Abstract
The potato leaf roll virus (PLRV) disease is a serious threat to successful potato production and is mainly controlled by integrated disease management; however, the use of chemicals is excessive and non-judicious, and it could be rationalized using a predictive model based on meteorological variables. The goal of the present investigation was to develop a disease predictive model based on environmental responses viz. minimum and maximum temperature, rainfall and relative humidity. The relationship between epidemiological variables and PLRV disease incidence was determined by correlation analysis, and a stepwise multiple regression was used to develop a model. For this purpose, five years (2010–2015) of data regarding disease incidence and epidemiological variables collected from the Plant Virology Section Ayub Agriculture Research Institute (AARI) Faisalabad were used. The model exhibited 94% variability in disease development. The predictions of the model were evaluated based on two statistical indices, residual (%) and root mean square error (RMSE), which were ≤±20, indicating that the model was able to predict disease development. The model was validated by a two-year (2015–2017) data set of epidemiological variables and disease incidence collected in Faisalabad, Pakistan. The homogeneity of the regression equations of the two models, five years (Y = −47.61 − 0.572x1 + 0.218x2 + 3.78x3 + 1.073x4) and two years (Y = −28.93 − 0.148x1 + 0.510x2 + 0.83x3 + 0.569x4), demonstrated that they validated each other. Scatter plots indicated that minimum temperature (5–18.5 °C), maximum temperature (19.1–34.4 °C), rainfall (3–5 mm) and relative humidity (35–85%) contributed significantly to disease development. The foliar application of salicylic acid alone and in combination with other treatments significantly reduced the PLRV disease incidence and its vector population over control. The salicylic acid together with acetamiprid proved the most effective treatment against PLRV disease incidence and its vector M. persicae.
1. Introduction
Potato (Solanum tuberosum L.) is one of the most important foods and vegetable crops in the world [1]. It is cultivated on 19.1 million hectares all over the world, with 381.7 million tons of tuber production, whereas, in Pakistan, 2.9 million tons of potatoes are produced from 0.15 million hectares of harvested area [2]. Its production is highly influenced by the attack of two viroids and 40 viruses [3]. One of the most severe viral diseases is caused by a potato leaf roll virus (PLRV), which is widely distributed in the potato growing regions of the world [4]. The virus is the type species of genus Polerovirus; it belongs to family Solemoviridae and was first identified by Somera et al. in 2021 [5]. It is efficiently transmitted by aphid species, particularly the green peach aphid M. persicae, in a circulative non-propagative manner and is restricted to the phloem tissues of infected plants [6]. The pathogen is responsible for 50% yield reduction in individual plants and over 20 million tons yield losses all over the world [7]. The primary symptoms of PLRV infections include rolling and yellowing of leaves, which may later roll inward. The secondary symptoms in the plant grown from infected tubers are the stunted growth of shoots and leaves rolling upward, starting from the oldest leaves [8]. The PLRV also causes net necrosis in the tubers and reduces crop quality. In Pakistan, 90% yield losses have been reported due to the PLRV disease incidence [9].
Efforts have been made by the plant pathologists and breeders to control PLRV disease incidence by adopting various techniques to ensure the production of virus-free seed potato stocks. These methods include specific growth strategies for seed production and storage, tissue culture and thermotherapy. The control of the virus vector by biopesticides, mineral oils and insecticides has been implemented successfully [10]. None of the varieties/advance lines have shown durable resistance against PLRV disease incidence in the country [11]. This is mainly due to the recurrent occurrence of the vector, continuous introductions of the viruses through imported seeds and the presence of diverse virus strains [12]. As a result, the use of insecticides to control the vector population has become an indispensable element for farmers all over the world, particularly in developing countries. A comprehensive study of the epidemiology of PLRV and its vector population is essential for justifying the application of insecticides. As an analytical tool, a predictive model provides an advanced prediction for vector populations and consequently helps in decisions making as to whether there is a need for insecticide application or not.
Epidemiology deals with the pathogen population on host plants under the impact of the environment at a particular time. Therefore, it is essential to investigate the influence of all the epidemiological variables that are involved in the development of a disease epidemic. For this purpose, detailed information regarding the pathogen, the host and the epidemiological variables, which may lead to the build-up of an epidemic, is of fundamental importance. Understanding the epidemiology of PLRV disease enables accurate prediction of its epidemic and determining the precise timing of application of chemicals in the light of most conducive environmental conditions. This would ultimately decrease pesticide use and thus promote environmentally friendly disease management. Hence, the main goal of the present study was to develop the epidemiological models based on environmental conditions of Faisalabad to predict PLRV disease incidence and to test the plant extracts/bio-pesticides/chemicals against PLRV disease incidence and Myzus persicae.
2. Materials and Methods
2.1. Development of Disease Predictive Model Based on Five-Year Data Set (2010–2015)
For the development of a disease predictive model, five years of data of PLRV disease incidence on three potato varieties, namely Desiree, Cardinal and Diamont, continuously cultivated for five years, and epidemiological variables data comprising minimum and maximum temperature, rainfall and relative humidity (RH) over six months, from November 2010 to April 2015, were collected from Plant Virology Section, Ayub Agriculture Research Institute (AARI), Faisalabad.
2.2. Model Evaluation
The model was evaluated based on a method described by Chatterjee and Hadi [13]. The following three steps were used during model evaluation: (i) comparison of physical theory with dependent variables and regression coefficients; (ii) comparison between observed and predicted values; and (iii) collection of new data to check predictions. The assessment of predictions was conducted through the root mean square error (RMSE) and error percentage as described by Chatterjee and Hadi [13]:
Pi and Oi are the predicted and observed values for the studied variables, respectively, whereas n is the total number of observations.
2.3. Collection of New Data Set
For the collection of a new data set, an experiment was conducted in the research field of the Department of Plant Pathology, University of Agriculture Faisalabad (UAF), during the autumn and spring crop seasons of 2015–2017 following the same procedures utilized in the preceding five years, as the soil type and environmental conditions of both places are almost identical. Three susceptible potato varieties—Cardinal, Diamant and Desiree—were sown during the winter planting in mid-October and spring planting in mid-January periods on the 25 × 25 experimental plots under randomized complete block design, with row-to-row and plant-to-plant distance of 75 and 20 cm, respectively. The crop was maintained in good conditions by following the recommended agronomic practices.
The disease incidence in the PLRV-infected plants was determined through visual inspection at every line in each plot after 15-day intervals during the 2015–2017 study period [14]. In each row, 10 plants demonstrating PLRV disease symptoms were selected and tagged, and the disease incidence was calculated using the expression given below.
2.4. Model Validation
For model validation, the PLRV disease incidence pertaining to the three potato varieties sown at the UAF experimental site noted during the 2015–2017 study period was used to develop a two-year model. This model was used to validate the five-year model by comparing the regression coefficients (R2) yielded by the F-test [15]. The data related to the epidemiological variables, namely minimum and maximum temperature, rainfall and relative humidity for the period covering November to April 2015–2017, were collected from UAF’s meteorological station (9610-B-1 Orion LX Weather Station).
2.5. Statistical Analysis
All obtained data were analyzed using Minitab V.17 (Minitab Inc., State College, PA, USA) and SPSS V.17 commercial software tools. The PLRV disease incidence and epidemiological variables were subjected to pairwise correlation and analysis of variance [16]. The least significant difference (LSD) test was adopted for the means separation (at p ≤ 0.05). A predictive model for PLRV disease incidence was developed on the basis of the epidemiological variables by performing stepwise multiple regression analysis [17]. Using the expressions below, coefficient of determination (R2) was calculated along with Adj. R2 to determine the strength of the relationship between individual epidemiological variables and the PLRV disease incidence and to test the model’s prediction accuracy [16]:
where n denotes the sample size, and k is the number of independent variables. Mean square error and Mallows’ Cp were also calculated to evaluate the influence of the independent variables included in the model using the following expressions [16]:
where p and n in the Cp equation are the number of beta coefficients and sample size in the model, respectively, whereas n and yi in mean square error (MSE) equation show the number of data values, observed values and predicted values, respectively. Average monthly values of all epidemiological variables and the PLRV disease incidence values were graphically plotted, and critical ranges conducive for disease development were determined.
2.6. Management Strategies of PLRV Disease Incidence and Its Vector
For effective management of PLRV disease and its vector Myzus persicae, biopesticides, mineral oils and insecticides namely SA (Salicylic acid) @ 200 mM or 27.4 g/L (T1), SA + Chemical (Acetameprid) @ 15 mL/20 L (T2), SA + Biocontrol (Tracer) @ 8 mL/20 L (T3), SA + Plant Extract (concentrated extract of Neem, a product from China) @ 5 mL/L (T4), SA + Mineral Oil (Dicer) @ 125 mL/20 L (T5) were sprayed on all three susceptible potato varieties—Cardinal, Diamant and Desiree—cultivated in the Research Area of Department of Plant Pathology, University of Agriculture Faisalabad (UAF), during the autumn and spring crop seasons of 2015–2017 with the help of hand knapsack sprayer on the PLRV-infested plants in the experimental area of UAF. The application of only distilled water served as control treatment (T6). The data on aphid population (apterae and alate aphids) and PLRV disease incidence were recorded before and after the 7-day application of treatments until the end of season by using the method described by Khan et al. [10]. The data were subjected to ANOVA, and the treatment means were compared with LSD test at p ≤ 0.05 [16].
3. Results
3.1. Development of PLRV Disease Predictive Model Based on Five-Year Data Set (2010–2015)
The data from five growing seasons (2010–2015) showed that all the epidemiological variables significantly contributed to PLRV disease incidence (Table 1).

Table 1.
Estimated Pearson’s correlation coefficients of the relationships between PLRV disease incidence in potatoes in Pakistan and environmental variables during the 2010–2015 field seasons.
A stepwise multiple regression model (Y = −47.61 − 0.572x1 + 0.218x2 + 3.78x3 + 1.073x4) based on a five-year data set exhibited 94% variability in the PLRV disease development (Table 2). This model could be used for PLRV disease prediction.

Table 2.
Summary of stepwise multiple regression model to predict PLRV disease incidence during 2010–2015.
3.2. Model Evaluation: Comparison of Physical Theory with Dependent Variables and Regression Coefficients
The model exhibited higher R2 (94.57%) and Adj. R2 (94.44%) values with lower standard error value ≤ 20 (Table 3).

Table 3.
Regression statistics of PLRV disease incidence during 2010–2015.
The F-distribution of the disease predictive model indicated significant regression statistics (Table 4).

Table 4.
ANOVA of PLRV disease predictive model based on five-year data set (2010–2015).
The minimum and maximum temperature, rainfall and relative humidity showed significant association with the PLRV disease model at p ≤ 0.05 (Table 5). The higher coefficient of regression (R2) value, lower standard error value and the significance of regression statistics exhibited that the model was able to predict PLRV disease incidence (Table 3, Table 4 and Table 5).

Table 5.
Coefficients of estimates, their standard error, t Stat and significance of multiple regression model during 2010–2015.
3.3. Model Evaluation
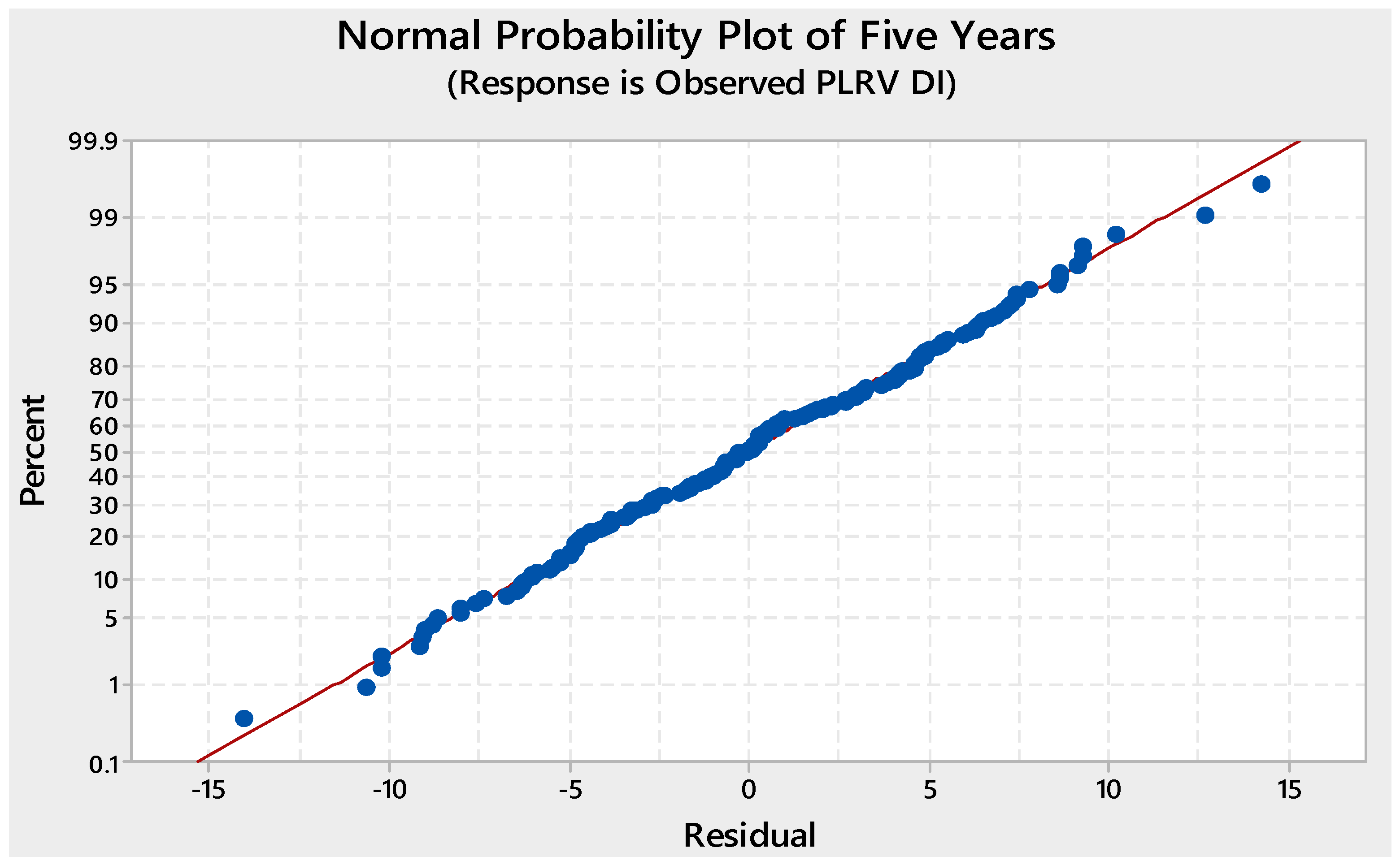
For the evaluation of the model, predictions were obtained using a regression model and evaluated based on two criteria: error (%) and root means square error (RMSE). The normal probability plot of the five-year model showed that most of the data points were around the reference line, while only a few data points, both at the higher and lower sides, deviated from the reference line, affecting the normal distribution of data. Overall, 15% residual was recorded, indicating a fair degree of matching between the observed and predicted data points (Figure 1).
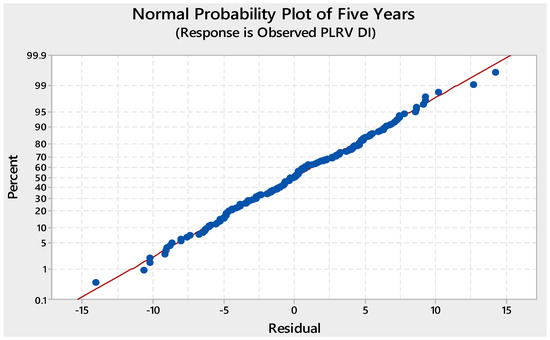
Figure 1.
Normal probability plot for five-year (2010–2015) model of potato leaf roll virus (PLRV) disease incidence.
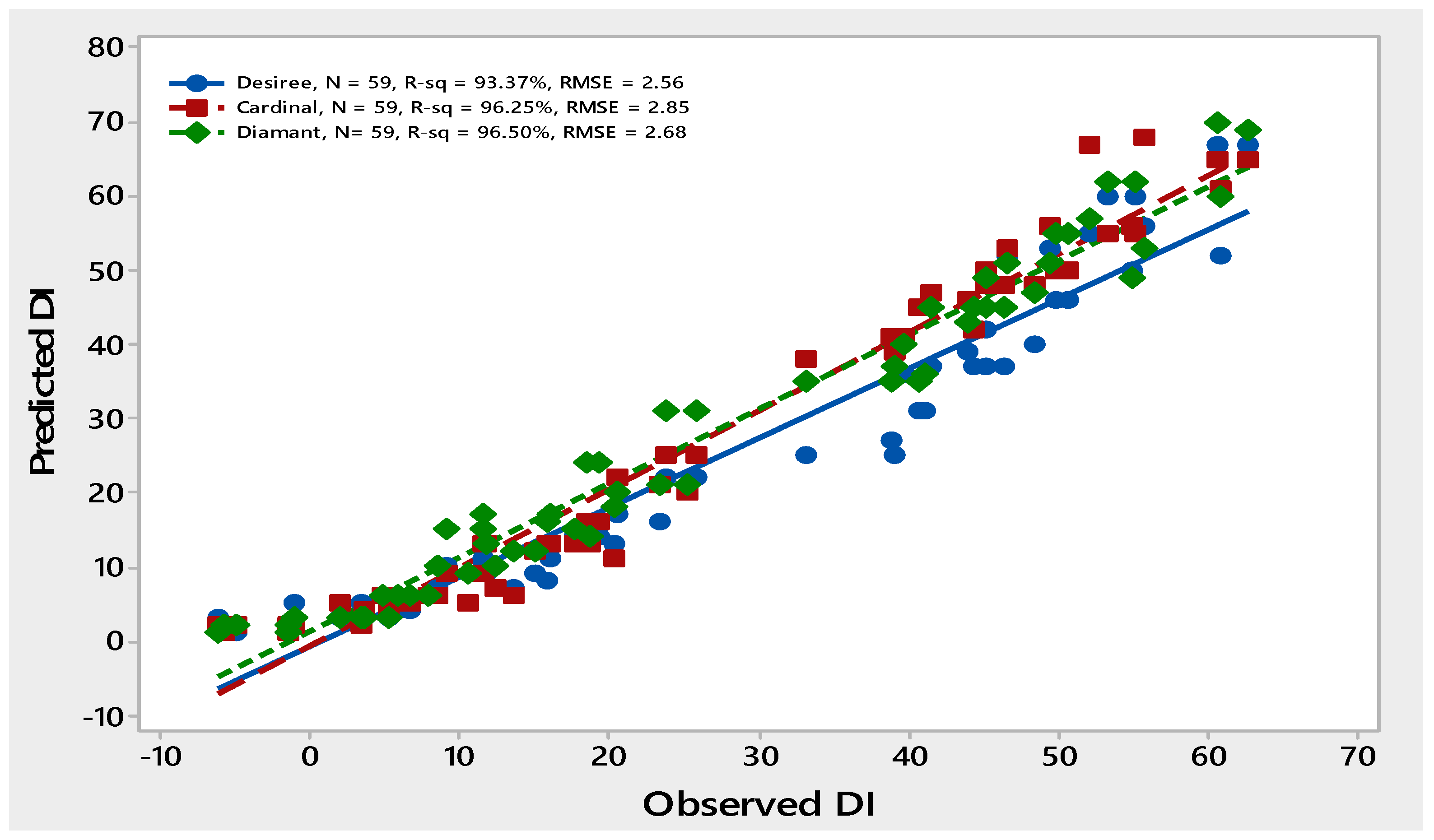
The higher R2 values > 90% and smaller RMSE values ≤ 20 of all three potato genotypes showed the close conformation between observed and predicted data points, indicating that the model was good at predicting PLRV disease incidence (Figure 2).
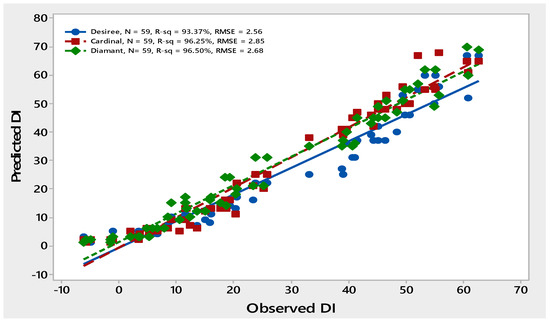
Figure 2.
Comparison of observed and predicted data points of PLRV disease incidence of three potato varieties—Desiree, Cardinal and Diamant—during years 2010–2015.
3.4. Model Validation
The stepwise multiple regression model based on a five-year data set was validated on the two-year data set collected from the UAF. The coefficients of determination (R2) of both models I and II indicated that environmental factors had significantly 94 and 89% impact on PLRV disease incidence, respectively. The regression equations of the two models demonstrated good proximity (Table 6).

Table 6.
Comparison of two multiple regression models for validation of PLRV disease incidence.
3.5. Characterization of Environmental Conditions Conducive for PLRV Disease during 2015–2017
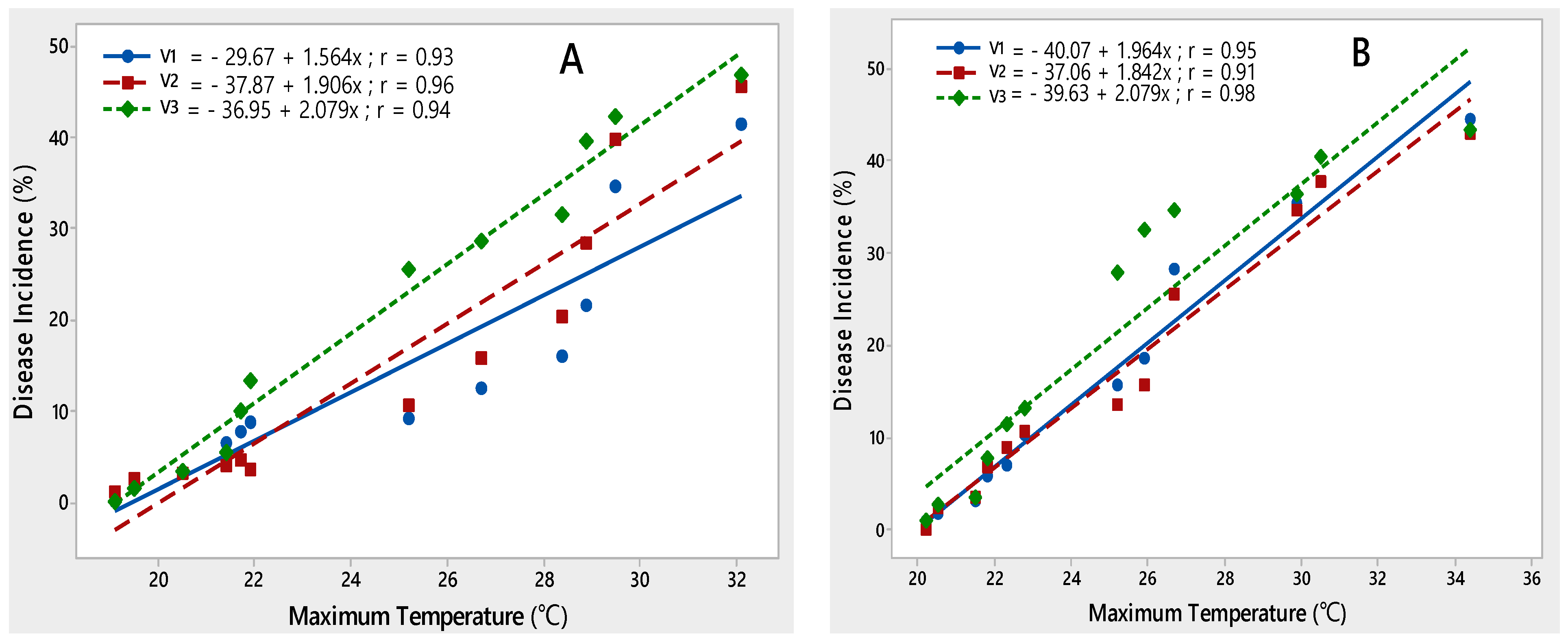
Three potato varieties, namely Desiree, Cardinal and Diamant, were employed for regression analysis to characterize critical ranges of epidemiological variables conducive for PLRV disease development. A significant relationship was observed between disease incidence and all environmental variables during both rating seasons. The maximum temperature contributed significantly to the development of PLRV disease on all potato varieties during 2015–2017. It was observed that with an increase in maximum temperature from 19.1–32.1 °C in 2015–2016 and 20.2–34.4 °C during 2016–2017, disease incidence also increased. This relationship was best explained by the linear regression model, as indicated by their correlation coefficient (r) values (Figure 3).
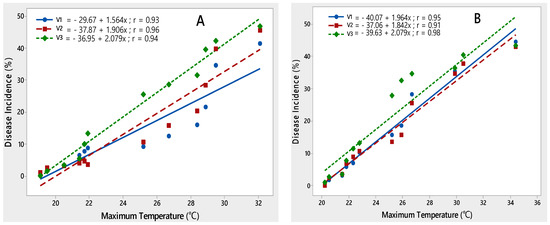
Figure 3.
Relationship between maximum temperature and PLRV disease incidence recorded on potato varieties V1 (Desiree), V2 (Cardinal) and V3 (Diamant) during 2015–2016 (A) and 2016–2017 (B).
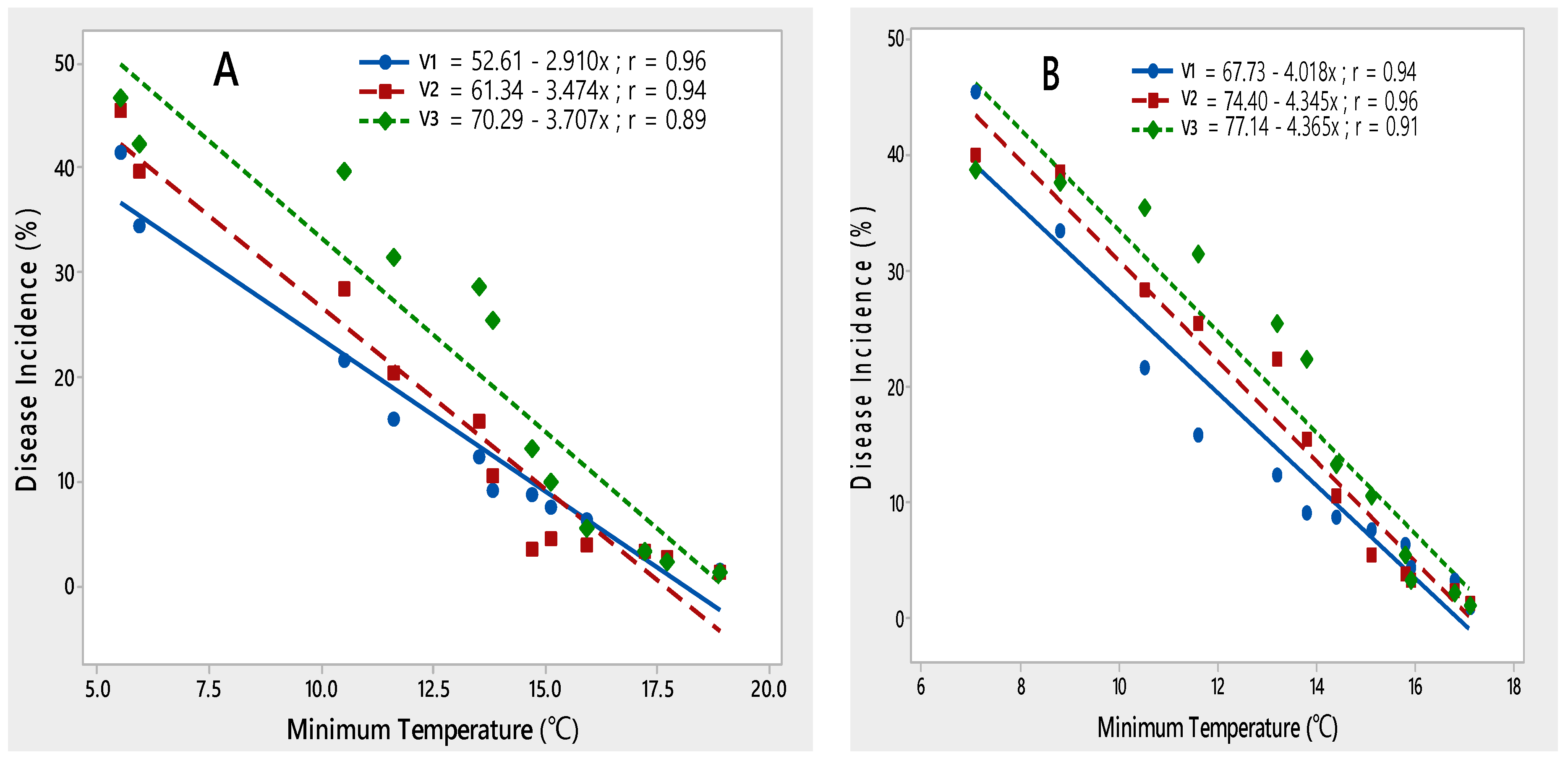
A negative linear relationship was observed between minimum temperature and PLRV disease incidence on all three potato varieties during both rating seasons of 2015–2017, indicating that with an increase in minimum temperature from 5 to 18.5 °C, disease incidence decreased (Figure 4).
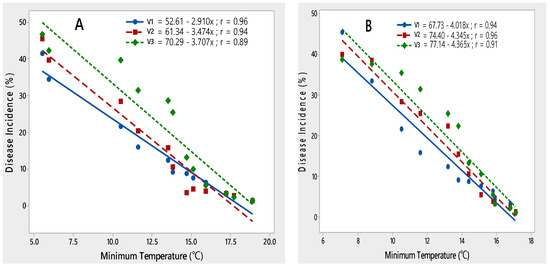
Figure 4.
Relationship between minimum temperature and PLRV disease incidence recorded on potato varieties V1 (Desiree), V2 (Cardinal) and V3 (Diamant) during 2015–2016 (A) and 2016–2017 (B).
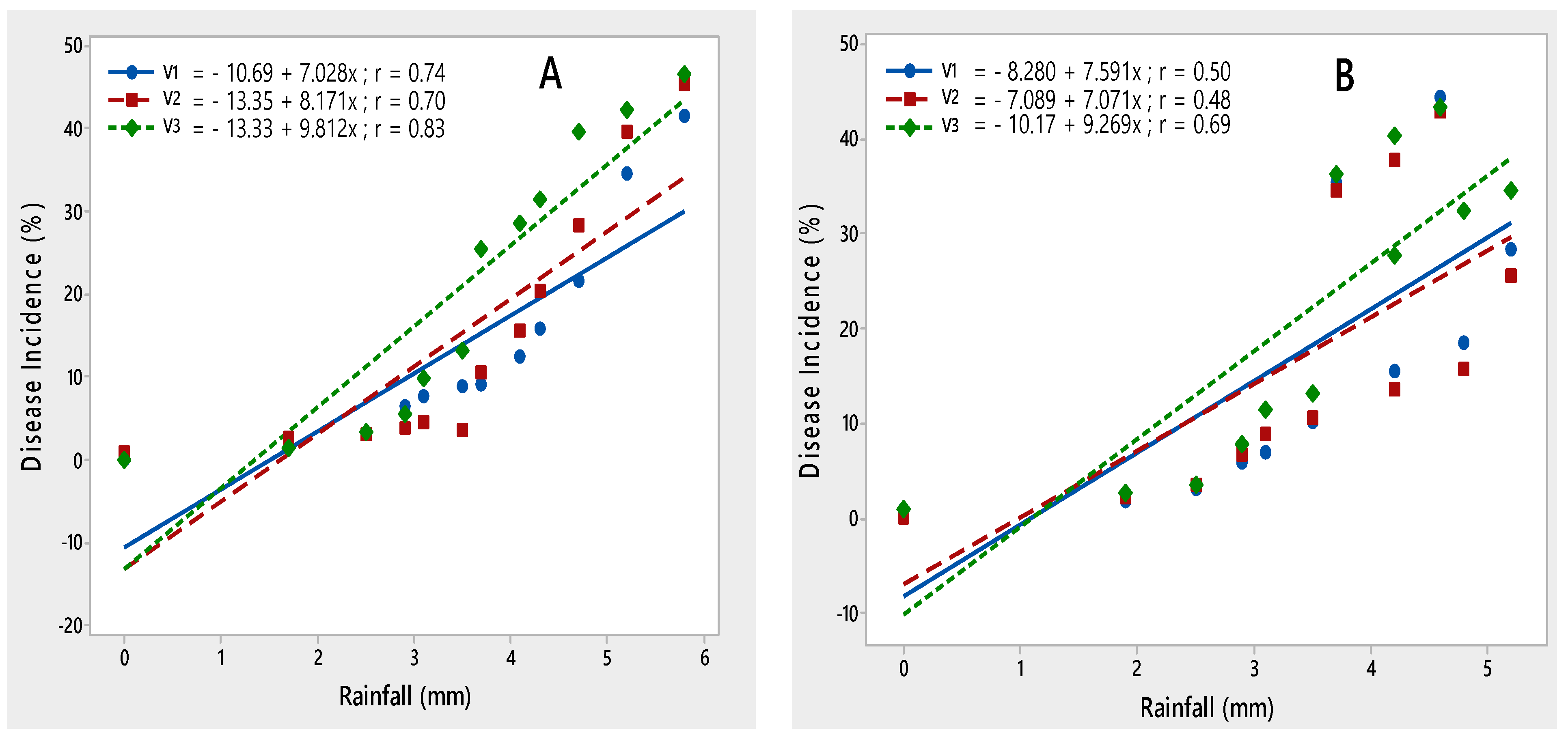
The impact of rainfall was recorded as significant with the PLRV disease development. The maximum disease was noted at 3–5 (mm) during both crop seasons; it demonstrated that disease incidence increased with an increase in rainfall, as demonstrated by their r values 0.74, 0.70 and 0.83 during 2015–2016 and 0.50, 0.48 and 0.69 during 2016–2017, respectively (Figure 5).
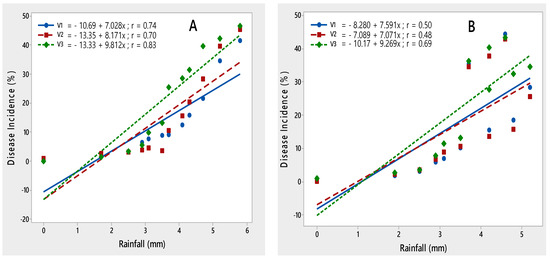
Figure 5.
Relationship between rainfall and PLRV disease incidence recorded on potato varieties V1 (Desiree), V2 (Cardinal) and V3 (Diamant) during 2015–2016 (A) and 2016–2017 (B).
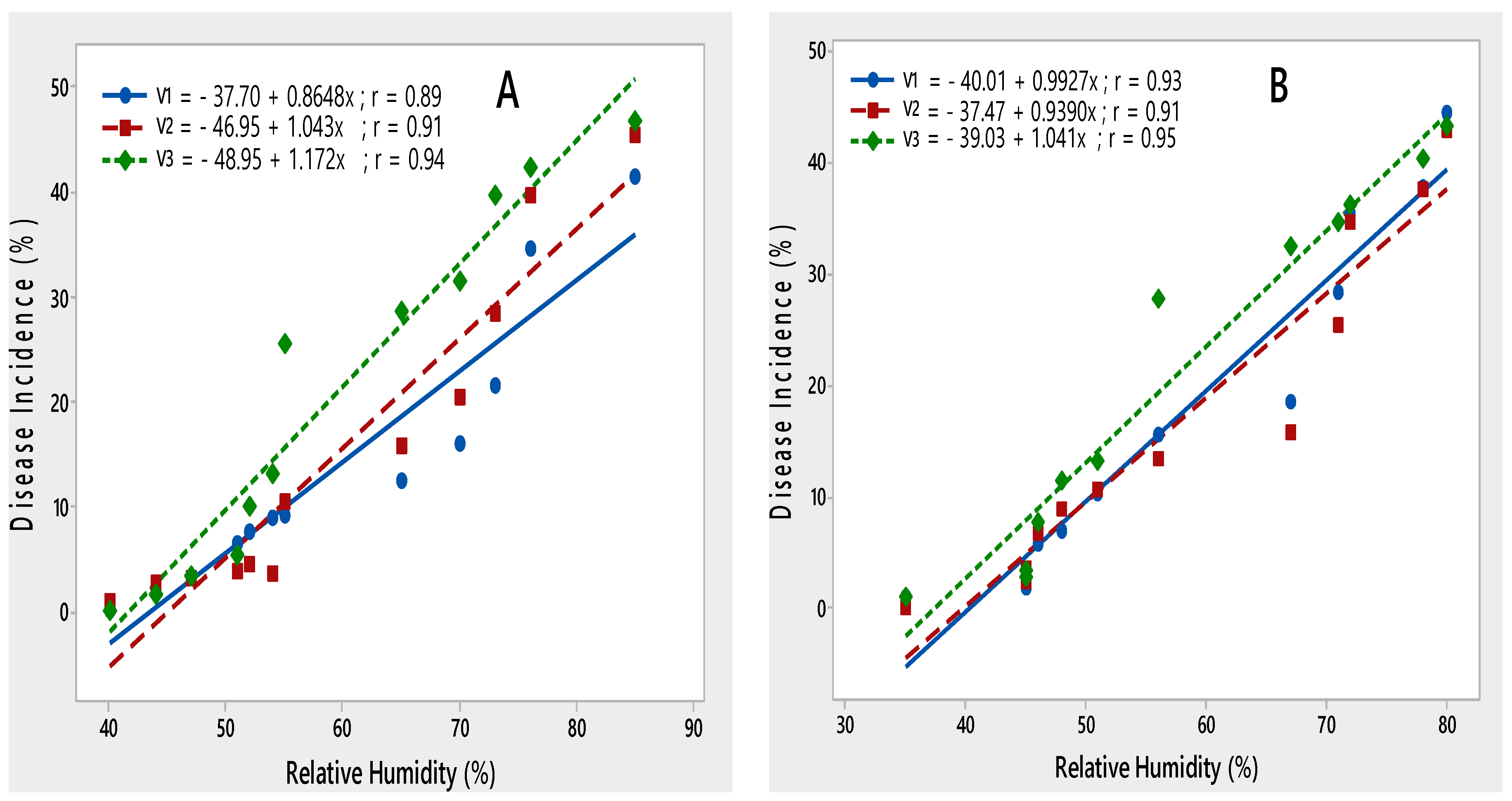
Relative humidity was positively correlated with disease incidence. During both rating seasons, disease incidence increased with an increase in relative humidity from 35 to 85% (Figure 6).
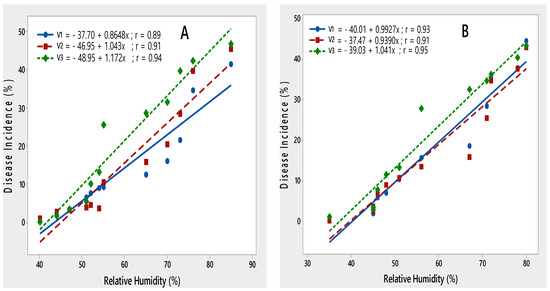
Figure 6.
Relationship between relative humidity and PLRV disease incidence recorded on potato varieties V1 (Desiree), V2 (Cardinal) and V3 (Diamant) during 2015–2016 (A) and 2016–2017 (B).
3.6. Management Strategies for PLRV and Its Vector
The exogenous application of salicylic acid alone and its combination with other treatments indicated a significant effect in controlling PLRV disease and its vector M. persicae as compared to the control. The salicylic acid in combination with acetamiprid proved the most effective in controlling PLRV disease incidence and its vector populations during both crop seasons of 2015–2017, followed by SA in combination with biocontrol (tracer), plant extract (neem), mineral oil, as compared to the salicylic acid alone and control (Table 7 and Table 8).

Table 7.
Comparison of treatments to control aphid populations during 2015–2017.

Table 8.
Comparison of treatments to control PLRV disease incidence during 2015–2017.
4. Discussion
Environmental conditions played a significant role in the development of pathogens on any crop; therefore, quantifying the relationship between PLRV disease incidence and epidemiological variables is important in early warning of its onset [10]. PLRV is significantly influenced by the epidemiological variables; however, the degree of correlation changes greatly by varieties and years. A significant correlation between epidemiological variables and PLRV disease incidence was observed in this investigation, in line with the findings of Khan and Abbas [18], who demonstrated a significant correlation of temperature (minimum and maximum), rainfall and relative humidity with PLRV disease incidence.
The significant correlation of temperature with PLRV disease incidence can be explained by the fact that it has a critical role in different aspects of disease development. The expression of viral disease symptoms was delayed at low temperatures in several plant species [19,20,21,22]. Szittya et al. (2003) described that temperature effect of plant–pathogen interactions and high temperature can either increase or decrease the disease resistance [21]. This reflects the effects of the same temperature variation on various plant–pathogen systems [23]. Virus resistance was compressed in plants at a higher temperature. For example, Capsicum chinense plants carrying the Tsw gene and tobacco plants carrying the N gene developed systemic infections of tomato spotted wilt virus (TSWV) and tobacco mosaic virus (TMV) at above 28 and 32 °C, respectively [24,25]. The increasing temperature alters the host plant physiology, phenology, morphology, nutritional status and metabolic pathways [26,27]. The rising heat stress and mean temperature reduced the effectiveness of temperature-sensitive single-gene resistance and increased general plant vulnerability to virus infection. Increased temperature also changes the virus multiplication, seed transmission and systemic movement of individual viruses present in mixed infection [27]. Jones (2014) showed that potato yellow vein virus (PYVV) and PLRV best adapted to hot regions; conversely, potato mop-top virus (PMTV) and Andean potato latent virus are projected for regions too cold for growth and development [28]. The significant relationship of relative humidity and rainfall with PLRV disease incidence was due, in part, to its key role in the survival, population growth, behavior and movement of virus vector [29]. Virus dispersal in crops is favored by the soft tender leaves and lush plant growth that develop under conditions of high relative humidity. Such plants are more vulnerable to viral infection as compared to the hard-leaved plants of low-humidity conditions. This is because wounds develop more readily when growth is soft, and viruses have to penetrate a plant’s protective cuticle through wounds before they can invade damaged cells [30].
The maximum temperature (19.1–34.4 °C), minimum temperature (5–18.5 °C), (rainfall (3–5 mm) and relative humidity (35–85%) appeared to be the main contributing epidemiological variables in the disease development, as these variables were retained after stepwise regression. The present multiple regression model explained 94% variability in PLRV disease development, whereas only 6% variability remained unexplained. The models that explain >80% variability are considered reliable and provide relatively accurate predictions [31]. The reason behind not explaining 100% variability might be due to the fact that regression models are empirical models. Khan and Abbas (2008) developed the multiple regression models and reported 60% unexplained variability in PLRV disease development when only environmental variables were used [18]. However, by including the primary source of virus inoculum and other biological factors as independent variables, the unexplained variability may be reduced [31]. Further, the present study was laid out under natural environmental conditions where the amounts of inoculum and infection efficiency were uncontrolled; an explanation of 100% variability was not possible. However, the current investigation remained successful in predicting PLRV disease because the model, with a large data set of five years, validated with a two-year data set, generated approximately precise predictions. The high coefficient of determination (R2) value 0.94 of the model indicated that it can be used in future for accurate prediction of PLRV disease.
Considering the management strategies of PLRV disease and its vector aphid, salicylic acid (SA) alone and its combination with other treatments, such as biopesticides, chemicals, mineral oils and neem extracts, significantly decreased the PLRV disease incidence and aphid population over control. It means that the application of salicylic acid is effective in controlling PLRV disease incidence by inducing systemic resistance in plants. Koo et al. [32] showed that exogenous application of SA provides tolerance to plants against several plant pathogens [33]. In tobacco, the foliar application of SA induced resistance against tobacco mosaic virus (TMV) [34]. The pathogenicity-related proteins are activated by the foliar application of SA against many plant viruses. After the application of SA, potato plants develop systemic acquired resistance (SAR), which results in the activation of plant defense mechanism [35]. The SA in combination with pesticide acetamiprid proved the most effective in controlling PLRV disease incidence and M. persicae populations. Acetamiprid has the ability to decrease the infection and dispersal rate of plant viruses during the pre-mortality phase [36]. Acetamiprid is very selective and provides an effective control against sucking pests, such as whiteflies and aphids, without negative impact on non-target insects [37]. The tracer in combination with SA and azadirachtin extracted from the seeds of the neem tree (Azadirechta indica) disturbs the feeding behavior of aphid and fecundity through repellent and antifeedant activity [38]. Mineral oil, which was the least effective in controlling PLRV disease incidence, does not kill aphids Myzus persicae but reduces the transmission by altering its behavior. Yang et al. [38] described that after 30 min of oil application, M. persicae was unable to transmit PVY in plants but could do so after 24 h, although with diminished ability.
5. Conclusions
It was concluded that a five-year model validated with a two-year data set exhibited 94% variability in the PLRV disease development. All environmental variables indicated a significant relationship with PLRV disease incidence. Regression analysis proved that there was a significant effect of average seasonal minimum temperature (5–18.5 °C), maximum temperature (19.1–34.4 °C), rainfall (3–5 mm) and relative humidity (35–85%) on PLRV disease development. The study concluded that PLRV disease can be managed when its vector is controlled. As the environmental conditions play crucial role in the development of the disease, the disease predictive models would be helpful for farmers in the proper management of the disease. The disease forecast model helps them decide whether to spray a crop right away or to wait for more days. Bio-pesticides, insecticides, oils, plant extracts and other chemicals often provide only short-term virus disease control; these materials can be more effectively utilized when the epidemiological components are understood. Thus, understanding the epidemiology of PLRV disease will enable us to predict its development, which will ultimately help farmers to improve plant protection measures more accurately.
Author Contributions
Conceptualization, Y.A., A.R. and M.I.; methodology, Y.A., A.R., H.M.A. and S.U.-A.; software, S.u.R.; validation, Y.A., A.R. and S.u.R.; formal analysis, Y.A. and H.M.A.; investigation, Y.A. and M.I.; resources, A.R., M.A.A. and M.M.; data curation, Y.A. and S.U.-A.; writing—original draft preparation, Y.A., A.R. and M.I.; writing—review and editing, S.Y.M.M., E.S.H.F., M.A.A. and M.M.; supervision, A.R. and M.A.A.; project administration, S.Y.M.M. and M.M.; funding acquisition, A.R., M.A.A. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research, King Khalid University, Saudi Arabia under grant no. (RGP.-2/135/42).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through research grant no. (RGP.-2/135/42).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| AARI | Ayub Agriculture Research Institute |
| MSE | Mean Square Error |
| PLRV | Potato Leaf Roll Virus |
| RH | Relative Humidity |
| RMSE | Root Mean Square Error |
| SA | Salicylic Acid |
| UAF | University of Agriculture Faisalabad |
References
- He, Z.; Larkin, R.; Honeycutt, W. Sustainable Potato Production: Global Case Studies; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- FAOSTAT. Food and Agricultural Organization Statistical Database, Crop Production. 2017. Available online: http://faostat3.fao.org/download/Q/QC/E (accessed on 22 July 2017).
- Jeffries, C.; Barker, H.; Khurana, S.M. Viruses and viroids. In Handbook of Potato Production, Improvement, and Postharvest Management; Food Products Press: New York, NY, USA, 2006; pp. 387–448. [Google Scholar]
- Gillen, A.M.; Novy, R.G. Molecular characterization of the progeny of Solanum etuberosum identifies a genomic region associated with resistance to potato leafroll virus. Euphytica 2007, 155, 403–415. [Google Scholar] [CrossRef]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C.; ICTV Report Consortium. ICTV virus taxonomy profile: Solemoviridae. J. Gen. Virol. 2021, 102, 001707. [Google Scholar]
- Lee, L.; Kaplan, I.B.; Ripoll, D.R.; Liang, D.; Palukaitis, P.; Gray, S.M. A surface loop of the potato leafroll virus coat protein is involved in virion assembly, systemic movement, and aphid transmission. J. Virol. 2005, 79, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Wales, S.; Platt, H.W.; Cattlin, N. Diseases, Pests and Disorders of Potatoes; Manson Publ. Ltd.: London, UK, 2008; pp. 75–76. [Google Scholar]
- Abbas, A.; Sohail, M.A.; Mubeen, M.; Alami, M.M.; Umer, M.; Khan, S.U. Plant Viruses in Gilgit-Baltistan (GB) Pakistan: Potential Future Research Direction. Plant Pathol. Microbiol. 2020, 10, 486. [Google Scholar] [CrossRef][Green Version]
- Bhutta, A.R.; Bhatti, M.F. Seed Potato Certification in Pakistan; Federal Seed Certification and Registration Department Ministry of Food Agriculture and Livestock: Islamabad, Pakistan, 2002; pp. 60–66.
- Khan, M.A.; Hannan, A.; Naqvi, S.A.; Khan, A.A.; Zulfiqar, M.A. Development and validation of potato leaf roll virus disease prediction model based on environmental factors for Faisalabad, Pakistan. Pak. J. Agric. Res. 2016, 29, 1–14. [Google Scholar]
- Qamar, N.; Khan, M.A.; Rashid, A. Screening of potato germplasm against potato virus X (PVX) and potato virus Y (PVY). Pak. J. Phytopathol. 2003, 2, 189–190. [Google Scholar]
- Kreuze, J.F.; Souza-Dias, J.A.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.; Jones, R.A. Viral Diseases in Potato. In The Potato Crop; Springer: Cham, Switzerland, 2020; pp. 389–430. [Google Scholar]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gabriel, W. The influence of temperature on the spread of aphid-borne potato virus diseases. Ann. Appl. Biol. 1965, 56, 461–475. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: New York, NY, USA, 2015. [Google Scholar]
- Steel, R.G.O.; Torrie, J.H.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Meyer, M.; Woodroofe, M. On the degrees of freedom in shape-restricted regression. Ann. Stat. 2000, 28, 1083–1104. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbas, W. Evaluation of multiple regression models based on epidemiological factors to predict M. persicae population and PLRV disease incidence. In International Conference of Plant Scientists; The Pakistan Botanical Society: Faisalabad, Pakistan, 2008; pp. 155–168. [Google Scholar]
- Saied, M.S.; Medany, M.A.; Abo El-Abbas, F.; El-Hammaday, M. Forecasting incidence potato leaf roll virus disease in Egypt. Egypt. J. Virol. 2005, 2, 201–213. [Google Scholar]
- Velázquez, K.; Renovell, A.; Comellas, M.; Serra, P.; García, M.L.; Pina, J.A.; Navarro, L.; Moreno, P.; Guerri, J. Effect of temperature on RNA silencing of a negative-stranded RNA plant virus: Citrus psorosis virus. Plant Pathol. 2010, 59, 982–990. [Google Scholar] [CrossRef]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Soustre-Gacougnolle, I.; Schmitt, C.; Perrin, M.; Burdloff, Y.; Chevalier, E.; Mutterer, J.; Himber, C.; Zervudacki, J.; Montavon, T.; et al. RNA silencing is resistant to low-temperature in grapevine. PLoS ONE 2013, 8, e82652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Erickson, F.L.; Dinesh-Kumar, S.P.; Holzberg, S.; Ustach, C.V.; Dutton, M.; Handley, V.; Corr, C.; Baker, B.J. Interactions between tobacco mosaic virus and the tobacco N gene. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 1999, 354, 653–658. [Google Scholar] [CrossRef]
- Roggero, P.; Pennazio, S.; Masenga, V.; Tavella, L. Resistance to tospoviruses in pepper. In Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera (CD-ROM); ANIC: Canberra, Australia, 2002; pp. 105–110. [Google Scholar]
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Chang. Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef]
- Jones, R.A.; Barbetti, M.J. Influence of climate change on, plant disease infections and epidemics caused by viruses and bacteria. Rev. Plant Sci. 2012, 22, 1–31. [Google Scholar] [CrossRef]
- Jone, R.A. Virus disease problems facing potato industries worldwide: Viruses found, climate change implications, rationalizing virus strain nomenclature, and addressing the Potato virus Y issue. In The Potato: Botany, Production and Uses; CABI: Wallingford, UK, 2014; pp. 202–224. [Google Scholar]
- Were, H.K.; Kabira, J.N.; Kinyua, Z.M.; Olubayo, F.M.; Karinga, J.K.; Aura, J.; Lees, A.K.; Cowan, G.H.; Torrance, L. Occurrence and distribution of potato pests and diseases in Kenya. Potato Res. 2013, 56, 325–342. [Google Scholar] [CrossRef]
- Jones, R.A. Future scenarios for plant virus pathogens as climate change progresses. Adv. Virus Res. 2016, 95, 87–147. [Google Scholar]
- Kumar, P.V. Development of weather-based prediction models for leaf rust in wheat in the Indo-Gangetic plains of India. Eur. J. Plant Pathol. 2014, 140, 429–440. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Murphy, A.M.; Carr, J.P. Salicylic acid has cell-specific effects on tobacco mosaic virus replication and cell-to-cell movement. Plant Physiol. 2002, 128, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Bhat, J.A.; Chandrashekar, N.; Papolu, P.K.; Rawat, S.; Grover, A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017, 39, 268. [Google Scholar] [CrossRef]
- Bethke, J.A.; Blua, M.J.; Redak, R.A. Effect of selected insecticides on Homalodisca coagulata (Homoptera: Cicadellidae) and transmission of oleander leaf scorch in a greenhouse study. J. Econ. Entomol. 2001, 94, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Bambhaniya, V.S.; Khanpara, A.V.; Patel, H.N. Bio-Efficacy of insecticides against sucking pests; whitefly and aphid infesting tomato. J. Pharmacogn. Phytochem. 2018, 7, 2051–2059. [Google Scholar]
- Nisbet, A.J.; Woodford, J.A.; Strang, R.H. The effects of azadirachtin on feeding by Myzus persicae. In Proceedings of the 8th International Symposium on Insect-Plant Relationships; Springer: Dordrecht, The Netherlands, 1992; pp. 179–180. [Google Scholar]
- Yang, Q.; Arthurs, S.; Lu, Z.; Liang, Z.; Mao, R. Use of horticultural mineral oils to control potato virus Y (PVY) and other non-persistent aphid-vectored viruses. Crop Prot. 2019, 118, 97–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).