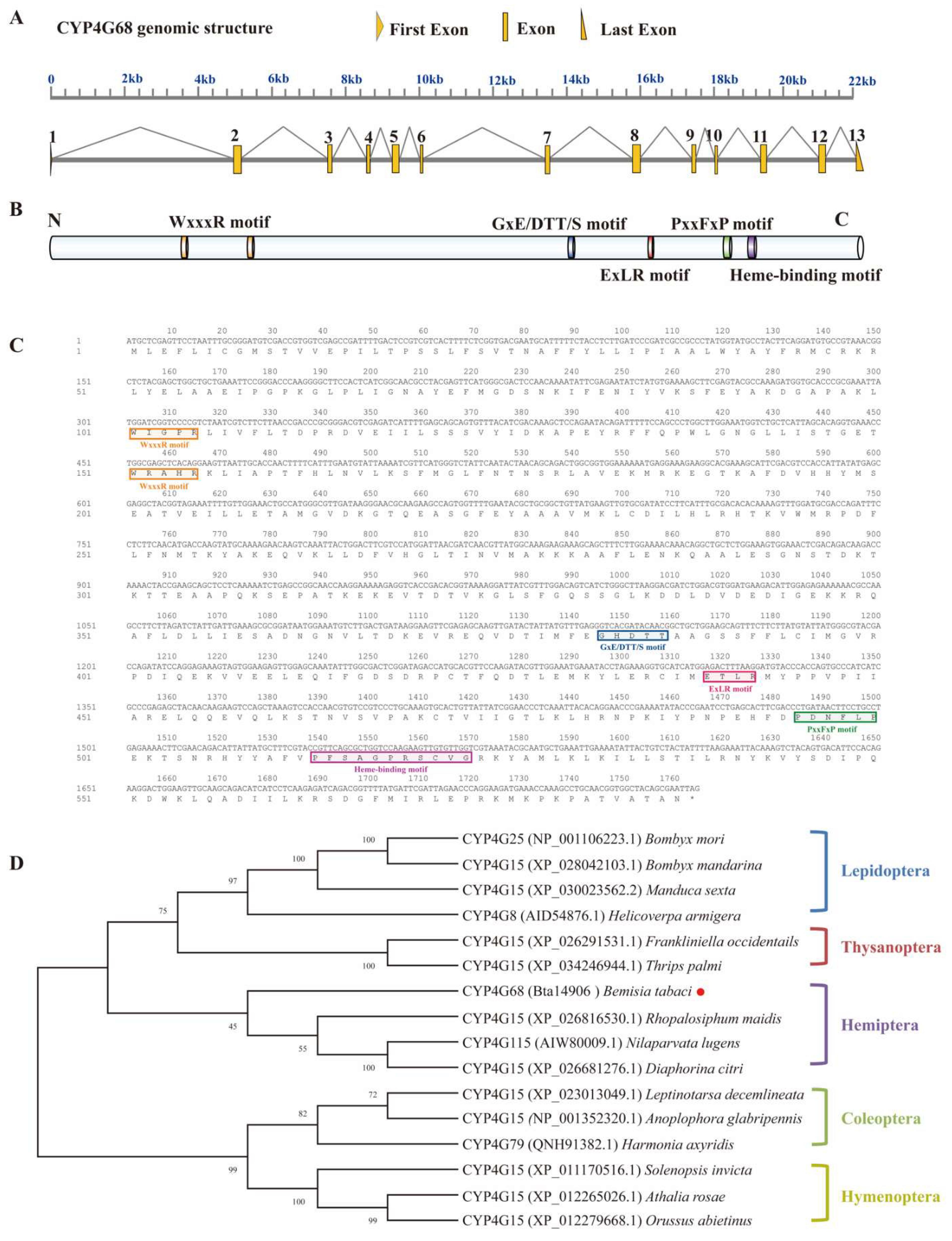
3.2. Gene Structure and Phylogenetic Analysis
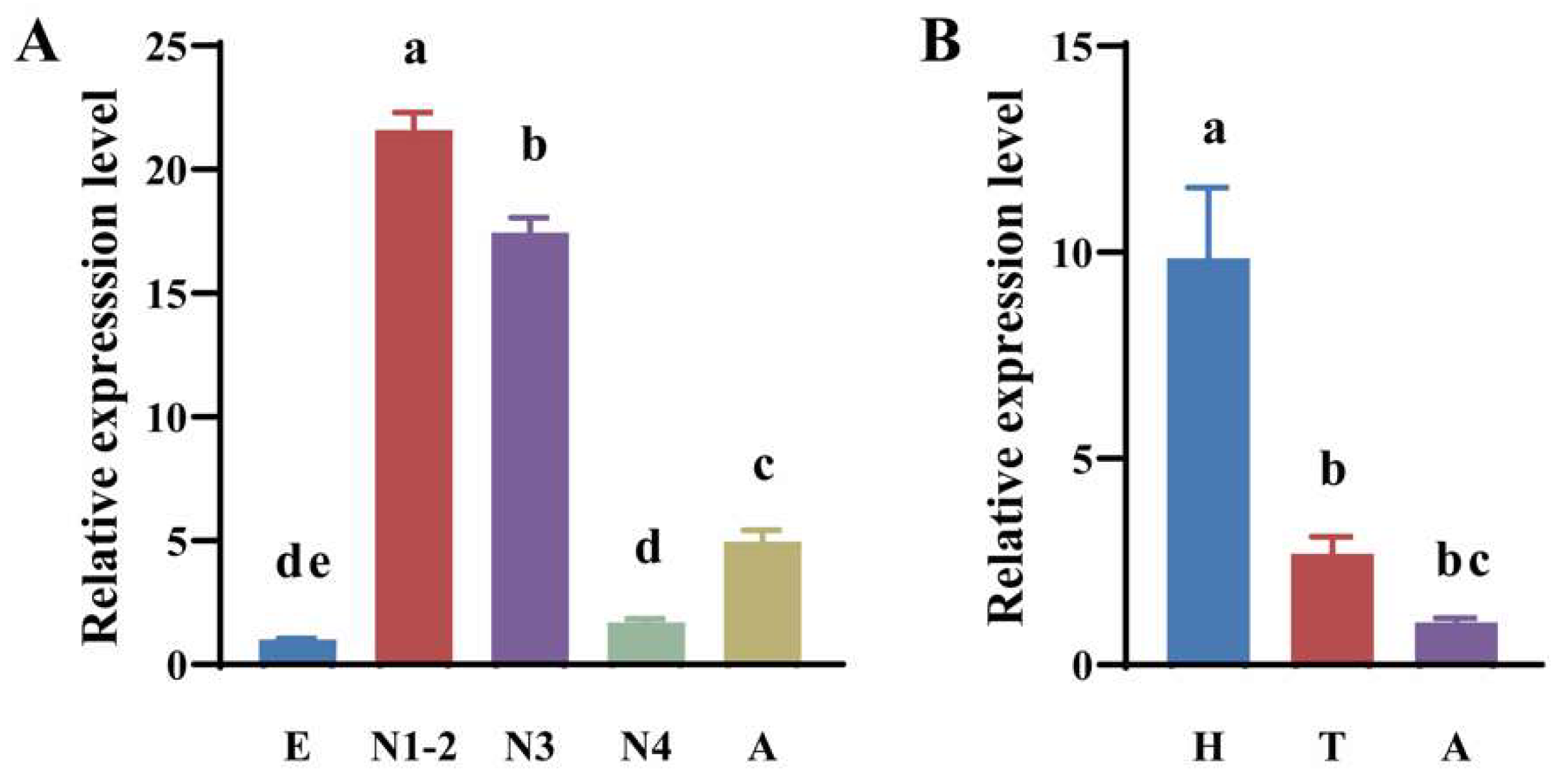
3.3. Spatiotemporal Expression Profiles
3.4. Functional Analysis of CYP4G68 in Laboratory Strains
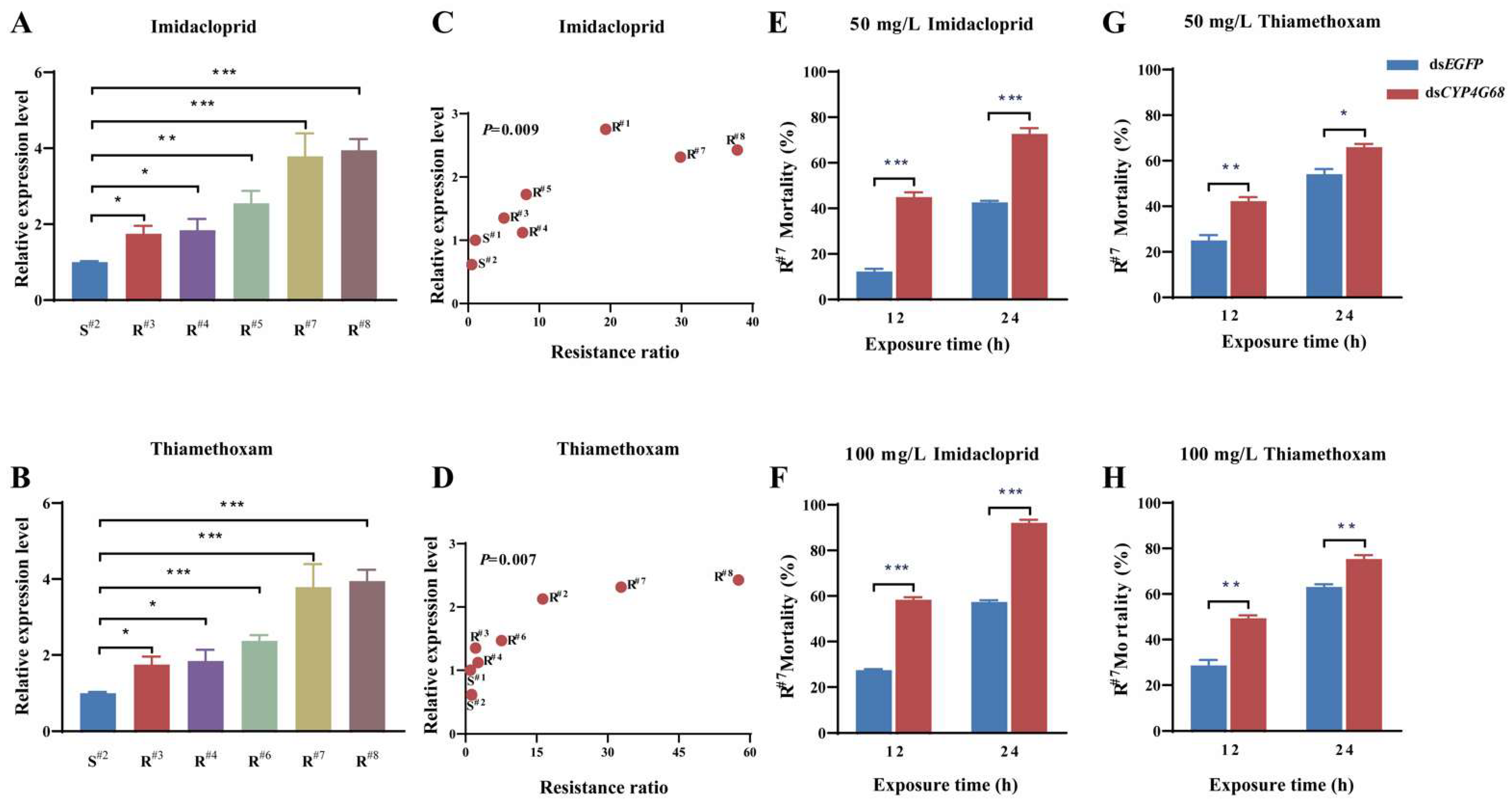
3.4.1. Response of CYP4G68 Expression to Imidacloprid and Thiamethoxam Exposure
3.4.2. Expression Analysis of CYP4G68 in Resistant and Susceptible Strains
3.4.3. Functional Analysis of CYP4G68 by RNAi
3.5. Field Validation of CYP4G68 Function
3.5.1. Relative Expression of CYP4G68 in Resistant and Susceptible Strains
3.5.2. Further Functional Validation of CYP4G68
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked Evolution: Can We Address the Sociobiological Dilemma of Pesticide Resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM); World Health Organization: Geneva, Switzerland, 2012; p. 130. [Google Scholar]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation That Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pesticides and Their Application: For the Control of Vectors and Pests of Public Health Importance; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hemingway, J.; Ranson, H.; Magill, A.; Kolaczinski, J.; Fornadel, C.; Gimnig, J.; Coetzee, M.; Simard, F.; Roch, D.K.; Hinzoumbe, C.K.; et al. Averting a Malaria Disaster: Will Insecticide Resistance Derail Malaria Control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef]
- Scott, J.G. Evolution of Resistance to Pyrethroid Insecticides in Musca Domestica. Pest Manag. Sci. 2017, 73, 716–722. [Google Scholar] [CrossRef]
- FAO. Guidelines on Prevention and Management of Pesticide Resistance; FAO: Rome, Italy, 2012; p. 55. [Google Scholar]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The Global Status of Insect Resistance to Neonicotinoid Insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide Resistance and Its Management in Bemisia tabaci Species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. 2019, 60, 241–255. [Google Scholar] [CrossRef]
- Du, T.; Fu, B.; Wei, X.; Yin, C.; Yang, J.; Huang, M.; Liang, J.; Gong, P.; Liu, S.; Xue, H.; et al. Knockdown of UGT352A5 Decreases the Thiamethoxam Resistance in Bemisia tabaci (Hemiptera: Gennadius). Int. J. Biol. Macromol. 2021, 186, 100–108. [Google Scholar] [CrossRef]
- Yang, N.; Xie, W.; Yang, X.; Wang, S.; Wu, Q.; Li, R.; Pan, H.; Liu, B.; Shi, X.; Fang, Y.; et al. Transcriptomic and Proteomic Responses of Sweetpotato Whitefly, Bemisia tabaci, to Thiamethoxam. PLoS ONE 2013, 8, e61820. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Gong, Y.; Ali, S.; Hou, M. Mechanisms of Resistance to Thiamethoxam and Dinotefuran Compared to Imidacloprid in the Brown Planthopper: Roles of Cytochrome P450 Monooxygenase and a P450 Gene CYP6ER1. Pestic. Biochem. Physiol. 2018, 150, 17–26. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.; Wang, Z.; Liu, J.; Zeng, X. Identification of Detoxification Genes in Imidacloprid-Resistant Asian Citrus Psyllid (Hemiptera: Lividae) and Their Expression Patterns under Stress of Eight Insecticides. Pest Manag. Sci. 2019, 75, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wen, Y.; Yang, B.; Zhang, Y.; Liu, S.; Liu, Z.; Han, Z. Biochemical Mechanisms of Imidacloprid Resistance in Nilaparvata lugens: Over-Expression of Cytochrome P450 CYP6AY1. Insect Biochem. Mol. Biol. 2013, 43, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zoh, M.G.; Gaude, T.; Prud’homme, S.M.; Riaz, M.A.; David, J.P.; Reynaud, S. Molecular Bases of P450-Mediated Resistance to the Neonicotinoid Insecticide Imidacloprid in the Mosquito Ae. aegypti. Aquat. Toxicol. 2021, 236, 105860. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional Analysis of Cytochrome P450 Genes Linked with Acetamiprid Resistance in Melon Aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhang, H.; Shan, T.; Shi, X.; Gao, X. The Overexpression of Three Cytochrome P450 Genes CYP6CY14, CYP6CY22 and CYP6UN1 Contributed to Metabolic Resistance to Dinotefuran in Melon/Cotton Aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2020, 167, 104601. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, Y.; Li, Y.; Li, W.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic Hormone Regulates Cytochrome P450-Mediated Imidacloprid Resistance in the Brown Planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef]
- Pang, R.; Xing, K.; Yuan, L.; Liang, Z.; Chen, M.; Yue, X.; Dong, Y.; Ling, Y.; He, X.; Li, X.; et al. Peroxiredoxin Alleviates the Fitness Costs of Imidacloprid Resistance in an Insect Pest of Rice. PLoS Biol. 2021, 19, e3001190. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Yang, J.; Du, T.; Yin, C.; Fu, B.; Huang, M.; Liang, J.; Gong, P.; Liu, S.; et al. Epitranscriptomic Regulation of Insecticide Resistance. Sci. Adv. 2021, 7, eabe5903. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed Activation of the Whitefly Transcription Factor CREB Leads to P450-mediated Imidacloprid Resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-Expression of Cytochrome P450 CYP6CM1 Is Associated with High Resistance to Imidacloprid in the B and Q Biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and Insecticide Resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, D. NlCYP4G76 and NlCYP4G115 Modulate Susceptibility to Desiccation and Insecticide Penetration through Affecting Cuticular Hydrocarbon Biosynthesis in Nilaparvata lugens (Hemiptera: Delphacidae). Front. Physiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanidou, V.; Kampouraki, A.; Maclean, M.; Blomquist, G.J.; Tittiger, C.; Juárez, M.P.; Mijailovsky, S.J.; Chalepakis, G.; Anthousi, A.; Lynd, A.; et al. Cytochrome P450 Associated with Insecticide Resistance Catalyzes Cuticular Hydrocarbon Production in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2016, 113, 9268–9273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Moreau, J.A.; Zina, J.M.; Mazgaeen, L.; Yoon, K.S.; Pittendrigh, B.R.; Clark, J.M. Identification and Interaction of Multiple Genes Resulting in DDT Resistance in the 91-R Strain of Drosophila melanogaster by RNAi Approaches. Pestic. Biochem. Physiol. 2018, 151, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect Cuticle: A Critical Determinant of Insecticide Resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Daniels, M.; Andrews, M.; Slater, R.; Lind, R.J.; Gorman, K.; Williamson, M.S.; Denholm, I. Age-Specific Expression of a P450 Monooxygenase (CYP6CM1) Correlates with Neonicotinoid Resistance in Bemisia tabaci. Pestic. Biochem. Physiol. 2011, 101, 53–58. [Google Scholar] [CrossRef]
- Nauen, R.; Vontas, J.; Kaussmann, M.; Wölfel, K. Pymetrozine Is Hydroxylated by CYP6CM1, a Cytochrome P450 Conferring Neonicotinoid Resistance in Bemisia tabaci. Pest Manag. Sci. 2013, 69, 457–461. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of Nitenpyram Resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Jin, R.; Mao, K.; Liao, X.; Xu, P.; Li, Z.; Ali, E.; Wan, H.; Li, J. Overexpression of CYP6ER1 Associated with Clothianidin Resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 154, 39–45. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Wu, H.; Zhang, H.; Zhang, J.; Ma, E. Knockdown of Cytochrome P450 CYP6 Family Genes Increases Susceptibility to Carbamates and Pyrethroids in the Migratory Locust, Locusta migratoria. Chemosphere 2019, 223, 48–57. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Zhang, X.; Ma, E.; Guo, Y.; Zhu, K.Y.; Zhang, J. RNA Interference of Cytochrome P450 CYP6F Subfamily Genes Affects Susceptibility to Different Insecticides in Locusta migratoria. Pest Manag. Sci. 2016, 72, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- le Goff, G.; Hilliou, F. Resistance evolution in Drosophila: The case of CYP6G1. Pest Manag. Sci. 2017, 73, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Riveron Miranda, J.; Mugenzi, L.M.J.; Djonabaye, D.; Wondji, M.J.; Tchoupo, M.; Tchapga, W.; Njiokou, F.; Wondji, C.S. Cytochrome P450 Metabolic Resistance (CYP6P9a) to Pyrethroids Imposes a Fitness Cost in the Major African Malaria Vector Anopheles funestus. Heredity 2020, 124, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Weedall, G.D.; Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R.; et al. A Cytochrome P450 Allele Confers Pyrethroid Resistance on a Major African Malaria Vector, Reducing Insecticide-Treated Bednet Efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, H.; Gao, H.; Zhang, Y.; Fan, D.; Fang, J.; Liu, Z. The Roles of CYP6AY1 and CYP6ER1 in Imidacloprid Resistance in the Brown Planthopper: Expression Levels and Detoxification Efficiency. Pestic. Biochem. Physiol. 2016, 129, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Morou, E.; Tsagkarakou, A.; Riga, M.; Nauen, R.; Paine, M.; Morin, S.; Vontas, J. Assessment of the Bemisia tabaci CYP6CM1vQ Transcript and Protein Levels in Laboratory and Field-Derived Imidacloprid-Resistant Insects and Cross-Metabolism Potential of the Recombinant Enzyme. Insect Sci. 2011, 18, 23–29. [Google Scholar] [CrossRef]
- Jones, D.R. Plant Viruses Transmitted by Whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Denholm, I.; Gorman, K.; Cenis, J.L.; Kontsedalov, S.; Ishaaya, I. Biotype Q of Bemisia tabaci Identified in Israel. Phytoparasitica 2003, 31, 94–98. [Google Scholar] [CrossRef]
- de Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Q.; Wang, S.; Chang, X.; Xie, W.; Xu, B.; Zhang, Y. Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in B-biotype Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Zhang, W.; Wu, Q.; Xu, B.; Chu, D. Analysis of Genetic Diversity among Different Geographical Populations and Determination of Biotypes of Bemisia tabaci in China. J. Appl. Entomol. 2005, 129, 121–128. [Google Scholar] [CrossRef]
- Luo, C.; Jones, C.M.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide Resistance in Bemisia tabaci Biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Feyereisen, R. Origin and Evolution of the CYP4G Subfamily in Insects, Cytochrome P450 Enzymes Involved in Cuticular Hydrocarbon Synthesis. Mol. Phylogenet. Evol. 2020, 143, 106695. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Jia, Z.; Ahmat, T.; Xie, L.; Jiang, W. Resistance to Neonicotinoid Insecticides and Expression Changes of Eighteen Cytochrome P450 Genes in Field Populations of Bemisia tabaci from Xinjiang, China. Entomol. Res. 2020, 50, 205–211. [Google Scholar] [CrossRef]
- Xie, W.; Yang, X.; Chen, C.; Yang, Z.; Guo, L.; Wang, D.; Huang, J.; Zhang, H.; Wen, Y.; Zhao, J.; et al. The Invasive MED/Q Bemisia tabaci Genome: A Tale of Gene Loss and Gene Gain. BMC Genomics 2018, 19, 68. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Xie, W.; Fu, B.; Xiao, S.; Tan, X.; Ji, Y.; Cheng, J.; Wang, R.; Liu, B.; Yang, X.; et al. Annual Analysis of Field-Evolved Insecticide Resistance in Bemisia tabaci across China. Pest Manag. Sci. 2021, 77, 2990–3001. [Google Scholar] [CrossRef]
- Fu, B.; Tao, M.; Xue, H.; Jin, H.; Liu, K.; Qiu, H.; Yang, S.; Yang, X.; Gui, L.; Zhang, Y.; et al. Spinetoram Resistance Drives Interspecific Competition between Megalurothrips usitatus and Frankliniella intonsa. Pest Manag. Sci. 2022. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Xie, W.; Li, R.M.; Zhou, X.M.; Wang, S.L.; Wu, Q.J.; Yang, N.N.; Xia, J.X.; Yang, Z.Z.; Guo, L.T.; et al. RNA Interference-Mediated Knockdown of the Hydroxyacid-Oxoacid Transhydrogenase Gene Decreases Thiamethoxam Resistance in Adults of the Whitefly Bemisia tabaci. Sci. Rep. 2017, 7, 41201. [Google Scholar] [CrossRef]
- Li, R.; Xie, W.; Wang, S.; Wu, Q.; Yang, N.; Yang, X.; Pan, H.; Zhou, X.; Bai, L.; Xu, B.; et al. Reference Gene Selection for QRT-PCR Analysis in the Sweetpotato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Lacasa-Plasencia, A.; Bielza-Lino, P.; Rodríguez-Del-Rincón, Á. Pyrethroid Resistance of Helicoverpa armigera in Spain: Current Status and Agroecological Perspective. Agric. Ecosyst. Environ. 2002, 93, 55–60. [Google Scholar] [CrossRef]
- Yang, X.; Xie, W.; Wang, S.; Wu, Q.; Pan, H.; Li, R.; Yang, N.; Liu, B.; Xu, B.; Zhou, X.; et al. Two Cytochrome P450 Genes Are Involved in Imidacloprid Resistance in Field Populations of the Whitefly, Bemisia tabaci, in China. Pestic. Biochem. Physiol. 2013, 107, 343–350. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Nelson, D.R.; Palli, S.R. A Specialist Herbivore Pest Adaptation to Xenobiotics through Up-Regulation of Multiple Cytochrome P450s. Sci. Rep. 2016, 6, 20421. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, C.; Tierney, K. Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Lu, K.; Song, Y.; Zeng, R. The Role of Cytochrome P450-Mediated Detoxification in Insect Adaptation to Xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Garrood, W.T.; Singh, K.S.; Randall, E.; Lueke, B.; Gutbrod, O.; Matthiesen, S.; Kohler, M.; Nauen, R.; Davies, T.G.E.; et al. Neofunctionalization of Duplicated P450 Genes Drives the Evolution of Insecticide Resistance in the Brown Planthopper. Curr. Biol. 2018, 28, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2017, 19, 38–54. [Google Scholar] [CrossRef]
- Nauen, R.; Wölfel, K.; Lueke, B.; Myridakis, A.; Tsakireli, D.; Roditakis, E.; Tsagkarakou, A.; Stephanou, E.; Vontas, J. Development of a Lateral Flow Test to Detect Metabolic Resistance in Bemisia tabaci Mediated by CYP6CM1, a Cytochrome P450 with Broad Spectrum Catalytic Efficiency. Pestic. Biochem. Physiol. 2015, 121, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kontsedalov, S.; Abu-Moch, F.; Lebedev, G.; Czosnek, H.; Horowitz, A.R.; Ghanim, M. Bemisia tabaci Biotype Dynamics and Resistance to Insecticides in Israel During the Years 2008–2010. J. Integr. Agr. 2012, 11, 312–320. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and Insecticide Resistance Status of the Whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, R.A. Field-Evolved Resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to Carbodiimide and Neonicotinoids in Pakistan. J. Econ. Entomol. 2017, 110, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fang, Y.; Mu, C.; Qu, C.; Li, F.; Wang, Z.; Luo, C. Baseline Susceptibility and Cross-Resistance of Cycloxaprid, a Novel Cis-Nitromethylene Neonicotinoid Insecticide, in Bemisia tabaci MED from China. Crop Prot. 2018, 110, 283–287. [Google Scholar] [CrossRef]
- Vassiliou, V.; Emmanouilidou, M.; Perrakis, A.; Morou, E.; Vontas, J.; Tsagkarakou, A.; Roditakis, E. Insecticide Resistance in Bemisia tabaci from Cyprus. Insect Sci. 2011, 18, 30–39. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Cao, L.; Gong, Y.; Hoffmann, A.A.; Wei, S. Toxicity of Seven Insecticides to Different Developmental Stages of the Whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) in Multiple Field Populations of China. Ecotoxicology 2018, 27, 742–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Xue, S.; An, S.; Zhang, K. Disruption of the Cytochrome P450 CYP6BQ7 Gene Reduces Tolerance to Plant Toxicants in the Red Flour Beetle, Tribolium castaneum. Int. J. Biol. Macromol. 2021, 172, 263–269. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, X.; Wu, H.; Silver, K.; Zhang, J.; Ma, E.; Zhu, K.Y. Transcriptome-Wide Survey, Gene Expression Profiling and Exogenous Chemical-Induced Transcriptional Responses of Cytochrome P450 Superfamily Genes in Migratory Locust (Locusta migratoria). Insect Biochem. Mol. Biol. 2018, 100, 66–77. [Google Scholar] [CrossRef]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A Brain-Specific Cytochrome P450 Responsible for the Majority of Deltamethrin Resistance in the QTC279 Strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Sztal, T.; Pasricha, S.; Sridhar, M.; Batterham, P.; Daborn, P.J. Characterization of Drosophila melanogaster Cytochrome P450 Genes. Proc. Natl. Acad. Sci. USA 2009, 106, 5731–5736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Shan, T.; Liu, Y.; Wang, C.; Shi, X.; Gao, X. Identification and Functional Analysis of a Cytochrome P450 Gene Involved in Imidacloprid Resistance in Bradysia odoriphaga Yang et Zhang. Pestic. Biochem. Physiol. 2019, 153, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, X.; Gao, X. Research Progresses on the Metabolism of Neonicotinoids Imidacloprid and Thiamethoxam. Chin. J. Pesticide Sci. 2012, 14, 587–596. [Google Scholar]
- Suchail, S.; de Sousa, G.; Rahmani, R.; Belzunces, L.P. In Vivo Distribution and Metabolisation of 14C-Imidacloprid in Different Compartments of Apis Mellifera L. Pest Manag. Sci. 2004, 60, 1056–1062. [Google Scholar] [CrossRef]
- Joußen, N.; Heckel, D.G.; Haas, M.; Schuphan, I.; Schmidt, B. Metabolism of Imidacloprid and DDT by P450 CYP6G1 Expressed in Cell Cultures of Nicotiana Tabacum Suggests Detoxification of These Insecticides in Cyp6g1-Overexpressing Strains of Drosophila Melanogaster, Leading to Resistance. Pest Manag. Sci. 2008, 64, 65–73. [Google Scholar] [CrossRef]
- Byrne, F.J.; Castle, S.; Prabhaker, N.; Toscano, N.C. Biochemical Study of Resistance to Imidacloprid in B Biotype Bemisia tabaci from Guatemala. Pest Manag. Sci. 2003, 59, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Benzidane, Y.; Touinsi, S.; Motte, E.; Jadas-Hécart, A.; Communal, P.Y.; Leduc, L.; Thany, S.H. Effect of Thiamethoxam on Cockroach Locomotor Activity Is Associated with Its Metabolite Clothianidin. Pest Manag. Sci. 2010, 66, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Salgado, V.L.; Kaussmann, M. Thiamethoxam Is a Neonicotinoid Precursor Converted to Clothianidin in Insects and Plants. Pestic. Biochem. Physiol. 2003, 76, 55–69. [Google Scholar] [CrossRef]
- Jones, C.M.; Haji, K.A.; Khatib, B.O.; Bagi, J.; Mcha, J.; Devine, G.J.; Daley, M.; Kabula, B.; Ali, A.S.; Majambere, S.; et al. The Dynamics of Pyrethroid Resistance in Anopheles arabiensis from Zanzibar and an Assessment of the Underlying Genetic Basis. Parasit. Vectors 2013, 6, 343. [Google Scholar] [CrossRef] [Green Version]
- Pittendrigh, B.; Aronstein, K.; Zinkovsky, E.; Andreev, O.; Campbell, B.; Daly, J.; Trowell, S.; Ffrench, R.H. Cytochrome P450 Genes from Helicoverpa armigera: Expression in a Pyrethroid-Susceptible and-Resistant Strain. Insect Biochem. Molec. Biol. 1997, 27, 507–512. [Google Scholar] [CrossRef]
- Müller, P.; Chouaïbou, M.; Pignatelli, P.; Etang, J.; Walker, E.D.; Donnelly, M.J.; Simard, F.; Ranson, H. Pyrethroid Tolerance Is Associated with Elevated Expression of Antioxidants and Agricultural Practice in Anopheles arabiensis Sampled from an Area of Cotton Fields in Northern Cameroon. Mol. Ecol. 2008, 17, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Zhang, L.; Liu, N. Overexpression of CYP4G19 Associated with a Pyrethroid-Resistant Strain of the German Cockroach, Blattella germanica (L.). Gene 2003, 314, 157–163. [Google Scholar] [CrossRef]
- Chen, N.; Pei, X.J.; Li, S.; Fan, Y.L.; Liu, T.X. Involvement of Integument-Rich CYP4G19 in Hydrocarbon Biosynthesis and Cuticular Penetration Resistance in Blattella germanica (L.). Pest Manag. Sci. 2020, 76, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Palli, S.R. Cap n Collar Transcription Factor Regulates Multiple Genes Coding for Proteins Involved in Insecticide Detoxification in the Red Flour Beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2017, 90, 43–52. [Google Scholar] [CrossRef]
- Zhang, R.L.; Guo, G.Z.; Geng, Y.J.; Huang, D.N.; Xue, C.F. Level of CYP4G19 Expression Is Associated with Pyrethroid Resistance in Blattella germanica. J. Parasitol. Res. 2010, 2010, 517534. [Google Scholar]
- He, C.; Liang, J.; Liu, S.; Wang, S.; Wu, Q.; Xie, W.; Zhang, Y. Changes in the Expression of Four ABC Transporter Genes in Response to Imidacloprid in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2019, 153, 136–143. [Google Scholar] [CrossRef]

| Insecticide | Strain | N a | Slope (±SE) | LC50 (mg/L) | 95% FL b | Df c | χ2 | RR d | Resistance Level |
|---|---|---|---|---|---|---|---|---|---|
| Imidacloprid | S#1 | 721 | 1.72 (±0.13) | 8.86 | 7.67–10.18 | 3 | 2.78 | - | Susceptible |
| S#2 | 787 | 1.86(±0.12) | 4.32 | 2.86–6.25 | 7 | 27.76 | 0.49 | Susceptible | |
| R#1 | 542 | 1.79(±0.17) | 171.35 | 108.05–320.69 | 3 | 8.99 | 19.34 | Moderate | |
| R#3 | 458 | 2.47 (±0.21) | 44.58 | 33.34–58.08 | 4 | 6.76 | 5.03 | Low | |
| R#4 | 653 | 1.57 (±0.14) | 67.89 | 39.04–98.53 | 5 | 10.22 | 7.66 | Low | |
| R#5 | 388 | 2.80 (±0.22) | 72.34 | 63.28–82.65 | 3 | 1.69 | 8.16 | Low | |
| R#6 | 430 | 1.93 (±0.16) | 6.37 | 4.17–9.13 | 4 | 8.73 | 0.72 | Susceptible | |
| R#7 | 671 | 1.39 (±0.10) | 264.47 | 175.12–424.48 | 5 | 14.54 | 29.85 | Moderate | |
| R#8 | 689 | 1.37 (±0.15) | 335.34 | 137.32–520.35 | 5 | 12.08 | 37.85 | High | |
| Thiamethoxam | S#1 | 548 | 3.05 (±0.24) | 7.01 | 5.72–8.43 | 4 | 5.08 | - | Susceptible |
| S#2 | 471 | 2.14 (±0.15) | 8.89 | 5.30–16.90 | 3 | 11.74 | 1.27 | Susceptible | |
| R#2 | 499 | 3.43 (±0.29) | 114.12 | 85.57–143.89 | 3 | 7.61 | 16.28 | Moderate | |
| R#3 | 486 | 1.62 (±0.12) | 14.75 | 12.24–17.81 | 3 | 1.83 | 2.1 | Low | |
| R#4 | 482 | 2.44 (±0.14) | 18.3 | 9.54–27.70 | 3 | 6.99 | 2.61 | Low | |
| R#5 | 602 | 1.36 (±0.11) | 11.98 | 7.18–17.45 | 4 | 7.78 | 1.71 | Susceptible | |
| R#6 | 491 | 3.05 (±0.23) | 53.11 | 37.37–76.09 | 4 | 3.35 | 7.58 | Low | |
| R#7 | 895 | 1.44 (±0.15) | 230.09 | 141.94–689.66 | 3 | 6.91 | 32.82 | High | |
| R#8 | 529 | 1.72 (±0.22) | 403.59 | 250.54–546.46 | 4 | 2.61 | 57.57 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Yang, J.; Hu, J.; Fu, B.; Gong, P.; Du, T.; Xue, H.; Wei, X.; Liu, S.; Huang, M.; et al. Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius). Agriculture 2022, 12, 473. https://doi.org/10.3390/agriculture12040473
Liang J, Yang J, Hu J, Fu B, Gong P, Du T, Xue H, Wei X, Liu S, Huang M, et al. Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius). Agriculture. 2022; 12(4):473. https://doi.org/10.3390/agriculture12040473
Chicago/Turabian StyleLiang, Jinjin, Jing Yang, Jinyu Hu, Buli Fu, Peipan Gong, Tianhua Du, Hu Xue, Xuegao Wei, Shaonan Liu, Mingjiao Huang, and et al. 2022. "Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius)" Agriculture 12, no. 4: 473. https://doi.org/10.3390/agriculture12040473
APA StyleLiang, J., Yang, J., Hu, J., Fu, B., Gong, P., Du, T., Xue, H., Wei, X., Liu, S., Huang, M., Yin, C., Ji, Y., He, C., Xie, W., Wang, R., Yang, X., & Zhang, Y. (2022). Cytpchrome P450 CYP4G68 Is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius). Agriculture, 12(4), 473. https://doi.org/10.3390/agriculture12040473









