Abstract
High-molecular-weight glutenin subunits (HMW-GSs) encoded by alleles at the Glu-A1, Glu-B1, and Glu-D1 loci confer unique end-use quality properties of common wheat (Triticum aestivum L.). Wheat accessions with the high-quality HMW-GSs combination of Ax2*/Bx7OE/Dx5 usually exhibit strong gluten characteristics. In order to stack these three high-quality subunit genes by molecular markers in strong gluten wheat breeding, an agarose gel-based multiplex PCR marker for these high-quality HMW-GSs and two agarose gel-based multiplex PCR markers detecting the homozygosity of Ax2* and Bx7OE subunits were developed. These markers were verified in an F2 segregating population from a cross between a medium-gluten winter wheat cultivar with the HMW-GSs combination of Ax null/Bx7 + By8/Dx4 + Dy12 and a strong-gluten spring wheat cultivar with the HMW-GSs combination of Ax2*/Bx7OE + By8*/Dx5 + Dy10. By integrating the newly established multiplex PCR markers and a published co-dominant PCR marker of the Dx5 subunit, a complete molecular marker selection system was established. After multiple rounds of molecular marker-assisted selection with the system, 17 homozygous winter wheat lines that stacked the three high-quality HMW-GSs were generated. The gluten strength of these homozygous lines was comparable to their strong-gluten parent, but significantly higher than that of their medium-gluten parent by measuring their lactic acid-sodium dodecyl sulfate solvent retention capacities of whole wheat meal. The multiplex PCR systems established in the present study can be used for molecular marker-assisted selection of strong gluten wheats.
1. Introduction
Wheat (Triticum aestivum L.) flour can be processed into various types of food products according to its gluten strength. Gluten, the main endosperm storage protein of wheat seeds, is composed of high molecular weight glutenin subunits (HMW-GSs), low-molecular-weight glutenin subunits (LMW-GSs) and gliadins [1]. HMW-GSs are the major determinant of dough elasticity, whereas LMW-GSs tend to contribute to both dough elasticity and extensibility [2]. Gliadins are associated with dough extensibility [3]. Dough elasticity is an important bread-making property of wheat [4]. HMW-GSs are encoded by three homoeologous complex loci Glu-1, Glu-A1, -B1 and -D1. Each locus has two tightly linked structural genes encoding a larger x-type subunit and a smaller y-type subunit [5]. Due to gene silencing and allelic variations, usually three to five HMW-GSs occur in wheat, and their composition varies among cultivars [6]. Locus Glu-D1 encodes two subunits, Glu-B1 two or one, and Glu-A1 one or none. Locus Glu-A1 is characterized by three alleles, namely, a, b and c [7]. Alleles a and b encode for glutenin subunits 1 and 2*, respectively, while c is a null allele [8]. Glu-A1a (Ax1) and Glu-A1b (Ax2*) are better than Glu-A1c (Ax null) in gluten strength [9]. At the Glu-D1 locus, the Glu-D1d allele (Dx5 + Dy10 subunits) confers superior mixing strength and bread-baking quality over the Glu-D1a allele (Dx2 + Dy12 subunits) [10,11]. At the Glu-B1 locus, the Glu-B1al allele (Bx7OE + By8* subunits) has been shown to enhance dough strength over the more common Glu-B1b allele (Bx7 + By8 subunits) [11] and another promising allele Glu-B1i (Bx17 + By18) [12]. Increased gluten strength associated with Glu-B1al allele is due to the increased amount of x-type subunit [11].
As the high-quality HMW-GSs, Ax1 and Dx5 + Dy10 have been widely used in wheat breeding in China, whereas Ax2* and Bx7OE + By8* are seldom [13,14]. Subunit Bx7OE is only used in strong gluten spring wheat breeding [12]. There is no report of this subunit in China’s strong gluten winter wheat varieties. Genotypes with the subunit combination Ax2*/Bx7OE + By8*/Dx5 + Dy10 exhibit higher gluten strength than those with other subunit combinations [15]. Therefore, the incorporation of these high-quality subunits and cold tolerance is an effective way to develop strong gluten winter wheat varieties in China.
The identification of HMW-GSs is the prerequisite for pyramiding high-quality HMW-GSs. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) has been routinely used to analyze HMW-GSs [16]. However, this method is difficult to distinguish HMW-GSs with similar electrophoretic mobility, such as By8 and By8* [17]. It is also not well suited to the high-throughput analysis of large numbers of samples. Efforts have been made to develop PCR-based assays to distinguish different HMW-GSs. The PCR-based assays for separate detection of subunits Ax2*, Bx7OE, and Dx5 have been developed [18,19,20]. Compared to a single PCR marker, multiplex PCR can simultaneously detect different target genes in one PCR reaction, which improves efficiency and reduces costs. A multiplex PCR for simultaneously detecting Ax2*, Bx7OE and Dx5 has been reported [21]. The marker for Bx7OE in that multiplex PCR is designed based on a 43-bp inDel in the promoter region [19]. Since the allelic variation in the promoter region is not the real cause of the Bx7OE phenotype [22], this marker is not suitable for detecting some materials with subunit Bx7OE. Thus, a new multiplex PCR system for simultaneously detecting subunits Ax2*, Bx7OE and Dx5 is required.
In the molecular marker-assisted selection (MAS) program, it is necessary to detect both presence and homozygosity of the target genes. At the Glu-A1 locus, a PAGE gel-based co-dominant marker and a Kompetitive Allele Specific PCR (KASP) marker have been developed [23,24]. These markers can be used to determine the homozygosity of progeny plants between Ax2* and Ax null subunits. Although the agarose gel electrophoresis is more convenient than PAGE, agarose gel based co-dominant markers for Ax2* are not available at present. Butow et al. [22] developed an agarose gel-based co-dominant MAR marker for the homozygosity test of the Bx7OE subunit at locus Glu-B1. As this MAR marker is also designed based on the 43-bp inDel in the promoter region, it is not suitable for determining the homozygosity of Bx7OE subunit in certain progenies. Recently, three KASP markers for subunit Bx7OE have been developed, but none of them was completely accurate [13,24,25]. Therefore, new co-dominant markers for accurately detecting homozygosity of Bx7OE subunit are also lacking. For the homozygosity detection of subunit Dx5, a KASP based and an agarose gel-based co-dominant markers are available [24,26].
Lactic acid-sodium dodecyl sulfate solvent retention capacity (LA-SDS SRC) is a rapid, small-scale SRC method to predict bread-making quality of hard winter wheat by combining the solutions used in the SDS sedimentation method AACC 56–70 and the centrifugation process within the SRC method AACC 56–11 [27]. The LA-SDS SRC assay shows a higher correlation with bread loaf volume compared to the SDS sedimentation method for both flour and meal of wheat [27], which has been further applied in analyzing wheat quality [28].
Although KASP marker is the most popular PCR-based molecular marker at present due to the advantages of no gel electrophoresis and high degree of automation, it is a single-plex marker and is not suitable for the simultaneous detection of multiple genes or loci. In addition, not all genes can develop ideal KASP markers. Therefore, the development of some agarose-based multiplex PCR markers is still needed. The present study was designed to develop an agarose gel-based multiplex PCR marker for simultaneously detecting the three high-quality HMW-GSs of Ax2*, Bx7OE and Dx5 and two agarose gel based multiplex PCR markers for separately detecting the homozygosity of Ax2* and Bx7OE subunits. These markers were evaluated for their application in MAS through the LA-SDS SRC analysis of homozygous lines stacking the three high-quality HMW-GSs.
2. Materials and Methods
2.1. Plant Materials
The plant materials included two wheat cultivars, Jimai 22 and Jinqiang 1, and their progeny plants and lines. Jimai 22 is a widely planted medium-gluten, high-yielding, and cold-resistant winter wheat variety. Jinqiang 1 is a strong-gluten spring wheat variety, which was derived from a mutant of the Canadian spring wheat line CSR17. The HMW-GSs compositions of the two cultivars are shown in Table 1. The F2 segregating population was used for validating the newly established multiplex PCR markers. The selected F6 lines were used to verify the effect of the MAS.

Table 1.
HMW-GS composition of Jimai 22 and Jinqiang 1.
2.2. DNA Extraction and Amplification
Genomic DNA was extracted from leaves following the protocol of Genomic DNA Mini Kit (Plant) (Tiangen Biotech Co., Ltd., Beijing, China). Amplification of DNA was performed in a BIO-RAD S1000TM thermal cycler in a total volume of 20 µL, consisting of 10 µL of 2× Taq PCR StarMix with loading dye (GenStar, Beijing, China), different primer combinations at matching concentrations, and 50–200 ng of template DNA. In multiplex PCR I, the primers included 5 pmol of each primer for Ax2*, and 3 pmol of each primer for Bx7OE and Dx5. In multiplex PCR II, the primers included 5 pmol of each primer for Ax2* and Ax null. In multiplex PCR III, the primers include 5 pmol of each primer for Bx7OE and 3 pmol of each primer for By8. Sequences of PCR primers and product sizes for the three multiplex PCR are shown in Table 2. All the PCR primers are taken from literatures listed in Table 2. The PCR programs were 3 min at 94 °C (initial denaturation), 32 cycles of 30 s at 94 °C, 30 s at 65 °C, 1 min for mutiplex PCR II and III at 72 °C, and 1.5 min for multiplex PCR I at 72 °C. An additional 7 min extension was performed after 32 cycles. The PCR products were electrophoresed in a 1% agarose gel with the TAE buffer (40 mm Tris–acetate, 1 mm EDTA), and visualized under ultraviolet light using a gel documentation system (Gel Doc XR+, Bio-Rad). The co-dominant PCR for Dx5 and electrophoretic separation of the PCR products were carried out using the method described by Ishikawa and Nakamura [26].

Table 2.
Sequences of PCR primers and fragment sizes for three multiplex PCR developed in this study.
2.3. Flour Characteristics
Whole grain samples were ground using a cyclotec mill CT410 (FOSS Scino (Suzhou) Co., Ltd., Suzhou, China) with a 1 mm stainless steel screen. Flour moisture was estimated using a HM-105L halogen moisture analyzer (Shanghai Hegong Scientific Instrument Co., Ltd., Shanghai, China). LA-SDS SRC was measured for the whole wheat meal (1 g) following the method as described by Seabourn et al. [27]. All LA-SDS SRC tests were performed in three replicates. The LA-SDS SRC values were calculated using the following formula:
Weight value (%) = (pellet weight/meal weight) × {[86/(100–percent meal moisture)]} −1) × 100.
2.4. Statistical Analysis
A one-way analysis of variance was performed for the LA-SDS SRC values using DPS software version 9.01 (Hangzhou Ruifeng Information Technology Co., Ltd., Hangzhou, China). The significance of difference was determined by the Duncan’s multiple range test at p < 0.05. The Chi-squared test was performed to examine the fitness of good for the segregation ratio of the markers for the loci of HMW-GSs.
3. Results
3.1. Establishment of a Multiplex PCR Marker I to Simultaneously Detect Ax2*, Bx7OE and Dx5 Subunits
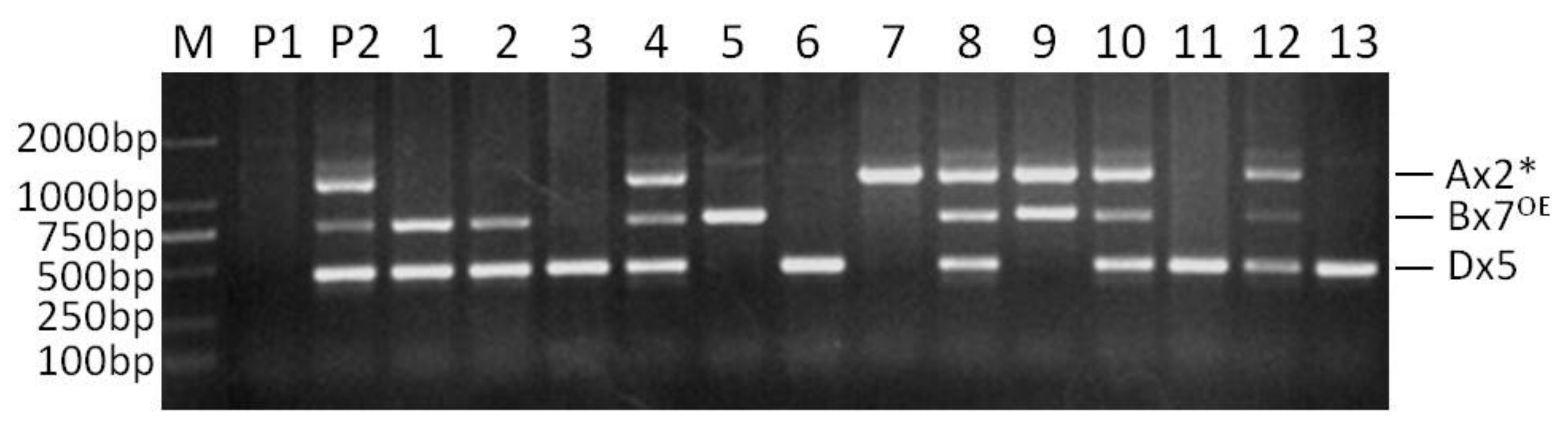
In the separate PCR analysis of the Ax2*, Bx7OE and Dx5 subunits, only one band of predicted size was amplified from the genotype containing the corresponding subunits. When the PCR assays were conducted with the three sets of primers together in a single reaction at the annealing temperature of 58 °C, optimal for the Ax2* and Dx5 markers [18], or 59 °C recommended for the Bx7OE marker [20], these primer pairs amplified their corresponding bands, but with obvious non-specific bands. As the annealing temperature increased, the non-specific amplified bands became weak. When the annealing temperature reached 65 °C, the non-specific amplified bands were almost invisible, and therefore this temperature was determined as the appropriate annealing temperature (Figure 1). The bands corresponding to Ax2*, Bx7OE and Dx5 were amplified in Jinqiang 1, but not in Jimai 22. The results of this multiplex PCR analysis were completely consistent with the subunit compositions of the two parents.
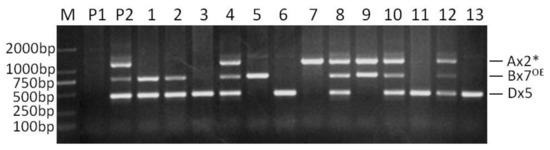
Figure 1.
Multiplex PCR detection of selected F2 plants from the cross of Jimai 22/Jinqiang 1. M: DNA ladder, P1: Jimai 22, P2: Jinqiang 1, 1–13: F2 plants of Jimai 22/Jinqiang 1.
In order to verify the validation of the multiple PCR, the F2 segregating population from a cross between Jimai 22 and Jinqiang 1 was tested with this set of markers. The three subunits of Ax2*, Bx7OE and Dx5 showed obvious segregation in the population (Figure 1). Among the 95 tested F2 plants, thirty-eight were positive for all the three subunits, and this segregation (38/95) was in agreement with the Mendelian segregation ratio of three genes (27/64) (χ2 = 0.11, p = 0.75), which confirms the validation of the multiplex PCR system (multiplex PCR I).
3.2. Establishment of Two Multiplex PCR Markers for Separately Detecting Ax2* and Bx7OE Homozygosity
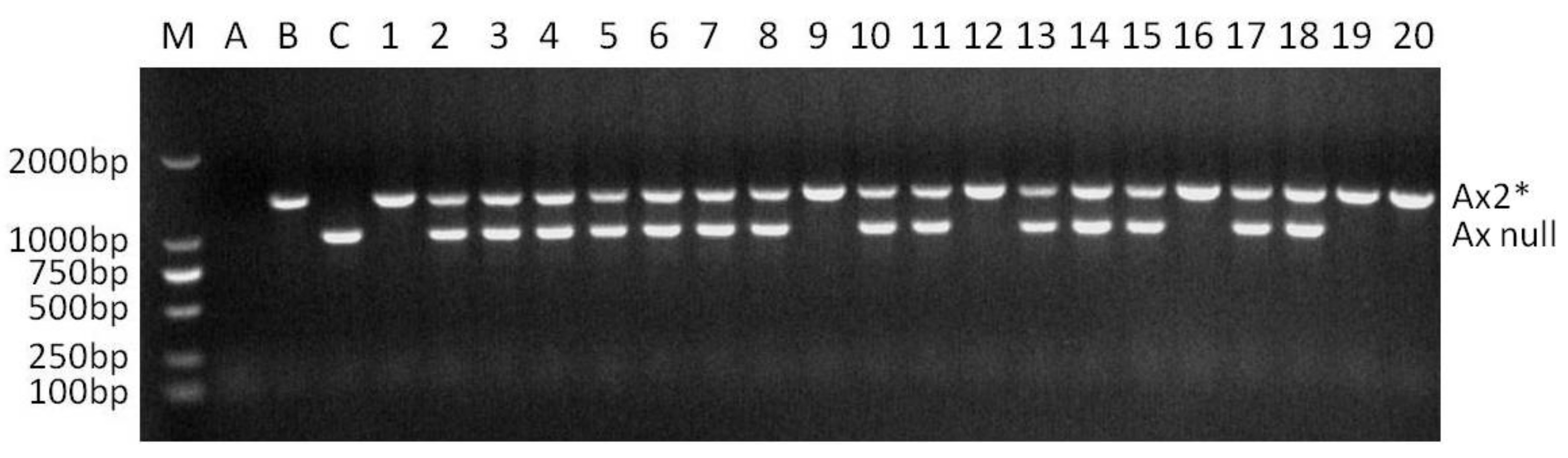
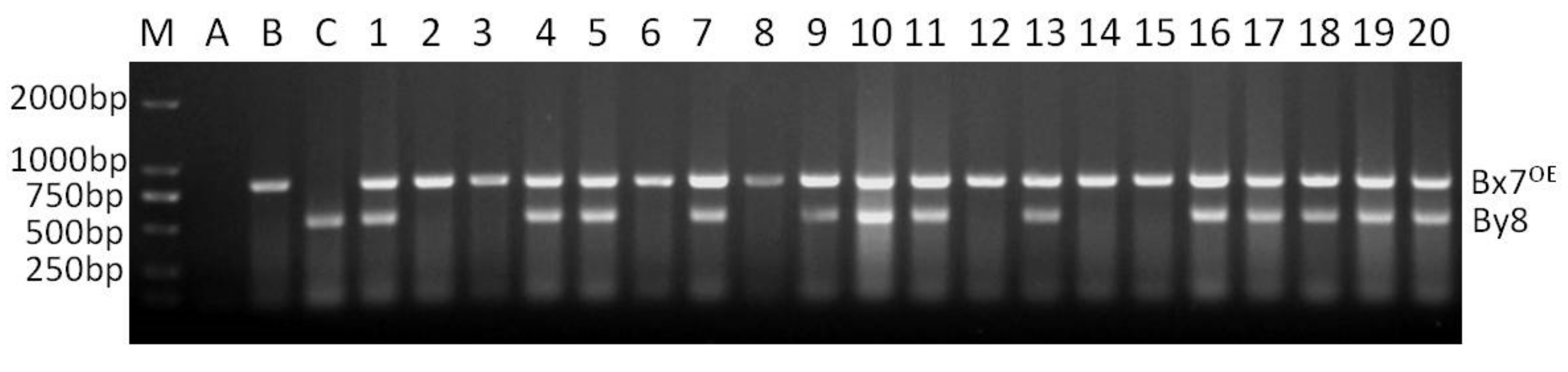
Locus Glu-A1 consists of two allelic variants, Ax2* and Ax null. When the two dominant PCR of Ax2* and Ax null were conducted in a single reaction with the optimized annealing temperature of 65 °C (named as multiplex PCR II), the two dominant markers functioned normally, and no non-specific bands were amplified. The band corresponding to Ax2* was amplified only in Jinqiang 1, and that corresponding to Ax null only in Jimai 22 (Figure 2). The multiplex PCR II was used to detect the homozygosity of the 64 F2 plants that were positive for Ax2* by multiplex PCR I (Figure 2). The number of Ax2* heterozygous and homozygous plants was 44 and 20, respectively, which is in agreement with the Mendelian segregation ratio of 2:1 for the two types of plants (χ2 = 0.049, p = 0.869). The multiplex PCR III for Bx7OE consisted of two dominant markers Bx7OE and By8. These markers also functioned normally without any non-specific bands when they were analyzed in a single PCR reaction with annealing temperature of 65 °C (Figure 3). In 65 Bx7OE positive F2 plants detected by multiplex PCR I, the number of Bx7OE heterozygous and homozygous plants was 42 and 23, respectively, which also agrees with the Mendelian segregation ratio of 2:1 for the two types of plants (χ2 = 0.048, p = 0.87).
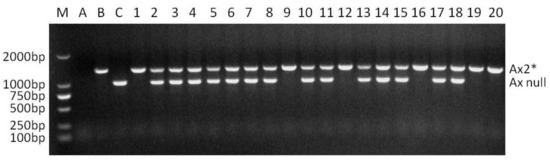
Figure 2.
Co-dominant PCR detection of Ax2* positive F2 plants from the cross of Jimai 22/Jinqiang 1. M: DNA ladder, A: without DNA template, B: Jinqiang 1, C: Jimai 22, 1–20: Ax2* positive F2 plants of Jimai 22/Jinqiang 1.
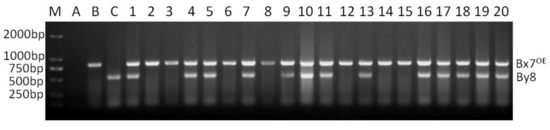
Figure 3.
Co-dominant PCR detection of Bx7OE positive F2 plants from the cross of Jimai 22/Jinqiang 1.M: DNA ladder, A: blank control, B: Jinqiang 1, C: Jimai 22, 1–20: Bx7OE positive F2 plants of Jimai 22/Jinqiang 1.
3.3. Application of Multiplex PCR Systems in the Breeding of Strong Gluten Winter Wheat
Starting from the F2 generation of the cross Jimai 22/Jinqiang 1, the progeny plants with cold tolerance and other superior agronomic traits including plant height and tiller number were selected and then further analyzed with the established multiplex PCR markers. The plants with Ax2*, Bx7OE and Dx5 subunits in the F2 population were easily screened out using multiple PCR marker I (Figure 1). Plants with Ax2*, Bx7OE and Dx5 alleles were further analyzed for the homozygosity of the three subunits with their corresponding multiplex PCR systems. Plants with homozygous or heterozygous Ax2* and Bx7OE subunits were distinguished clearly (Figure 2 and Figure 3). The multiplex PCR marker for Dx5 subunit homozygosity detection was selected from a published paper [26]. Once a high-quality subunit was homozygous, it would not be detected in the next generation. With this MAS system, 17 F6 lines containing all the three high-quality subunits were obtained.
3.4. Quality Analysis of Selected Lines with the Three High-Quality Subunits
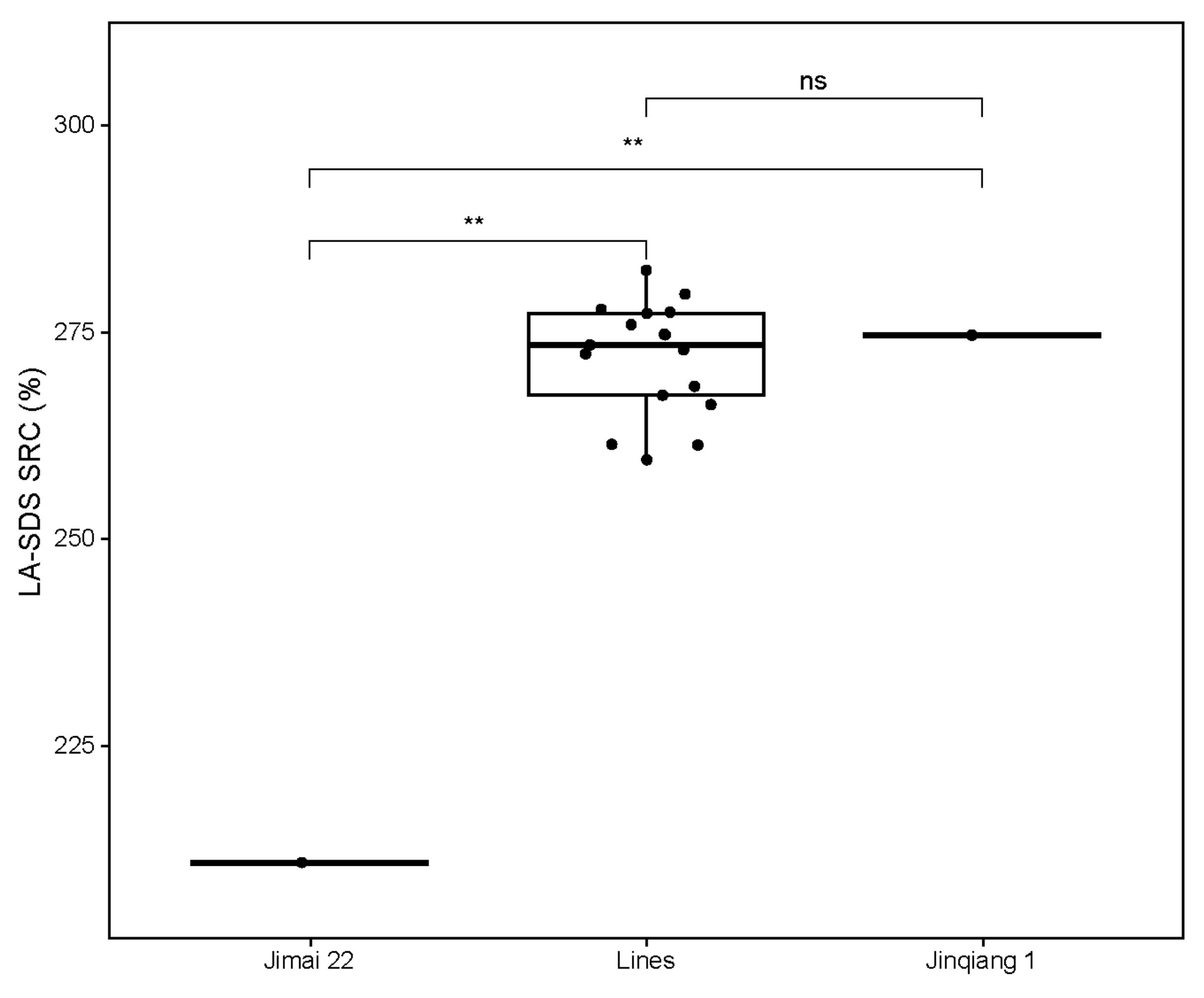
Values of LA-SDS SRC were determined to predict the qualities of selected stable lines with the three high-quality subunits and their parents. As expected, the SRC value of the strong gluten parent Jinqiang 1 was significantly higher than that of the medium gluten parent Jimai 22 (Figure 4). The SRC values of the selected lines were significantly higher than that of Jimai 22, but comparable to Jinqiang 1, indicating that the strong-gluten characteristics are mainly determined by the three high-quality subunits of Ax2*, Bx7OE and Dx5 from Jinqiang 1 in this breeding population.
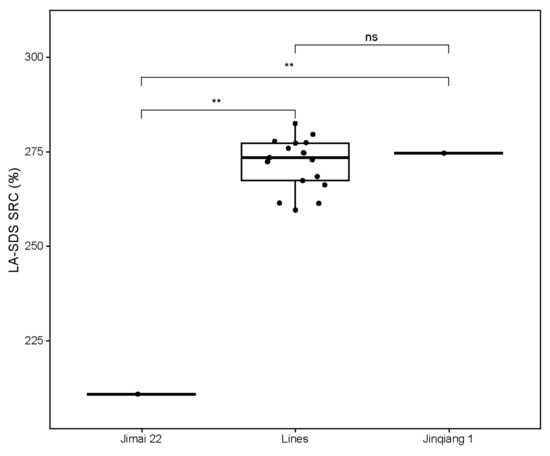
Figure 4.
A box plot for the LA-SDS SRC values of Jimai 22 and Jinqiang 1 and the 17 progeny lines stacking the three high-quality subunits of Ax2*, Bx7OE and Dx5. Black dots: parents or progeny lines, The significant difference between the progeny lines and Jimai 22 are shown by double asterisks at p < 0.01, ns: no significant difference at p < 0.01.
4. Discussion
4.1. Establishment of the MAS System Further Improves the Efficiency of Breeding
The multiplex PCR I we established consists of three dominant PCR markers for separately detecting Ax2*, Bx7OE and Dx5 subunits. Although a multiplex PCR for detecting these subunits has been reported [21], the Bx7OE markers used in the two multiplex PCR systems are different. Some genotypes with Bx7OE cannot be detected with the Bx7OE marker used in the reported multiplex PCR marker, because the promoter region with which the Bx7OE marker was designed is not the real cause of the Bx7OE phenotype [22]. In the multiplex PCR I, the dominant marker for Bx7OE was designed according to the right boundary sequence of a retroelement causing the Bx7OE phenotype, and it was completely consistent with the Bx7OE phenotype [20]. The validation of this multiplex PCR marker was confirmed from a F2 segregating population.
High annealing temperature is an important factor for the successful establishment of multiplex PCR I. In an early published the multiplex PCR marker that was expected to detect Ax2*, Bx17 and Dx5 subunits, the 1.3-kb band produced with the Ax2* primers in the single primer pair assay was replaced by a 344-bp band due to mismatch annealing of the Bx17 forward primer (CAACAGCCAGGACAATT) to the Ax2* template (CAGCAGCCAGGACAATT) [18]. In order to recover the 1.3-kb specific band, the dominant marker for Dx5 subunit in the above multiplex PCR was replaced by another Dx5 dominant marker [30], and the 1.3-kb specific band was successfully amplified despite the presence of the 344-bp non-specific band [31], indicating that the degree of interference between different primer pairs in a multiplex PCR marker is different. Increased annealing temperature from 58 °C to 60 °C might also play a role in this improvement. During establishing multiplex PCR I, we noticed that when the annealing temperature was 58 °C, there were obvious non-specific bands. As the annealing temperature increased, the non-specific bands became weak. At the annealing temperature of 65 °C, thenon-specific bands were almost invisible. Although other measures could also be used to prevent non-specific amplification, such as the use of different brands of Taq enzymes, the use of additives, etc., using a high annealing temperature might be the most economical method. Therefore, in multiplex PCR II and III, we also used an annealing temperature of 65 °C. Such high an annealing temperature seems rarely been used in previous PCR markers. Our suggestion is to try to use a high annealing temperature as long as sufficient amplification products can be obtained.
In molecular marker-assisted breeding, in addition to needing to know whether the target gene exists, it is also important to determine whether the target gene is homozygous in segregating generations. In multiplex PCR II, due to the 399 bp difference between the amplified fragments of the two dominant markers for Ax2* and Ax null, it is easy to screen out the Ax2* homozygous individuals in the segregating population by agarose gel electrophoresis. Obviously this agarose gel-based multiplex PCR marker is easier to manipulate than the previously published acrylamide gel-based marker [23]. For breeding units with KASP detection equipment, breeders can choose the more economical and rapid KASP marker [24] to detect the homozygosity of the Ax2* subunit in the segregating population. For the detection of Bx7OE homozygosity, our multiplex PCR III is suitable for homozygosity detection of Bx7OE in all segregating population from two parents with Bx7OE and By8 subunits, respectively. While the previously published agarose gel-based co-dominant marker for Bx7OE [22] is only suitable for such partial segregating population, because some parents with Bx7OE subunit cannot be detected with this marker [20]. Although some KASP markers for the Ax2* and Dx5 subunits can accurately detect homozygosity of these two subunits in segregating population [24], the present KASP markers for Bx7OE [13,24,25] are not suitable for such detections in some segregating population due to the generation of false positives. One [24] of the KASP markers for Bx7OE consists of two pairs of primers. One amplified a wheat housekeeping gene, and the other was allele-specific for Bx7OE. This KASP marker is actually a dominant marker only excludes the generation of false negatives, thus not suitable for homozygosity detection. For these segregating population for which present KASP markers are not suitable, the agarose gel-based multiplex PCR III marker may be the best supplement.
Multiplex PCR III was developed for two closely linked loci, because there is no report of the recombination of Bx7OE and By8 subunits at present. In the segregating population we examined, no individuals with a recombination between Bx7OE and By8 were detected. The molecular assay results in this study suggest that this co-dominant marker development strategy is feasible. This strategy may also be feasible for the development of some KASP markers. The multiplex PCR III was specially developed for the parents containing By8 subunits, and new multiplex PCR markers need to be developed for the parents with other By subunits. Since multiplex PCR is highly dependent on some factors such as primer combination, concentration and annealing temperature, care should be taken to optimize these parameters when creating new multiplex PCR markers.
In the present study, by integrating these newly developed multiplex PCR markers and a published co-dominant PCR marker for Dx5, a MAS system was established. Using this system, we can scientifically determine the number of molecular assays and the scale of next-generation planting. This not only saves the time and cost of molecular detection, but also significantly improves the efficiency of molecular marker-assisted selection.
4.2. Pyramiding the Three High-Quality Subunits of Ax2*, Bx7OE and Dx5 Is an Effective Way to Develop Strong Gluten Wheat Varieties
The composition of wheat HMW-GSs was directly related to the bread processing quality of wheat [10]. Ma et al. [15] compared the gluten parameters of different HMW-GSs combinations in 128 soft winter wheat varieties from the eastern USAand found that the materials containing the three high-quality subunit combination of Ax2*, Bx7OE and Dx5 had the highest SDS sedimentation value. The strong gluten parent Jinqiang 1 also possesses this the HMW-GSs combination. By crossing a high-yield winter wheat variety Jimai 22 with other HMW-GSs combinations with the aid of MAS, 17 stable lines with similar qualities as the strong gluten parent Jinqiang 1 were developed, suggesting that stacking the three high-quality subunits is an effective way to develop new wheat varieties with strong gluten.
As a high-quality HMW-GS, Bx7OE has not been widely used in breeding in most wheat growing countries [13]. The strong-gluten wheat cultivars containing the three high quality subunits of Ax2*, Bx7OE and Dx5 are rare at present [15]. The multiplex PCR markers established in the present study will be effective in developing strong-gluten wheat cultivars via MAS.
5. Conclusions
In the present study, we established a new agarose gel-based multiplex PCR system for detecting three high-quality HMW-GSs of Ax2*, Bx7OE and Dx5 and two matching agarose gel-based multiplex PCR markers detecting the homozygosity of Ax2* and Bx7OE subunits. Their validation and effectiveness were confirmed from a breeding population. These new established multiplex PCR systems can be used for molecular marker-assisted selection of strong gluten wheats.
Author Contributions
J.C. conceived and designed the experiments; C.Y., C.Z., F.D., Y.L., F.Y. and B.J. carried out the experiments; J.C., H.Z. and M.L. analyzed the data; J.C. wrote the manuscript; J.C., H.W., C.B. and S.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Key Research and Development Program of Hebei Province (20326348D) and Hebei Modern Agricultural Science and Technology Innovation Project (2021110205).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branlard, G.; Dardevet, M.; Saccomano, R.; Lagoutte, F.; Gourdon, J. Genetic diversity of wheat storage proteins and bread wheat quality. Euphytica 2001, 119, 59–67. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.; Liu, C.L.; Chang, C.; Wang, C.P.; You, M.S.; Li, B.Y.; Liu, G.T. PCR-based markers for identification of HMW-GS at Glu-B1x loci in common wheat. J. Cereal Sci. 2008, 47, 394–398. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Law, C.N. Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin, Part 1: Allelic variation in subunits amongst varieties of wheat (Triticum aestivum). Theor. Appl. Genet. 1981, 60, 229–236. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.C.; Yan, Y.M.; Appels, R.; Mahmood, T.; He, Z.H. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1 and Glu-D1 which code for highmolecular-weight subunits of glutenin in hexaploid wheat. Cereal Res.Commun. 1983, 11, 29–35. [Google Scholar]
- Thompson, R.D.; Bartels, D.; Harberd, N.P.; Flavell, R.B. Characterisation of the multigenic family coding for HMW glutenin subunits in wheat using cDNA clones. Theor. Appl. Genet. 1983, 67, 87–96. [Google Scholar] [CrossRef]
- Luo, C.; Griffin, W.B.; Branlard, G.; McNeil, D.L. Comparison of low- and high molecular-weight wheat glutenin allele effects on flour quality. Theor. Appl. Genet. 2001, 102, 1088–1098. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunits composition and bread-making quality of British grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Butow, B.J.; Ma, W.; Gale, K.R.; Cornish, G.B.; Rampling, L.; Larroque, O.; Morell, M.K.; Bekes, F. Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high-molecular-weight glutenin allele has a major impact on wheat flour dough strength. Theor. Appl. Genet. 2003, 107, 1524–1532. [Google Scholar] [CrossRef]
- Zhao, H.B.; Gao, C.J.; Song, W.F.; Zhang, Y.B.; Gao, D.D.; Zhang, X.M.; Zhao, L.J.; Yang, X.F.; Liu, D.J.; Song, Q.J.; et al. Quality differences between NILs of wheat variety Longmai 20 possessing HMW-GS 7OE + 8* and 17 + 18. Cereal Res.Commun. 2020, 48, 493–498. [Google Scholar] [CrossRef]
- Rasheed, A.; Jin, H.; Xiao, Y.G.; Zhang, Y.; Hao, Y.F.; Zhang, Y.; Hickey, L.; Morgounov, A.I.; Xia, X.C.; He, Z.H. Allelic effects and variations for key bread-making quality genes in bread wheat using high-throughput molecular markers. J. Cereal Sci. 2019, 85, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Sun, G.L.; Liu, W.H.; Sun, D.K.; Peng, Y.C.; Ren, X.F. High-molecular-weight glutenin subunit compositions in current Chinese commercial wheat cultivars and the implication on Chinese wheat breeding for quality. Cereal Chem. 2020, 97, 762–771. [Google Scholar] [CrossRef]
- Ma, F.; Kim, J.; Cho, E.; Brown-Guedira, G.; Park, C.S.; Baik, B.K. HMW-GS composition and rye translocations of U.S. eastern soft winter wheat and their associations with protein strength. J. Cereal Sci. 2019, 89, 102799. [Google Scholar] [CrossRef]
- Bietz, J.A.; Shephard, K.W.; Wall, J.S. Single-kernel analysis of glutenin: Use in wheat genetics and breeding. Cereal Chem. 1975, 52, 513–532. [Google Scholar]
- Lei, Z.S.; Gale, K.R.; He, Z.H.; Gianibelli, C.; Larroque, O.; Xia, X.C.; Butow, B.J.; Ma, W. Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat. J. Cereal Sci. 2006, 43, 94–101. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Gale, K.R. Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 2003, 134, 51–60. [Google Scholar] [CrossRef]
- Radovanovic, N.; Cloutier, S. Gene-assisted selection for high molecular weight glutenin subunits in wheat doubled haploid breeding programs. Mol. Breed. 2003, 12, 51–59. [Google Scholar] [CrossRef]
- Ragupathy, R.; Naeem, H.A.; Reimer, E.; Lukow, O.M.; Sapirstein, H.D.; Cloutier, S. Evolutionary origin of the segmental duplication encompassing the wheat Glu-B1 encoding the overexpressed Bx7 (Bx7OE) high molecular weight glutenin subunit. Theor. Appl. Genet. 2008, 116, 283–296. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, G.Y.; Yu, L.; Zhang, A.M.; Sun, J.Z.; Yang, W.L.; Liu, D.C.; Guo, X.L. Establishment of multiplex PCR system for high molecular weight glutenin subunits inwheat. Mol. Plant Breed. 2008, 6, 363–369. [Google Scholar]
- Butow, B.J.; Gale, K.R.; Ikea, J.; Juhasz, A.; Bedo, Z.; Tamas, L.; Gianibelli, M.C. Dissemination of the highly expressed Bx7 glutenin subunit (Glu-B1al allele) in wheat as revealed by novel PCR markers and RP-HPLC. Theor. Appl. Genet. 2004, 109, 1525–1535. [Google Scholar] [CrossRef]
- Liu, S.; Chao, S.; Anderson, J. New DNA markers for high molecular weight glutenin subunits in wheat. Theor. Appl. Genet. 2008, 118, 177–183. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.E.; Gao, F.M.; Zhai, S.N.; Jin, H.; Liu, J.D.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.C.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- Ravel, C.; Faye, A.; Ben-Sadoun, S.; Ranoux, M.; Dardevet, M.; Dupuits, C.; Exbrayat, F.; Poncet, C.; Sourdille, P.; Branlard, G. SNP markers for early identification of high molecular weight glutenin subunits (HMW-GSs) in bread wheat. Theor. Appl. Genet. 2020, 133, 751–770. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nakamura, T. A new co-dominant PCR-based marker to identify the high-molecular-weight glutenin subunit combination “5+10” of common wheat. Wheat Inf. Serv. 2007, 103, 1–16. [Google Scholar]
- Seabourn, B.W.; Xiao, Z.S.; Tilley, M.; Herald, T. A rapid, small-scale sedimentation method to predict breadmaking quality of hard winter wheat. Crop Sci. 2012, 52, 1306–1315. [Google Scholar] [CrossRef] [Green Version]
- Wilson, T.L.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Tilley, M. Nitrogen and sulfur effects on hard winter wheat quality and asparagine concentration. J. Cereal Sci. 2020, 93, 102969. [Google Scholar] [CrossRef]
- Lafiandra, D.; Tucci, G.F.; Pavoni, A.; Turchetta, T.; Margiotta, B. PCR analysis of x- and y-type genes present at the complex Glu-A1 locus in durum and bread wheat. Theor. Appl. Genet. 1997, 94, 235–240. [Google Scholar] [CrossRef]
- Anderson, D.D.; Greene, F.C. The characterization and comparativeanalysis of high-molecular-weight glutenin genes from genomes A andB of a hexaploid bread-wheat. Theor. Appl. Genet. 1989, 77, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.X.; Zhang, X.K.; Xia, X.C.; Zhang, P.Z.; He, Z.H. Development of multiplex PCR and identification of major quality genes in cultivars from Yellow and Huai rivervalley wheat region. Sci. Agric. Sin. 2008, 41, 643–653. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).