In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Growing Conditions
- ■
- 1% (w/v) sucrose (control, non-inducing medium);
- ■
- 0.5% (w/v) Bran and Brain (B&B) CF 70 fiber and 1% (w/v) lyophilized mushrooms (Agaricus bisporus basidiocarps);
- ■
- 1% (w/v) corn silage;
- ■
- 1% (w/v) chopped barley, oats and triticale (a hybrid of wheat and rye);
- ■
- 1% (w/v) corn flour.
2.2. Determination of Total Proteins
2.3. Determination of Glucanase Activity
2.4. Determination of Xylanase Activity
2.5. Determination of Cellulase Activity
2.6. In Vitro Feed Digestion
- ■
- Control-fiber: incubation in presence of SM 1X, 1% (w/v) lyophilized mushrooms and 0,5% (w/v) wheat fiber;
- ■
- Control-sucrose: incubation in the presence of SM 1X and distilled water;
- ■
- T22-sucrose: incubation in the presence of SM 1X and 5 mL of the enzymatic mixture produced by T. afroharzianum strain T22 grown in liquid culture (SM 1X + 1% sucrose, as described in Section 2.1) for 14 d;
- ■
- T22-fiber: incubation in the presence of SM 1X, 5 mL of the enzymatic mixtures produced by T. afroharzianum strain T22 grown in liquid culture (SM 1X + 1% lyophilized mushrooms + 0, 5% wheat fiber, as described in Section 2.1) for 14 d.
2.7. Statistical Analysis
3. Results
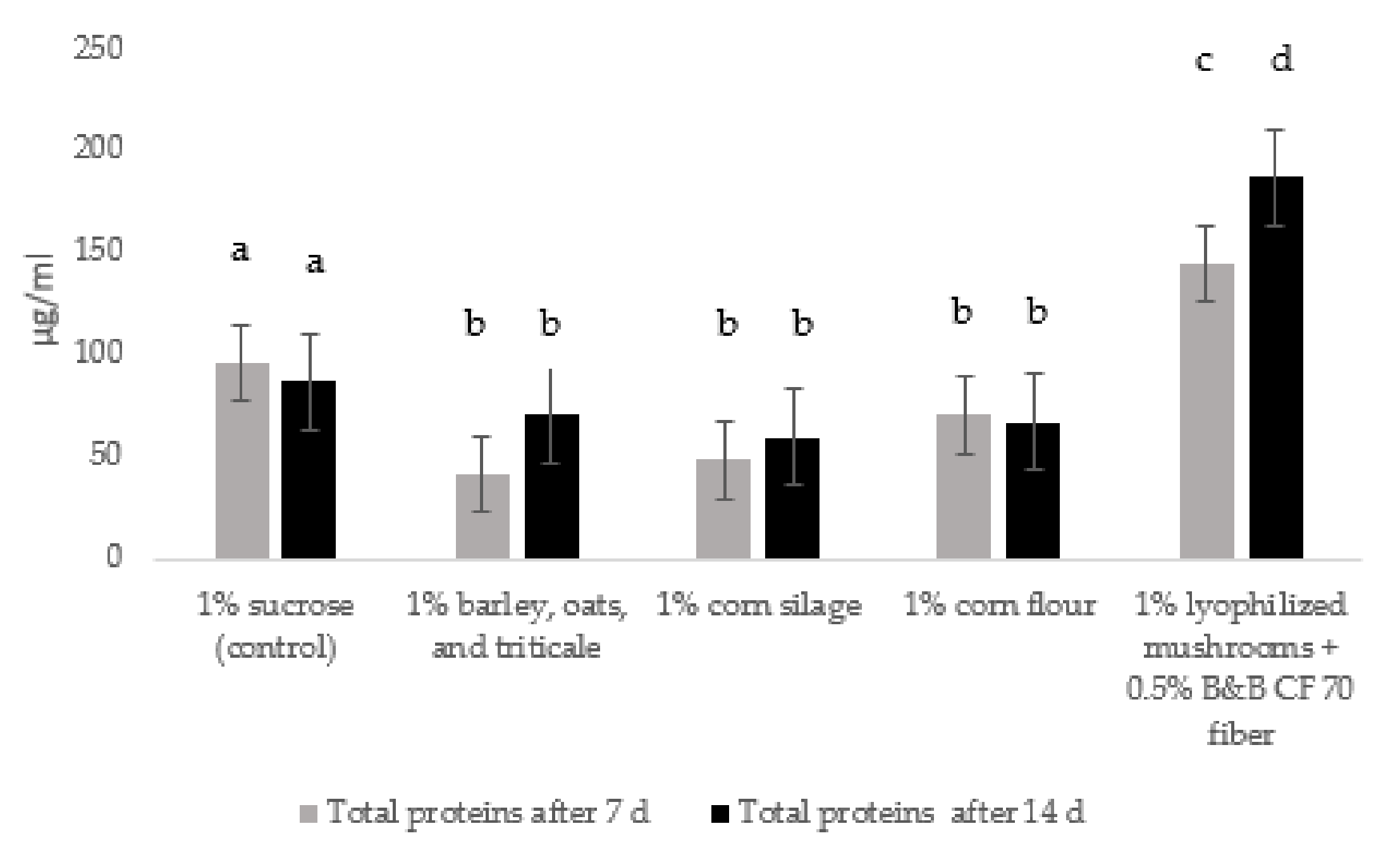
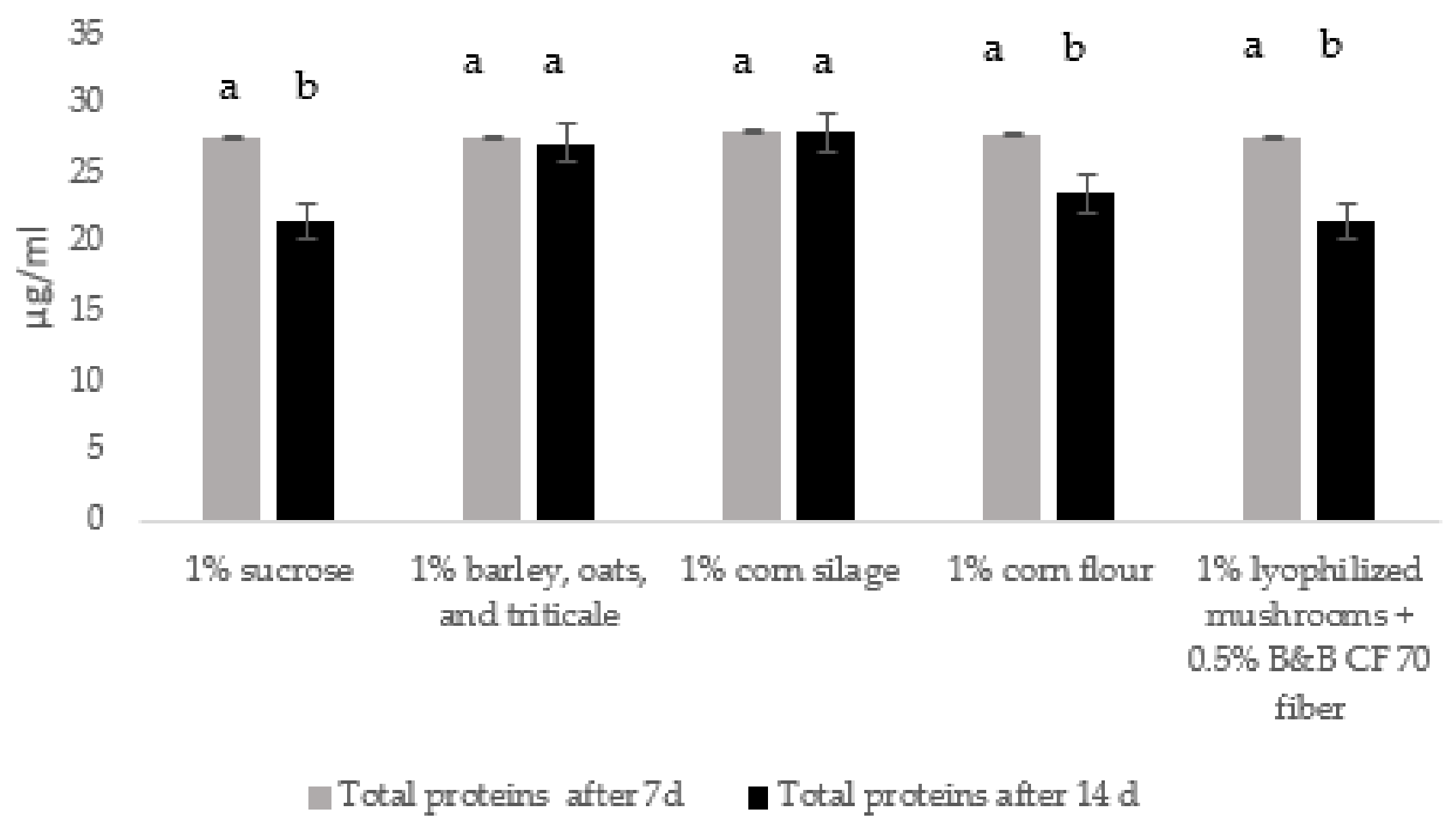
3.1. Production of Enzymatic Mixtures by Trichoderma Strains
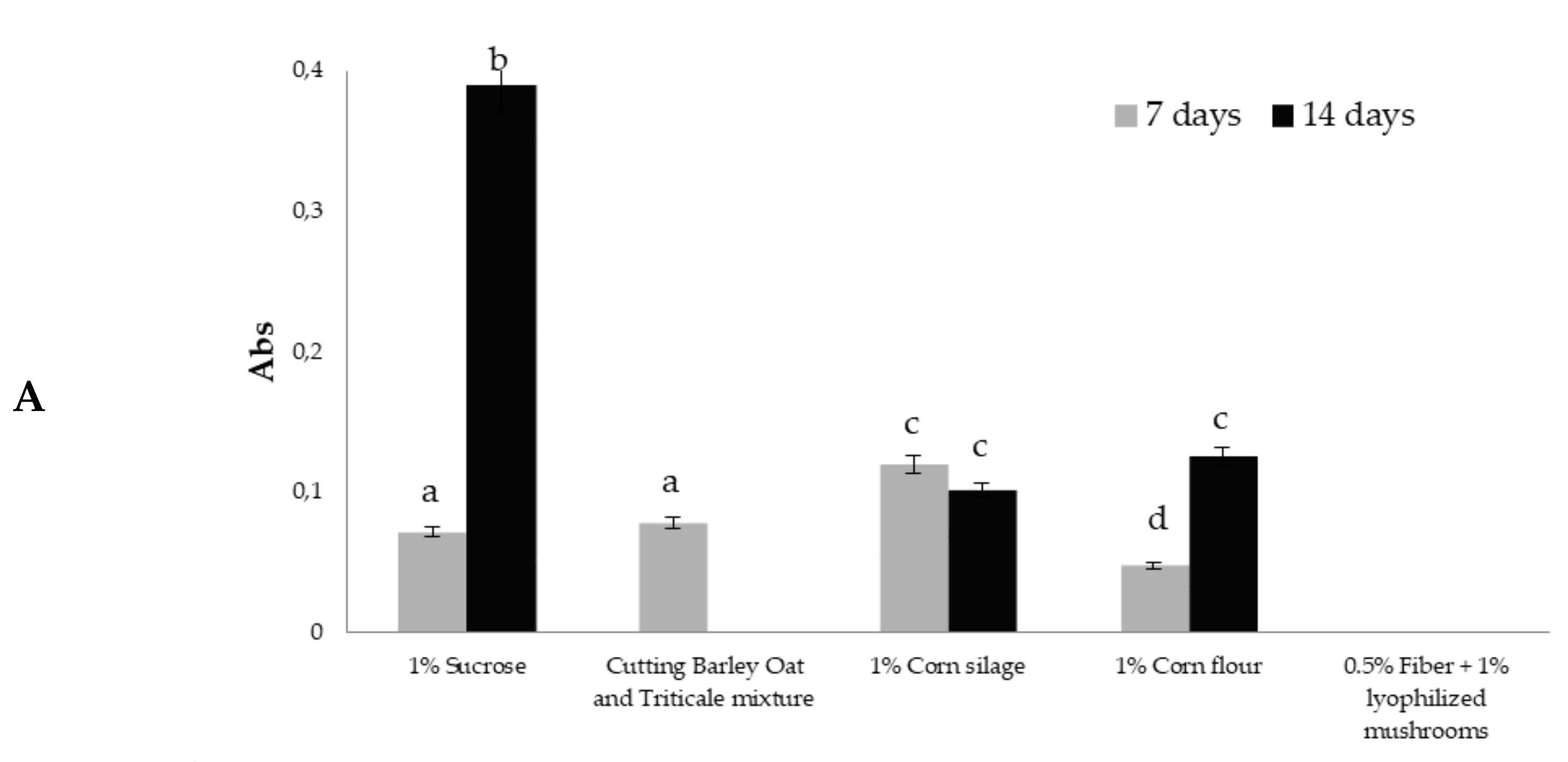
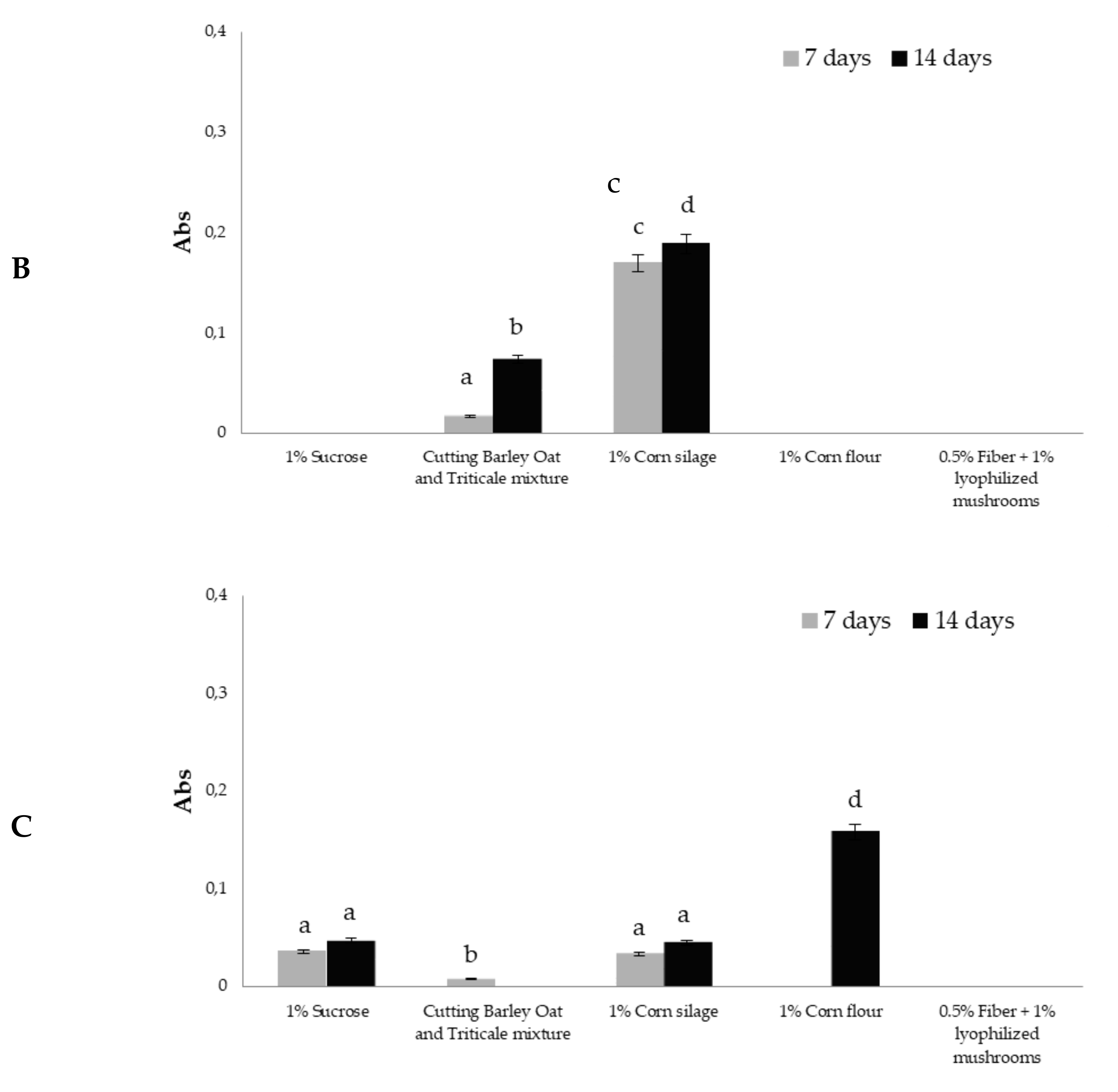
3.2. Glucanase Activity in Trichoderma Culture Filtrates
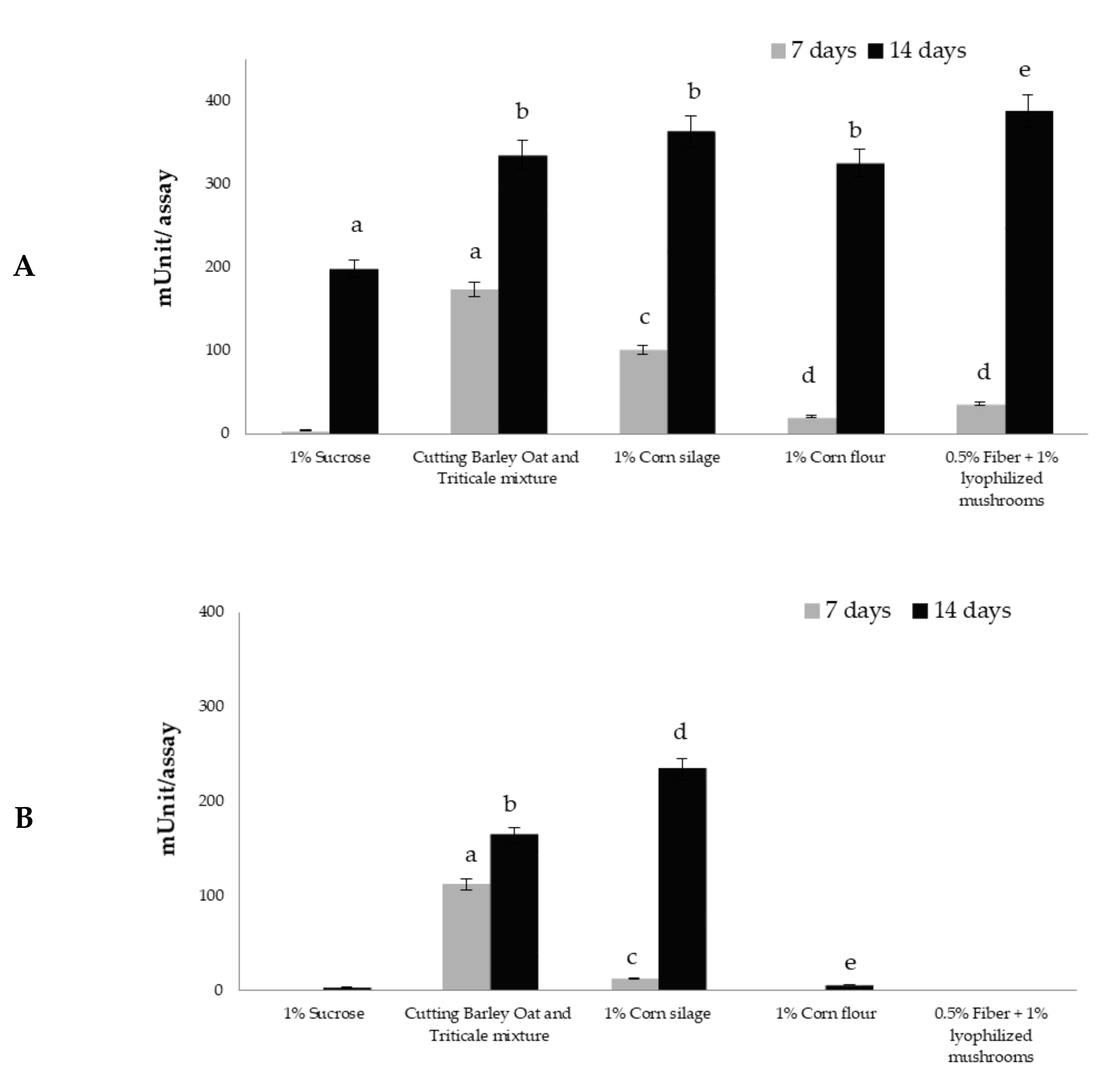
3.3. Xylanase Activity in Trichoderma Culture Filtrates
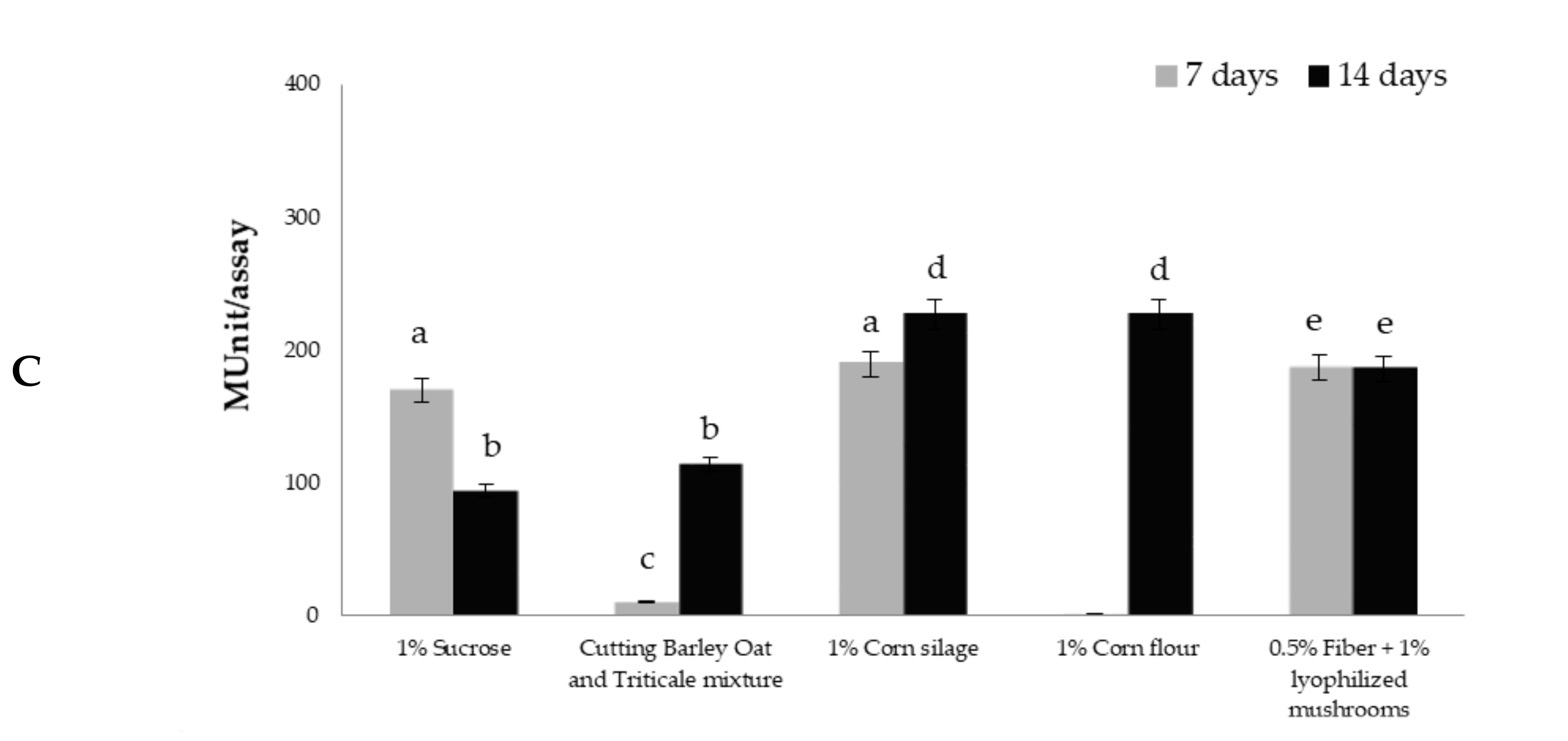
3.4. Cellulase Activity in Trichoderma Culture Filtrates
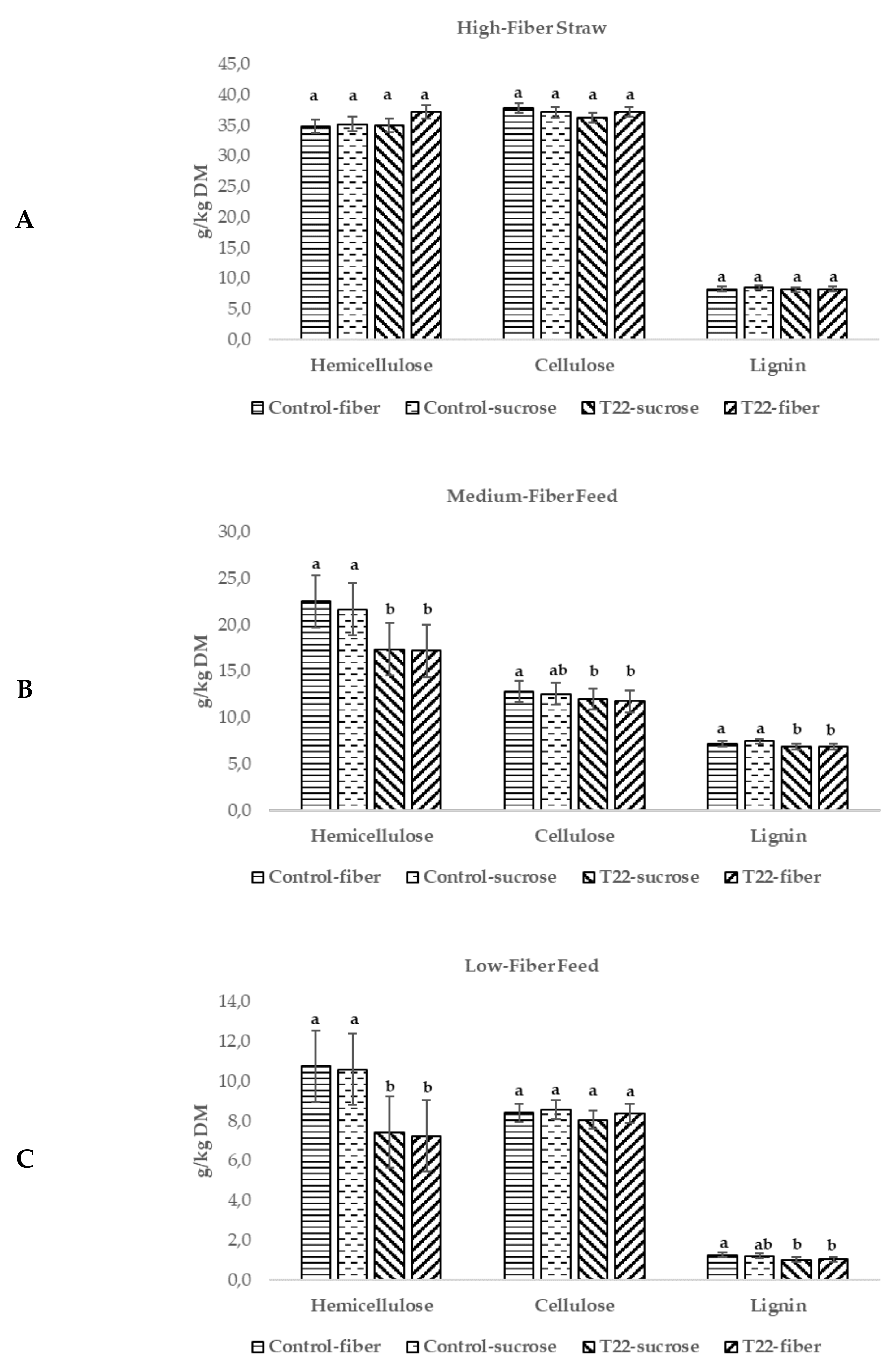
3.5. In Vitro Feed Digestion by Enzymatic Mixtures Produced by Strain T22
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tullo, E.; Finzi, A.; Guarino, M. Environmental impact of livestock farming and Precision Livestock Farming as a mitigation strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Bragaglio, A.; Napolitano, F.; Pacelli, C.; Pirlo, G.; Sabia, E.; Serrapica, F.; Serrapica, M.; Braghieri, A. Environmental Impacts of Italian Beef Production: A Comparison between Different Systems. J. Clean. Prod. 2018, 172, 4033–4043. [Google Scholar] [CrossRef]
- Serrapica, F.; Masucci, F.; Romano, R.; Napolitano, F.; Sabia, E.; Aiello, A.; Di Francia, A. Effects of Chickpea in Substitution of Soybean Meal on Milk Production, Blood Profile and Reproductive Response of Primiparous Buffaloes in Early Lactation. Animals 2020, 10, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzun, P.; Masucci, F.; Serrapica, F.; Varricchio, M.L.; Pacelli, C.; Claps, S.; Di Francia, A. Use of Mycorrhizal Inoculum under Low Fertilizer Application: Effects on Forage Yield, Milk Production, and Energetic and Economic Efficiency. J. Agric. Sci. 2018, 156, 127–135. [Google Scholar] [CrossRef]
- Uzun, P.; Masucci, F.; Serrapica, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Esposito, G.; Di Francia, A. The Inclusion of Fresh Forage in the Lactating Buffalo Diet Affects Fatty Acid and Sensory Profile of Mozzarella Cheese. J. Dairy Sci. 2018, 101, 6752–6761. [Google Scholar] [CrossRef]
- Irawan, A.; Sofyan, A.; Ridwan, R.; Hassim, H.A.; Respati, A.N.; Wardani, W.W.; Astuti, W.D.; Jayanegara, A. Effects of different lactic acid bacteria groups and fibrolytic enzymes as additives on silage quality: A meta-analysis. Bioresour. Technol. Rep. 2021, 14, 100654. [Google Scholar] [CrossRef]
- Carrillo-Díaz, M.I.; Miranda-Romero, L.A.; Chávez-Aguilar, G.; Zepeda-Batista, J.L.; González-Reyes, M.; García-Casillas, A.C.; Tirado-González, D.N.; Tirado-Estrada, G. Improvement of Ruminal Neutral Detergent Fiber Degradability by Obtaining and Using Exogenous Fibrolytic Enzymes from White-Rot Fungi. Animals 2022, 12, 843. [Google Scholar] [CrossRef]
- Vittorazzi, P.C., Jr.; Marques, J.A.; Takiya, C.S.; Chesini, R.G.; Bugoni, M.; da Silva, G.G.; Renno, F.P. Increasing doses of carbohydrases: Effects on rumen fermentation, nutrient digestibility, and performance of mid-lactation cows. J. Dairy Sci. 2021, 104, 12508–12519. [Google Scholar] [CrossRef]
- Estrada, G.T.; Haro, I.M.; Vázquez, C.R.C.; Martínez, G.D.M.; González, D.N.T. Degradación in situ y patrones de fermentación del rastrojo de maíz (Zea mays L.) tratado con enzimas exógenas en vacas Holstein. Intercienc. Rev. Cienc. Tecnol. Am. 2015, 40, 716–721. [Google Scholar]
- Farooq, S.; Shah, M.A.; Ganaie, T.A.; Mir, S.A. Exogenous Enzymes. In Food Biopolymers: Structural, Functional and Nutraceutical Properties; Gani, A., Ashwar, B.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 319–338. [Google Scholar]
- Bajaj, P.; Mahajan, R. Cellulase and xylanase synergism in industrial biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 8711–8724. [Google Scholar] [CrossRef]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; de Vries, R.P. Fungal xylanolytic enzymes: Diversity and applications. Bioresour. Technol. 2022, 344, 126290. [Google Scholar] [CrossRef]
- Azzaz, H.H.; Abd El Tawab, A.M.; Khattab, M.S.A.; Szumacher-Strabel, M.; Cieślak, A.; Murad, H.A.; Kiełbowicz, M.; El-Sherbiny, M. Effect of Cellulase Enzyme Produced from Penicilliumchrysogenum on the Milk Production, Composition, Amino Acid, and Fatty Acid Profiles of Egyptian Buffaloes Fed a High-Forage Diet. Animals 2021, 11, 3066. [Google Scholar] [CrossRef]
- Velázquez-De Lucio, B.S.; Hernández-Domínguez, E.M.; Villa-García, M.; Díaz-Godínez, G.; Mandujano-Gonzalez, V.; Mendoza-Mendoza, B.; Álvarez-Cervantes, J. Exogenous Enzymes as Zootechnical Additives in Animal Feed: A Review. Catalysts 2021, 11, 851. [Google Scholar] [CrossRef]
- Almaamory, Y.A.; Sundos, F.M.; Abass, E.R. Effect of Exogenous Enzymes (Labazyme and Bio SB-Gold) in Rumen Fermentation and Blood Parameters of Awassi Lambs. Plant Arch. 2020, 20, 13–16. [Google Scholar]
- Mohamed, D.E.A.; Borhami, B.E.; El-Shazly, K.A.; Sallam, S.M.A. Effect of Dietary Supplementation with Fibrolytic Enzymes on the Productive Performance of Early Lactating Dairy Cows. J. Agric. Sci. 2013, 5, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Kholif, A.M.; Aziz, H.A. Influence of Feeding Cellulytic Enzymes on Performance, Digestibility and Ruminal Fermentation in Goats. Anim. Nutr. Feed Technol. 2014, 14, 121–136. [Google Scholar]
- Song, S.D.; Chen, G.J.; Guo, C.H.; Rao, K.Q.; Gao, Y.H.; Peng, Z.L.; Zhang, Z.F.; Bai, X.; Wang, Y.; Wang, B.X.; et al. Effects of exogenous fibrolytic enzyme supplementation to diets with different NFC/NDF ratio ratios on the growth performance, nutrient digestibility and ruminal fermentation in Chinese domesticated black goats. Anim. Feed Sci. Technol. 2018, 236, 170–177. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Baah, J.; Wilde, R.; Beauchemin, K.A.; Rode, L.M.; Shelford, J.A.; Kamande, G.M.; Cheng, K.J. Effects of Tween 80 on In Vitro Fermentation of Silages and Interactive Effects of Tween 80, Monensin and Exogenous Fibrolytic Enzymeson Growth Performance by Feedlot Cattle. Asian-Australas. J. Anim. Sci. 2003, 16, 968–978. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Evaluation of the Importance of the Digestibility of Neutral Detergent Fiber from Forage: Effects on Dry Matter Intake and Milk Yield of Dairy Cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Kung, L.; Cohen, M.A.; Rode, L.M.; Treacher, R.J. The Effect of Fibrolytic Enzymes Sprayed onto Forages and Fed in a Total Mixed Ratio to Lactating Dairy Cows1. J. Dairy Sci. 2002, 85, 2396–2402. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.D.; Phipps, R.H.; Deaville, E.R.; Jones, A.K.; Humphries, D.J. Whole-Crop Wheat for Dairy Cows: Effects of Crop Maturity, a Silage Inoculant and an Enzyme Added before Feeding on Food Intake and Digestibility and Milk Production. J. Anim. Sci. 2002, 74, 307–318. [Google Scholar] [CrossRef]
- Vicini, J.L.; Bateman, H.G.; Bhat, M.K.; Clark, J.H.; Erdman, R.A.; Phipps, R.H.; Van Amburgh, M.E.; Hartnell, G.F.; Hintz, R.L.; Hard, D.L. Effect of Feeding Supplemental Fibrolytic Enzymes or Soluble Sugars with Malic Acid on Milk Production. J. Dairy Sci. 2003, 86, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Titi, H.H. Evaluation of Feeding a Fibrolytic Enzyme to Lactating Dairy Cows on Their Lactational Performance during Early Lactation. Asian-Australas. J. Anim. Sci. 2003, 16, 677–684. [Google Scholar] [CrossRef]
- Elwakeel, E.A.; Titgemeyer, E.C.; Johnson, B.J.; Armendariz, C.K.; Shirley, J.E. Fibrolytic Enzymes to Increase the Nutritive Value of Dairy Feedstuffs. J. Dairy Sci. 2007, 90, 5226–5236. [Google Scholar] [CrossRef] [Green Version]
- Arriola, K.G.; Kim, S.C.; Staples, C.R.; Adesogan, A.T. Effect of Fibrolytic Enzyme Application to Low- and High-Concentrate Diets on the Performance of Lactating Dairy Cattle. J. Dairy Sci. 2011, 94, 832–841. [Google Scholar] [CrossRef]
- Holtshausen, L.; Chung, Y.-H.; Gerardo-Cuervo, H.; Oba, M.; Beauchemin, K.A. Improved Milk Production Efficiency in Early Lactation Dairy Cattle with Dietary Addition of a Developmental Fibrolytic Enzyme Additive. J. Dairy Sci. 2011, 94, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.-S.; Beauchemin, K.A.; Schulze, H. Use of an in Vitro Fermentation Bioassay to Evaluate Improvements in Degradation of Alfalfa Hay Due to Exogenous Feed Enzymes. Anim. Feed Sci. Tech. 2007, 135, 315–328. [Google Scholar] [CrossRef]
- Duarte, E.R.; Maia, H.A.R.; Freitas, C.E.S.; da Silva Alves, J.M.; Valério, H.M.; Cota, J. Hydrolysis of lignocellulosic forages by Trichoderma longibrachiatum isolate from bovine rumen. Biocatal. Agric. Biotechnol. 2021, 36, 102135. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma Species: Versatile Plant Symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. An Environmentally Friendly Practice Used in Olive Cultivation Capable of Increasing Commercial Interest in Waste Products from Oil Processing. Antioxidants 2020, 9, 466. [Google Scholar] [CrossRef]
- Dini, I.; Marra, R.; Cavallo, P.; Pironti, A.; Sepe, I.; Troisi, J.; Scala, G.; Lombari, P.; Vinale, F. Trichoderma Strains and Metabolites Selectively Increase the Production of Volatile Organic Compounds (VOCs) in Olive Trees. Metabolites 2021, 11, 213. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Spices, Condiments, Extra Virgin Olive Oil and Aromas as Not Only Flavorings, but Precious Allies for Our Wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New Strategies in the Cultivation of Olive Trees and Repercussions on the Nutritional Value of the Extra Virgin Olive Oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef]
- Cavallo, P.; Dini, I.; Sepe, I.; Galasso, G.; Fedele, F.L.; Sicari, A.; Bolletti Censi, S.; Gaspari, A.; Ritieni, A.; Lorito, M.; et al. An Innovative Olive Pâté with Nutraceutical Properties. Antioxidants 2020, 9, 581. [Google Scholar] [CrossRef]
- Zafra, G.; Cortés-Espinosa, D.V. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environ. Sci. Pollut. Res. Int. 2015, 22, 19426–19433. [Google Scholar] [CrossRef]
- Dini, I.; Pascale, M.; Staropoli, A.; Marra, R.; Vinale, F. Effect of Selected Trichoderma Strains and Metabolites on Olive Drupes. Appl. Sci. 2021, 11, 8710. [Google Scholar] [CrossRef]
- Harman, G.E.; Lorito, M.; Lynch, J.M. Uses of Trichoderma spp. to alleviate or remediate soil and water pollution. Adv. Appl. Microbiol. 2004, 56, 313–330. [Google Scholar] [CrossRef]
- Gupta, V.G.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.S.; Druzhinina, I.; Tuohy, M. Biotechnology and Biology of Trichoderma; Newnes: Boston, MA, USA, 2014; ISBN 0444595945. [Google Scholar]
- Sakita, G.Z.; Bompadre, T.F.V.; Dineshkumar, D.; de Mello Tavares Lima, P.; Filho, A.L.A.; Campioni, T.S.; de Oliva Neto, P.; Neto, H.B.; Louvandini, H.; Abdalla, A.L. Fibrolytic enzymes improving in vitro rumen degradability of tropical forages. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1267–1276. [Google Scholar] [CrossRef]
- Díaz, A.; Ranilla, M.J.; Giraldo, L.A.; Tejido, M.L.; Carro, M.D. Treatment of tropical forages with exogenous fibrolytic enzymes: Effects on chemical composition and in vitro rumen fermentation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Ideal Feedstock and Fermentation Process Improvements for the Production of Lignocellulolytic Enzymes. Processes 2021, 9, 38. [Google Scholar] [CrossRef]
- Li, W.; Cheng, P.; Zhang, J.; Zhao, L.; Ma, Y.; Ding, K. Synergism of microorganisms and enzymes in solid-state fermentation of animal feed. A review. J. Anim. Feed Sci. 2021, 30, 3–10. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Enhancing the Quality of Total Mixed Ration Containing Cottonseed or Rapeseed Meal by Optimization of Fermentation Conditions. Fermentation 2021, 7, 234. [Google Scholar] [CrossRef]
- Zilio, E.M.C.; Del Valle, T.A.; Ghizzi, L.G.; Takiya, C.S.; Dias, M.S.S.; Nunes, A.T.; Silva, G.G.; Rennó, F.P. Effects of Exogenous Fibrolytic and Amylolytic Enzymes on Ruminal Fermentation and Performance of Mid-Lactation Dairy Cows. J. Dairy Sci. 2019, 102, 4179–4189. [Google Scholar] [CrossRef] [PubMed]
- Feed Enzymes Market by Type (Phytase, Carbohydrase, and Protease), Livestock (Poultry, Swine, Ruminants, and Aquatic Animals), Source (Microorganism, Plant, and Animal), Form (Dry and Liquid), and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/feed-enzyme-market-1157.html?gclid=CjwKCAjw4qCKBhAVEiwAkTYsPHL4phTwHlcNXccyF96D70nXnpyBnPqS6jGS6k0Knz9RaNB6HNGK8hoCY48QAvD_BwE (accessed on 25 September 2021).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Rinne, M.; Jaakkola, S.; Huhtanen, P. Grass maturity effects on cattle fed silage-based diets. 1. Organic matter digestion, rumen fermentation and nitrogen utilization. Anim. Feed Sci. Tech. 1997, 67, 1–17. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. (Eds.) Trichoderma And Gliocladium, Volume 2: Enzymes, Biological Control and Commercial Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Trichoderma enzymes for food industries. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 339–344. [Google Scholar]
- Jung, H.G. Forage digestibility: The intersection of cell wall lignification and plant tissue anatomy. In Proceedings of the III International Symposium Advances on Research Techniques for Ruminant Nutrition, Pirassununga, Brazil, 24–25 March 2011; pp. 137–160. [Google Scholar]
- Feng, P.; Hunt, C.W.; Pritchard, G.T.; Julien, W.E. Effect of enzyme preparations on in situ and in vitro degradation and in vivo digestive characteristics of mature cool-season grass forage in beef steers. J. Anim. Sci. 1996, 74, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.Z.; Beauchemin, K.A.; Rode, L.M. Effects of an enzyme feed additive on extent of digestion and milk production of lactating dairy cows. J. Dairy Sci. 1999, 82, 391–403. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Rode, L.M.; Yang, W.Z.; McAllister, T.A. Use of feed enzymes in ruminant nutrition. In Proceedings of the 33rd Pacific Northwest Nutrition Conference, Vancouver, BC, Canada, 1998; pp. 121–135. [Google Scholar]
- Pech-Cervantes, A.A.; Ogunade, I.M.; Jiang, Y.; Estrada-Reyes, Z.M.; Arriola, K.G.; Amaro, F.X.; Staples, C.R.; Vyas, D.; Adesogan, A.T. Effects of a xylanase-rich enzyme on intake, milk production, and digestibility of dairy cows fed a diet containing a high proportion of bermudagrass silage. J. Dairy Sci. 2021, 104, 7671–7681. [Google Scholar] [CrossRef]
- Tirado-González, D.N.; Tirado-Estrada, G.; Miranda-Romero, L.A.; Ramírez-Valverde, R.; Medina-Cuéllar, S.E.; Salem, A.Z.M. Effects of addition of exogenous fibrolytic enzymes on digestibility and milk and meat production—A systematic review. Ann. Anim. Sci. 2021, 21, 1159–1192. [Google Scholar] [CrossRef]
- Napolitano, A.; Lanzuise, S.; Ruocco, M.; Arlotti, G.; Ranieri, R.; Knutsen, S.H.; Lorito, M.; Fogliano, V. Treatment of Cereal Products with a Tailored Preparation of Trichoderma Enzymes Increases the Amount of Soluble Dietary Fiber. J. Agric. Food Chem. 2006, 54, 7863–7869. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Shao, J.; Feng, H.; Zhang, R.; Shen, Q. Extracellular proteins of Trichoderma guizhouense elicit an immune response in maize (Zea mays) plants. Plant Soil 2020, 449, 133–149. [Google Scholar] [CrossRef]
- Sharma, A.; Salwan, R.; Sharma, V. Extracellular proteins of Trichoderma and their role in plant health. S. Afr. J. Bot. 2022, 147, 359–369. [Google Scholar] [CrossRef]
- Lorito, M.; Hayes, C.K.; Di Pietro, A.; Woo, S.L.; Harman, G.E. Purification, Characterization, and Synergistic Activity of a Glucan 1,3-Beta-Glucosidase and an N-Acetyl-Beta-Glucosaminidase from Trichoderma Harzianum. Phytopathology 1994, 84, 398–405. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Cavalheiro, G.F.; de Queiroz Vieira, E.R.; Gandra, J.R.; de Tonissi e Buschinelli de Goes, R.H.; da Paz, M.F.; Fonseca, G.G.; Leite, R.S.R. Catalytic properties of xylanases produced by Trichoderma piluliferum and Trichoderma viride and their application as additives in bovine feeding. Biocatal. Agric. Biotechnol. 2019, 19, 101161. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Beauchemin, K.A.; Nsereko, V.L.; Rode, L.M.; Iwaasa, A.D.; Yang, W.Z.; McAllister, T.A.; Wang, Y. Synergy between ruminal fibrolytic enzymes and enzymes from Trichoderma longibrachiatum. J. Dairy Sci. 2000, 83, 1310–1321. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Ravindran, V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase1. Poult. Sci. 2014, 93, 1186–1196. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannaccone, F.; Alborino, V.; Dini, I.; Balestrieri, A.; Marra, R.; Davino, R.; Di Francia, A.; Masucci, F.; Serrapica, F.; Vinale, F. In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants. Agriculture 2022, 12, 573. https://doi.org/10.3390/agriculture12050573
Iannaccone F, Alborino V, Dini I, Balestrieri A, Marra R, Davino R, Di Francia A, Masucci F, Serrapica F, Vinale F. In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants. Agriculture. 2022; 12(5):573. https://doi.org/10.3390/agriculture12050573
Chicago/Turabian StyleIannaccone, Francesco, Vittoria Alborino, Irene Dini, Anna Balestrieri, Roberta Marra, Rosario Davino, Antonio Di Francia, Felicia Masucci, Francesco Serrapica, and Francesco Vinale. 2022. "In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants" Agriculture 12, no. 5: 573. https://doi.org/10.3390/agriculture12050573
APA StyleIannaccone, F., Alborino, V., Dini, I., Balestrieri, A., Marra, R., Davino, R., Di Francia, A., Masucci, F., Serrapica, F., & Vinale, F. (2022). In Vitro Application of Exogenous Fibrolytic Enzymes from Trichoderma Spp. to Improve Feed Utilization by Ruminants. Agriculture, 12(5), 573. https://doi.org/10.3390/agriculture12050573










