Bioconversion in Ryegrass-Fescue Hay by Pleurotus ostreatus to Increase Their Nutritional Value for Ruminant
Abstract
:1. Introduction
2. Materials and Methods
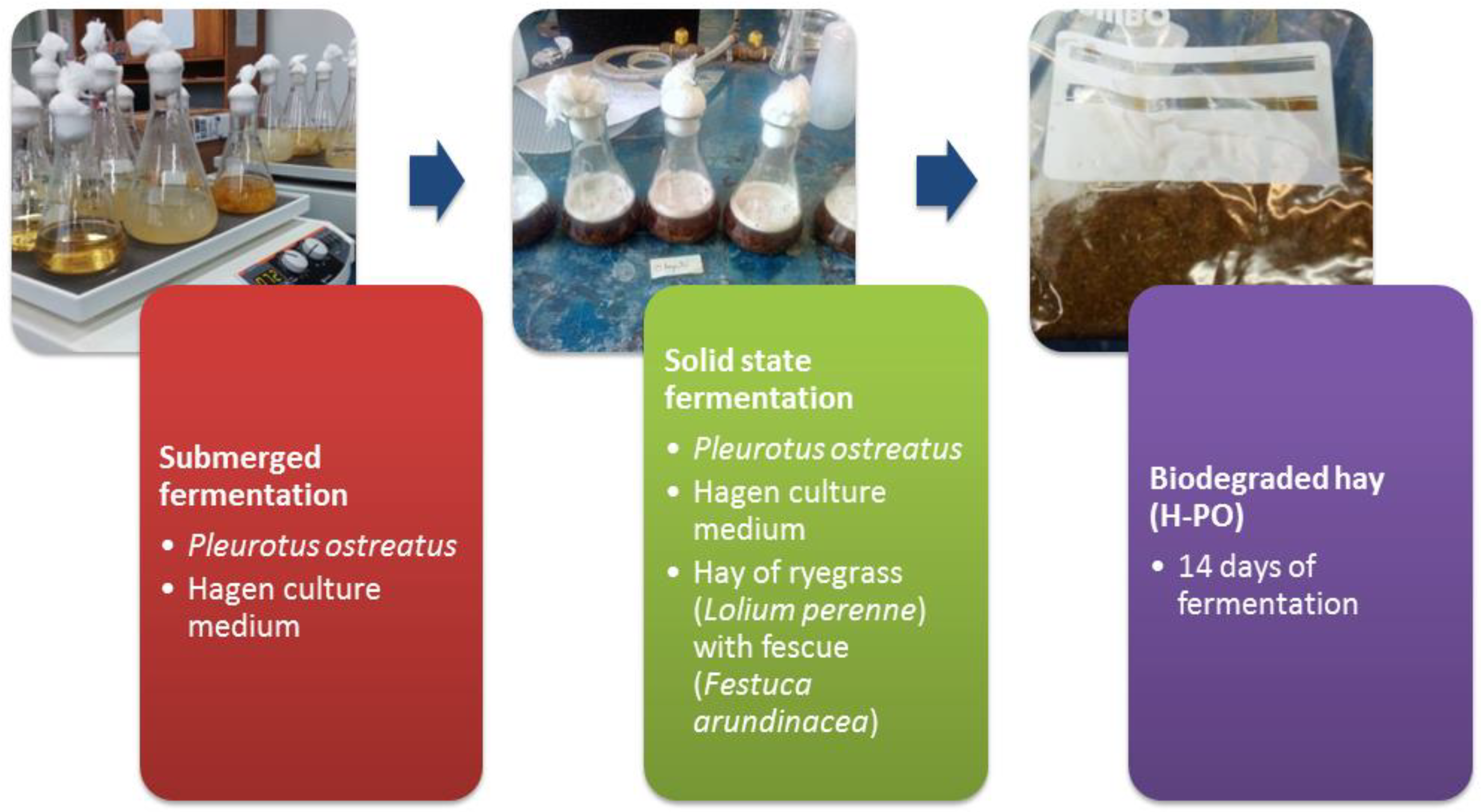
2.1. Biological Material
2.2. Growth Activation of P. ostreatus
2.3. Cultivation in Plates
2.4. Submerged Fermentation
2.5. Substrate for Solid State Fermentation
2.6. Solid State Fermentation (SSF)
2.7. Crude Enzymatic (CE) Obtainment from Submerged Fermentation
2.8. Crude Enzymatic (CE) Obtainment from Solid State Fermentation (SSF)
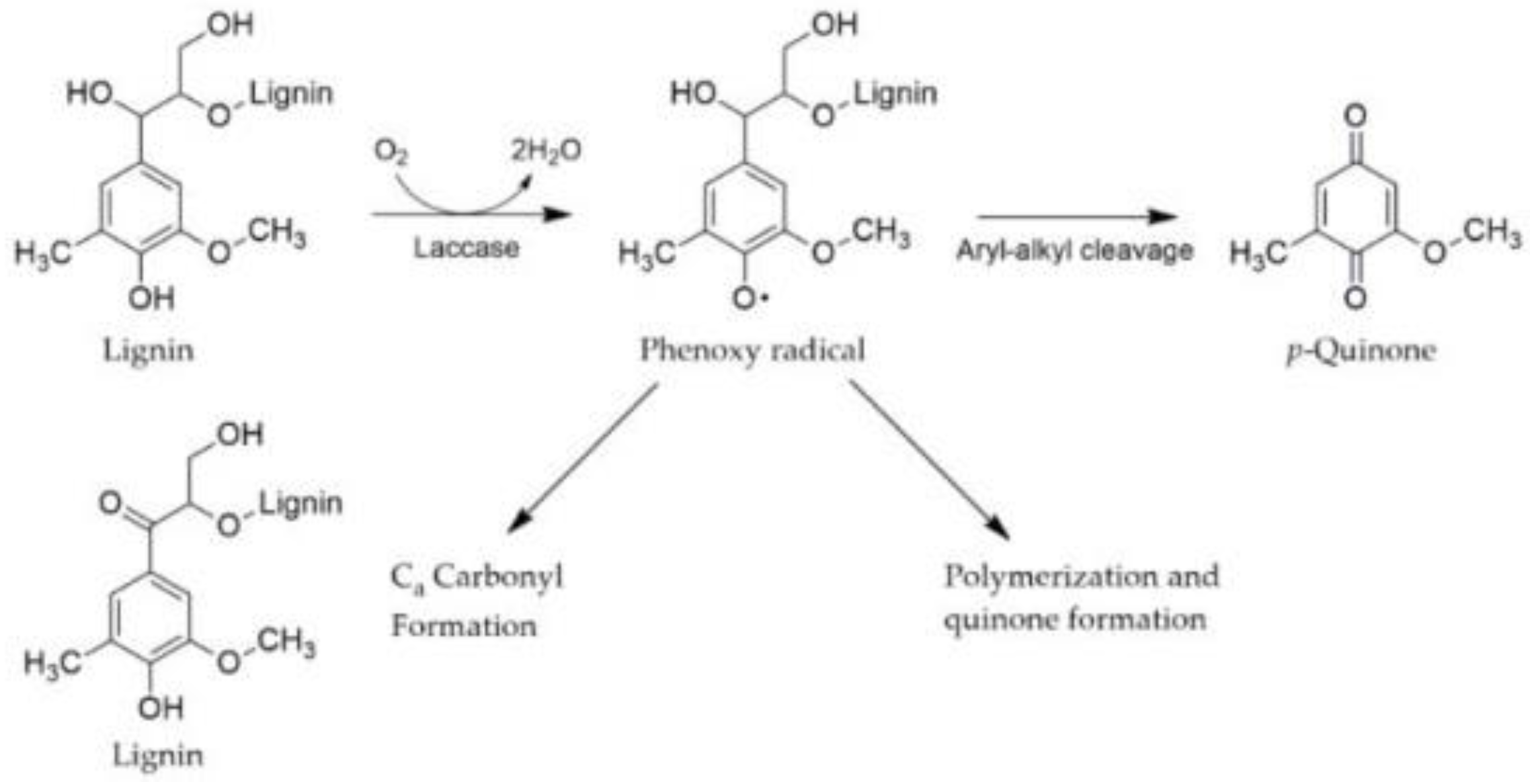
2.9. Laccase (Lac EC 1.10.3.2) Enzymatic Activity
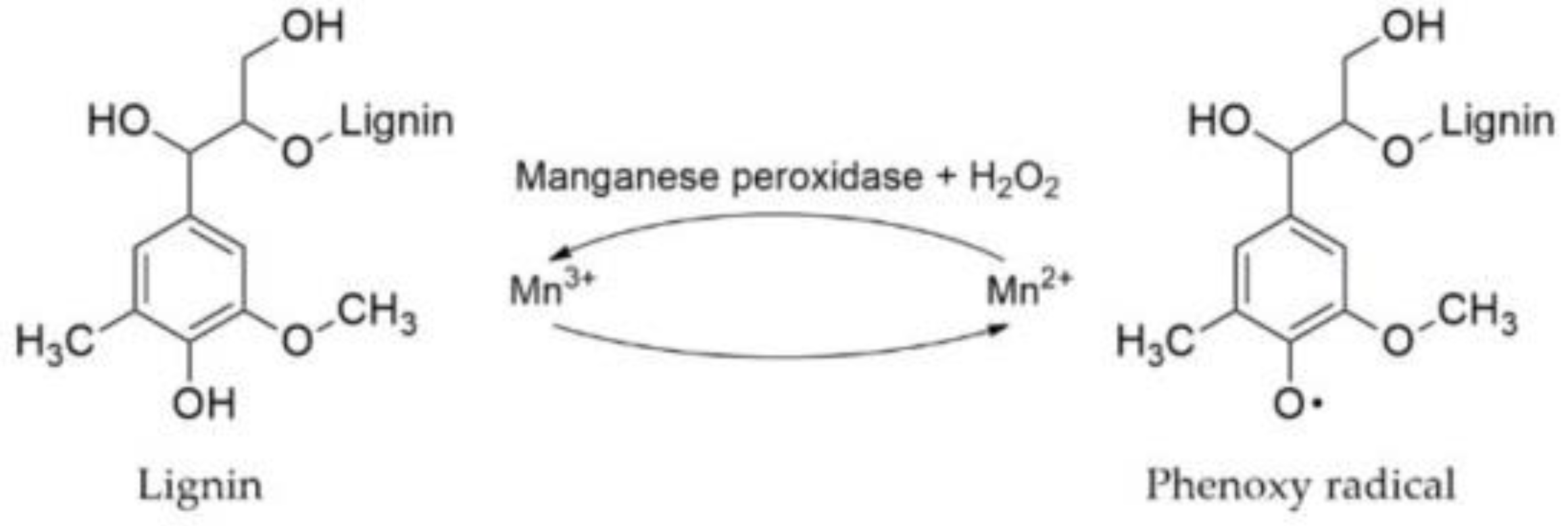
2.10. Manganese Peroxidase (MnP EC 1.11.1.13) Enzymatic Activity
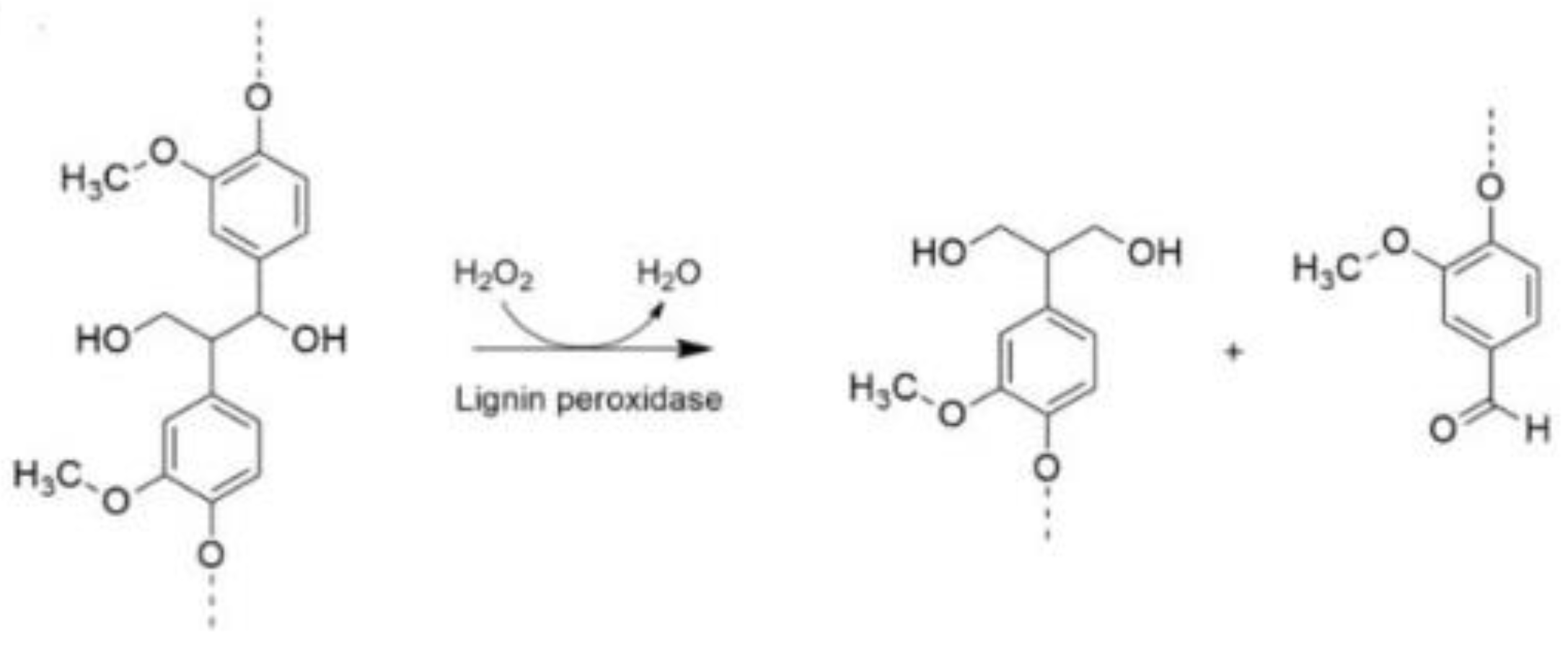
2.11. Lignin Peroxidase (LiP EC 1.11.1.14) Enzymatic Activity
2.12. Chemical Composition of Hay before and after Solid State Fermentation (SSF)
2.13. Structural Analysis of Ryegrass Hay with Fescue
2.14. Statistical Analysis
3. Results
3.1. Chemical Composition of Ryegrass-Fescue Hay before and after 14 Day Solid State Fermentation by Action of P. ostreatus
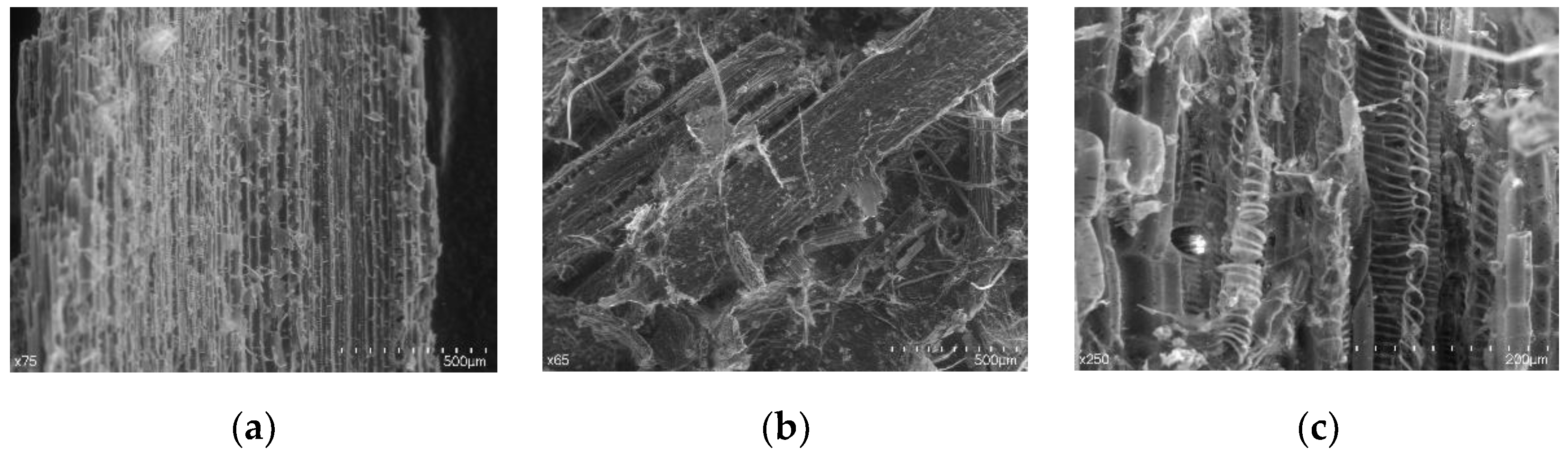
3.2. Structural Analysis
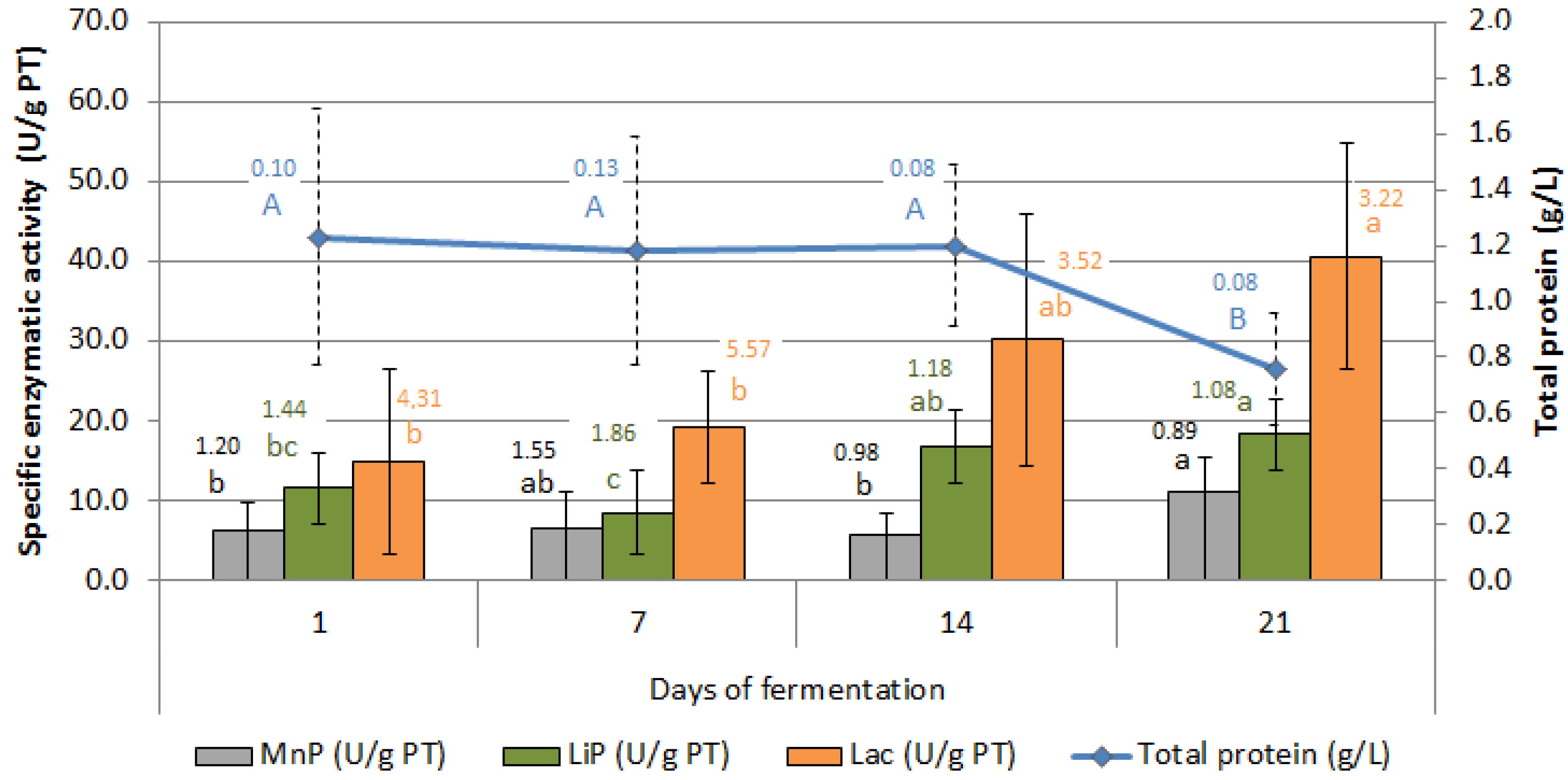
3.3. Enzymatic Activity of P. ostreatus in Submerged Fermentation
3.4. Enzymatic Activity of P. ostreatus in SSF
4. Discussion
4.1. Bioconversion of Hay by the Action of P. ostreatus and Structural Analysis
4.2. Enzymatic Activity of P. ostreatus in Submerged Fermentation
4.3. Enzymatic Activity of P. ostreatus in SSF
4.4. Producer-Level Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callejo, A. Conservación de Forrajes (II): Fundamentos de la henificación. Frisona Española 2017, 220, 104–109. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Ortiz, M. Aproximaciones a la comprensión de la degradación de la lignina. Orinoquia 2009, 13, 137–144. [Google Scholar]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzym. Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Arora, D.S.; Sharma, R.K. Effect of different supplements on bioprocessing of wheat straw by Phlebia brevispora: Changes in its chemical composition, in vitro digestibility and nutritional properties. Bioresour. Technol. 2011, 102, 8085–8091. [Google Scholar] [CrossRef]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef]
- Howard, R.L.; Abotsi, E.; van Rensburg, E.J.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Hong, Y.; Dashtban, M.; Chen, S.; Song, R.; Qin, W. Enzyme production and lignin degradation by four basidiomycetous fungi in submerged fermentation of peat containing medium. Int. J. Biol. 2012, 4, 172–180. [Google Scholar] [CrossRef]
- Arora, D.S.; Sharma, R.K. Comparative ligninolytic potential of Phlebia species and their role in improvement of in vitro digestibility of wheat straw. J. Anim. Feed Sci. 2009, 18, 151–161. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production though the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Have, R.T.; Teunissen, P.J. Oxidation mechanisms involved in lignin degradation by white rot fungi. Chem. Rev. 2001, 101, 3397–3413. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyski, A. Lignin degradation: Microorganisms, enzymes involved genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Cho, N.S.; Luterek, J.; Wilkolazka, A.; Wojtas-Wasilewska, M.; Matuszewska, A.; Hofrichter, M.; Wesenberg, D.; Rogalski, J. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar]
- Shrivastava, B.; Nandal, P.; Sharma, A.; Jain, K.K.; Khasa, Y.P.; Das, T.K.; Mani, V.; Kewalramani, N.J.; Kundu, S.S.; Kuhad, R.C. Solid state bioconversion of wheat straw into digestible and nutritive ruminant feed by Ganoderma sp. rckk02. Bioresour. Technol. 2012, 107, 347–351. [Google Scholar] [CrossRef]
- Díaz-Godínez, G.; Téllez-Téllez, M.; Sánchez, C.; Díaz, R. Characterization of the solid-state and liquid fermentation for the production of laccases of Pleurotus ostreatus. In Fermentation Processes; Jozala, A., Ed.; IntechOpen: London, UK, 2017; Chapter 4; pp. 57–74. [Google Scholar]
- Cueva, C.M. Aprovechamiento De Residuos De Plátano, Cacao Y Maíz Como Sustratos Para La Producción Del Hongo “Pleurotus ostreatus”, En La Comunidad La Magdalena De Francisco De Orellana; Presentado Para Optar El Grado Académico De: Ingeniera En Biotecnología Ambiental. Facultad De Ciencias, Escuela Superior Politécnica de Chimborazo: Orellana, Ecuador, 2018. Available online: http://dspace.espoch.edu.ec/handle/123456789/10172 (accessed on 11 April 2020).
- Chan-Cupul, W.; Heredia-Abarca, G.P.; Rodríguez-Vázquez, R. Aislamiento y evaluación de la actividad enzimática ligninolítica de acromicetos del estado de Veracruz, México. Rev. Int. Contam. Ambient. 2016, 32, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Ergun, S.O.; Urek, R.O. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann. Agrar. Sci. 2017, 15, 273–277. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia-Amezquita, L.E.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 1–10. [Google Scholar] [CrossRef]
- Shraddha, R.S.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Iandolo, D.; Piscitelli, A.; Sannia, G.; Faraco, V. Enzyme Production by Solid Substrate Fermentation of Pleurotus ostreatus and Trametes versicolor on Tomato Pomace. Appl. Biochem. Biotechnol. 2011, 163, 40–51. [Google Scholar] [CrossRef] [Green Version]
- García, A. Estudio De La Degradación De Residuos Lignocelulósicos Derivados Del Procesamiento Industrial Del Cranberry (Vaccinium macrocarpon Ait.). Ph.D. Thesis, Universidad Austral de Chile, Valdivia, Chile, 2006. [Google Scholar]
- Rodriguez-Barbosa, J.; dos Santos-Freitas, M.M.; da Silva-Martins, L.H.; de Carvalho-Junior, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.A.; Buglione, M.B.; Filippi, M.V.; Reynoso, L.C.; Rodríguez, G.E.; Agüero, M.S. Evaluación del crecimiento mycelial de Pleurotus ostreatus y Agrocybe aegerita sobre orujos de pera. An. Biol. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Tuyen, D.V.; Phuong, H.N.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Effect of fungal treatments of fibrous agricultural by-products on chemical composition and in vitro rumen fermentation and methane production. Bioresour. Technol. 2013, 129, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Sharma, R.K.; Chandra, P. Biodelignification of wheat straw and its effect on in vitro digestibility and antioxidant properties. Int. Biodeterior. Biodegrad. 2011, 65, 352–358. [Google Scholar] [CrossRef]
- Fazaeli, H.; Mahmodzadeh, H.; Azizi, A.; Jelan, Z.A.; Liang, J.B.; Rouzbehan, Y.; Osman, A. Nutritive value of wheat straw treated with Pleurotus fungi. Asian-Australas. J. Anim. Sci. 2004, 17, 1681–1688. [Google Scholar]
- Hoa, H.T.; Wang, C.L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcón, J.; Águila, S.; Arancibia-Ávila, P.; Fuentes, O.; Zamorano-Ponce, E.; Hernández, M. Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains. Z. Nat. C 2003, 58, 62–64. [Google Scholar] [CrossRef]
- González-Caloch, I.; Nava-Galicia, S.; Díaz-Godínez, R.; Garrido-Bazán, V.; Tlecuitl-Beristain, S.; Bibbins-Martínez, M.D. Determinación de la actividad de oxidasas producidas por Oxyporus latemarginatus crecido en fermentación sumergida en presencia y ausencia de colorante amarillo azo. Rev. Latinoam. El Ambiente Y Las Cienc. 2015, 6, 72–88. [Google Scholar]
- Rahman, M.M.; Lourenco, M.; Hassim, H.A.; Baars, J.J.P.; Sonnenberg, A.S.M.; Cone, J.W.; De Boever, J.; Fievez, V. Improving ruminal degradability of oil palm fronds using white rot fungi. Anim. Feed Sci. Technol. 2011, 169, 157–166. [Google Scholar] [CrossRef]
- Akpinar, M.; Urek, R.O. Production of ligninolytic enzymes by solid-state fermentation using Pleurotus eryngii. Prep. Biochem. Biotechnol. 2012, 42, 582–597. [Google Scholar] [CrossRef]
- Akinfemi, A.; Ogunwole, O.A. Chemical composition and in vitro digestibility of rice straw treated with Pleurotus ostreatus, Pleurotus pulmonarius and Pleurotus tuber-regium. Slovak J. Anim. Sci. 2012, 45, 14–20. [Google Scholar]
- Ardón, C. La Producción De Hongos Comestibles. Master’s Thesis, Universidad de San Carlos de Guatemala, Ciudad de Guatemala, Guatemala, 2007. [Google Scholar]
- Páez, M. Determinación De La Actividad Enzimática De Lacasa Y Lignina Peroxidasa De Hongos Degradadores De Colorantes Seleccionados Para El Tratamiento De Aguas Residuales De La Industria Textil; Memora de título para obtención de título de Ingeniería en Biotecnología; Escuela Politécnica del Ejército: Sangolqui, Ecuador, 2012. [Google Scholar]
- Usnayo, P. Optimización De Medios De Cultivo, Para La Producción De Enzimas Ligninolíticas, Por Cepas Fúngicas Aisladas Del Altiplano Boliviano. Trabajo de grado para optar al título de Lic. En Bioquímica. Bachelor’s Thesis, Universidad Mayor de San Andrés, La Paz, Bolivia, 2007. [Google Scholar]
- Téllez-Téllez, M.; Fernández, F.J.; Montiel-González, A.M.; Sánchez, C.; Días-Godínez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef] [PubMed]
- García-Oduardo, N.; Bermúdez-Savon, R.; Téllez-Suarez, I.; Chávez-Toledano, M.; Perraund-Gaime, I. Enzimas lacasa en inóculos de Pleurotus spp. RTQ 2017, 27, 39–45. [Google Scholar]
- AOAC (Association of Official Analytical Chemists Official). Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1997. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- InfoStat Versión 2017. Grupo InfoStat, FCA. Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. Available online: http://www.infostat.com.ar (accessed on 19 March 2020).
- NRC. Nutrient Requirement of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Anrique, R. Composición De Alimentos Para El Ganado Bovino; Cuarta Ed. Consorcio Lechero; Imprenta América Ltd.: Región Metropolitana, Chile, 2014. [Google Scholar]
- Jonathan, S.G.; Okorie, A.N.; Garuba, E.O.; Babayemi, O.J. Bioconversion of sorghum stalk and rice straw into value added ruminant feed using Pleurotus pulmonarius. Nat. Sci. 2012, 10, 10–16. [Google Scholar]
- Shabtay, A.; Hadar, Y.; Eitam, H.; Brosh, A.; Orlov, A.; Tadmor, Y.; Izhaki, I.; Jerem, Z. The potential of Pleurotus-treated olive mill solid waste as cattle feed. Bioresour. Technol. 2009, 100, 6457–6464. [Google Scholar] [CrossRef]
- Calsamiglia, S. Nuevas Bases Para La Utilización De La Fibra En Dieta De Rumiantes. Avances En Nutrición Y Alimentación Animal: Xiii Curso De Especialización Fedna/Coord; Rebollar, P.G., Beorlegui, C.d., Mateos, G.G., Eds.; Universidad Autónoma de Barcelona: Barcelona, Spain, 1997; pp. 3–19. [Google Scholar]
- Carro-Travieso, M.D.; de Evan, T.; González-Cano, J. Emisiones de metano en los animales rumiantes: Influencia de la dieta. Rev. Albéitar 2018, 220, 32–35. [Google Scholar]
- Bonilla-Cárdenas, J.A.; Lemus-Flores, C. Emisión de metano entérico por rumiantes y su contribución al calentamiento global y al cambio climático. Revisión. Rev. Mex. Cienc. Pecu. 2012, 3, 215–246. [Google Scholar]
- Fazaeli, H.; Azizi, A.; Amile, M. Nutritive value index of treated wheat straw with Pleurotus Fungi fed to sheep. Pak. J. Biol. Sci. 2006, 9, 2444–2449. [Google Scholar] [CrossRef] [Green Version]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of sorghum stover into animal feed with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef] [Green Version]
- Cofré, P.; Velasco, R. Forrajeras: Calidad Y Costos De Producción [En Línea]. Informativo Agropecuario Bioleche INIA Quilamapu. 2007. Available online: https://biblioteca.inia.cl/handle/123456789/34598 (accessed on 25 March 2020).
- Zuo, S.; Niu, D.; Zheng, M.; Jiang, D.; Tian, P.; Li, R.; Xu, C. Effect of Irpex lacteus, Pleurotus ostreatus and Pleurotus cystidiosus pretreatment of corn stover on its improvement of the in vitro rumen fermentation. J. Sci. Food Agric. 2018, 98, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R. Efecto Del Ph Inicial De Desarrollo De Pleurotus Ostreatus En Fermentación Sumergida Sobre Su Actividad De Lacasas. Master’s Thesis, Instituto politécnico nacional Tlaxcala, Tlaxcala, Mexico, 2009. [Google Scholar]
- Guillén-Navarro, G.K.; Márquez-Rocha, F.J.; Sanchez-Vásquez, J.E. Producción de biomasa y enzimas ligninolíticas por Pleurotus ostreatus en cultivo sumergido. Rev. Iberoam. Micol. 1998, 15, 302–306. [Google Scholar] [PubMed]
- Vyas, B.R.M.; Molitois, H.P. Involvement of an Extracellular H2O2-Dependent Ligninolytic Activity of the White Rot Fungus Pleurotus ostreatus in the Decolorization of Remazol Brilliant Blue R. Appl. Environ. Microbiol. 1995, 61, 3919–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Duarte, L.D. Evaluación De Enzimas Degradadoras De Lignina Producidas Por Aislamientos Fúngicos De Cultivos De Arroz; Trabajo de Grado Presentado Para Optar al Titulo de Microbiología Industrial; Pontificia Universidad Javeriana: Bogota, Colombia, 2012. [Google Scholar]
- France, A.; Cañumir, J.A.; Cortez, M. Produccion De Hongos Ostras (Pleurotus ostreatus); No. 23; Boletin INIA—Instituto de Investigaciones Agropecuarias: Primera Edición; Rodríguez, H., Ed.; Imprenta Trama: Chillán, Chile, 2000. [Google Scholar]


| Determinations * | Units | Ryegrass-Fescue Hay | Fermentation Residue |
|---|---|---|---|
| Proximal analysis | |||
| Dry matter (DM) | g/100g | 91.29 | 14.52 |
| Total ashes (TA) | g/100g | 4.41 | 3.83 |
| Crude protein (CP) a | g/100g | 4.73 | 5.16 |
| Etheric extract (EE) | g/100g | 1.30 | 1.10 |
| Non-fibrous carbohydrates (NFC) b | g/100g | 20.84 | 25.04 |
| Metabolizable energy (ME) c | Mcal/kg DM | 1.94 | 1.95 |
| Van Soest analysis | |||
| Neutral detergent fiber (aNDFom) | g/100g | 68.72 | 64.87 |
| Acid detergent fiber (aADFom) | g/100g | 42.45 | 42.05 |
| Acid detergent lignin d | g/100g | 5.88 | 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo-Neira, R.; Muñoz-Nuñez, E.; Quiroz-Carreno, S.; Avila-Stagno, J.; Alarcon-Enos, J. Bioconversion in Ryegrass-Fescue Hay by Pleurotus ostreatus to Increase Their Nutritional Value for Ruminant. Agriculture 2022, 12, 534. https://doi.org/10.3390/agriculture12040534
Astudillo-Neira R, Muñoz-Nuñez E, Quiroz-Carreno S, Avila-Stagno J, Alarcon-Enos J. Bioconversion in Ryegrass-Fescue Hay by Pleurotus ostreatus to Increase Their Nutritional Value for Ruminant. Agriculture. 2022; 12(4):534. https://doi.org/10.3390/agriculture12040534
Chicago/Turabian StyleAstudillo-Neira, Rita, Evelyn Muñoz-Nuñez, Soledad Quiroz-Carreno, Jorge Avila-Stagno, and Julio Alarcon-Enos. 2022. "Bioconversion in Ryegrass-Fescue Hay by Pleurotus ostreatus to Increase Their Nutritional Value for Ruminant" Agriculture 12, no. 4: 534. https://doi.org/10.3390/agriculture12040534






