Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analytical Methods for the Determination of the Four Fungicides in Pears
2.2.1. Sample Preparation
2.2.2. Determination of the Four Fungicides
2.2.3. Method Validation
2.3. Field Trials
2.4. Dietary Risk Evaluation
2.5. Data Analysis
3. Results
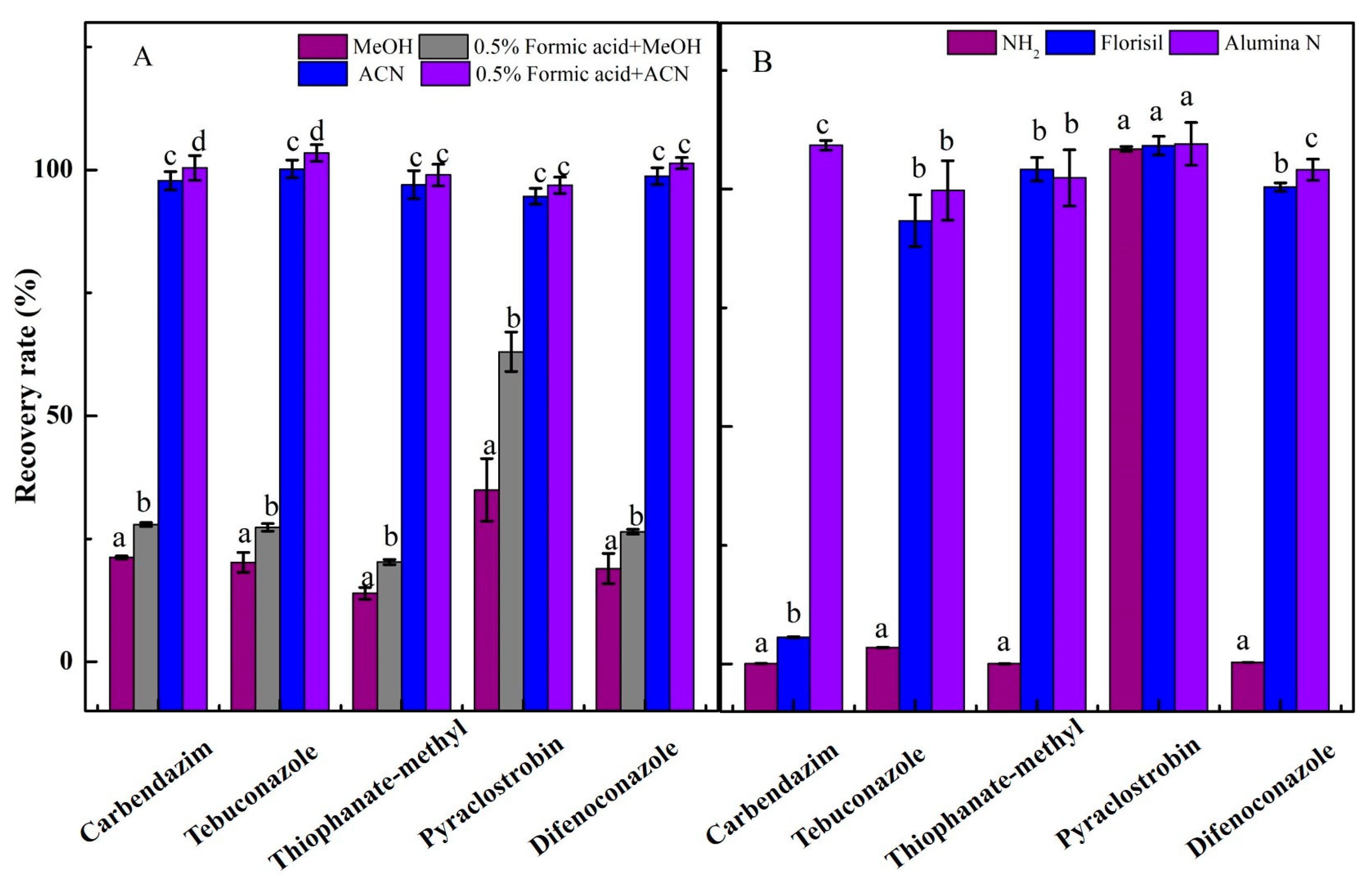
3.1. Optimization of the Extraction and Cleanup Procedure
3.2. Method Validation
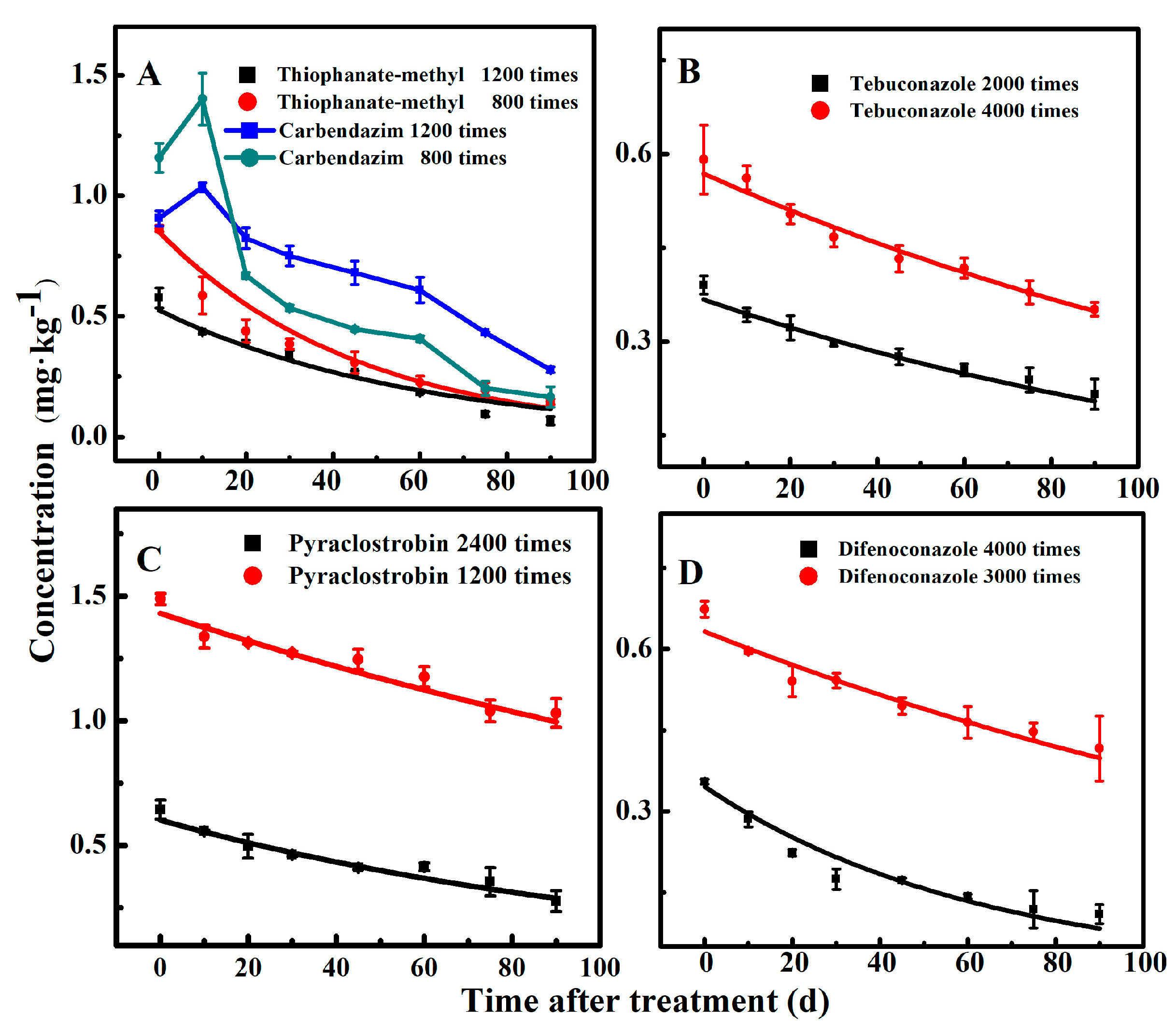
3.3. Dissipation Behaviors of the Fungicides in Pears
3.4. Fungicide Residues of Commercial Pear Samples
3.5. Dietary Intake Risk of Fungicides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Li, X.J.; Wang, T.T.; Gao, W.Y. Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2015; pp. 573–608. ISBN 978-0-12-408117-8. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/zh/#data/QCL/visualize (accessed on 16 July 2021).
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct. 2010, 1, 73–83. [Google Scholar] [CrossRef]
- Lesellier, E.; Destandau, E.; Grigoras, C.; Fougère, L.; Elfakir, C. Fast separation of triterpenoids by supercritical fluid chromatography/evaporative light scattering detector. J. Chromatogr. A 2012, 1268, 157–165. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Sutton, T.B.; Aldwinckle, H.S.; Agnello, A.M.; Walgenbach, J.F. Compendium of Apple and Pear Diseases and Pests, 2nd ed.; American Phytopathological Society: St. Paul, MN, USA, 2014; pp. 20–21. ISBN 978-0-89054-433-4. [Google Scholar]
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial Spoilage of Fruits: A Review on Causes and Prevention Methods. Food Rev. Int. 2020, 4, 1–22. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.F.; Marcone, M. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricker, S.M.; Gossen, B.D.; McDonald, M.R. Risk assessment of secondary metabolites produced by fungi in the genus Stemphylium. Can. J. Microbiol. 2021, 67, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ji, D.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Advances and Strategies for Controlling the Quality and Safety of Postharvest Fruit. Engineering 2021, 7, 1177–1184. [Google Scholar] [CrossRef]
- Khosla, K. Prevalence, incidence and management of fruit rot of pears incited by Acremonium kiliense. Plant Dis. Res. 2018, 2, 168–173. [Google Scholar]
- Fang, Q.; Wu, R.; Hu, G.; Lai, A.; Wu, K.; Zhang, L.; Cao, H. Dissipation behavior, residue distribution and risk assessment of three fungicides in pears. J. Sci. Food Agric. 2020, 100, 1757–1763. [Google Scholar] [CrossRef]
- Gupta, R.C. Veterinary Toxicology Basic and Clinical Principles, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 569–580. ISBN 978-0-12-811411-7. [Google Scholar]
- Freitas, R.M.; Linhares, B.S.; Oliveira, J.M.; Leite, J.P.V.; da Matta, S.L.P.; Gonçalves, R.V.; Freitas, M.B. Tebuconazole-induced toxicity and the protective effect of Ficus carica extract in Neotropical fruit-eating bats. Chemosphere 2021, 275, 129985. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Liu, S.H.; Liao, M.H.; Chen, R.M.; Liu, P.Y.; Ueng, T.H. Effects of tebuconazole on cytochrome P450 enzymes, oxidative stress, and endocrine disruption in male rats. Environ. Toxicol. 2018, 33, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, A.H.; Salazar, G.; Cooper, E.M.; Stapleton, H.M.; Zylka, M.J. Choice of vehicle affects pyraclostrobin toxicity in mice. Chemosphere 2019, 218, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Mu, X.; Chai, T.; Wang, K.; Zhang, J.; Zhu, L.; Li, X.; Wang, C. Occurrence and origin of sensitivity toward difenoconazole in zebrafish (Danio reio) during different life stages. Aquat. Toxicol. 2015, 160, 57–68. [Google Scholar] [CrossRef]
- Jia, K.; Cheng, B.; Huang, L.; Xiao, J.; Bai, Z.; Liao, X.; Lu, H. Thiophanate-methyl induces severe hepatotoxicity in zebrafish. Chemosphere 2020, 248, 125941. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, T.; Zhao, J.; Zhang, L.; Chen, R.; Wu, Y. Determination of eight pesticides in Lycium barbarum by LC–MS/MS and dietary risk assessment. Food Chem. 2017, 218, 192–198. [Google Scholar] [CrossRef]
- Dong, M.; Ma, L.; Zhan, X.; Chen, J.; Huang, L.; Wang, W.; Zhao, L. Dissipation rates and residue levels of diflubenzuron and difenoconazole on peaches and dietary risk assessment. Regul. Toxicol. Pharm. 2017, 108, 104447. [Google Scholar] [CrossRef]
- Lin, H.; Liu, L.; Zhang, Y.; Shao, H.; Li, H.; Li, N.; Guo, Y. Residue behavior and dietary risk assessment of spinetoram (XDE-175-J/L) and its two metabolites in cauliflower using QuEChERS method coupled with UPLC–MS/MS. Ecotoxicol. Environ. Saf. 2020, 202, 110942. [Google Scholar] [CrossRef]
- Announcement, No. 2308 of the Ministry of Agriculture of the People’s Republic of China. Available online: http://agrichem.cn/news/2015/10/13/2015101315371558875.shtml (accessed on 14 October 2021).
- De, A.; Mridha, D.; Ray, I.; Joardar, M.; Das, A.; Chowdhury, N.R.; Roychowdhury, T. Fluoride exposure and probabilistic health risk assessment through different agricultural food crops from Fluoride Endemic Bankura and Purulia Districts of West Bengal, India. Front. Environ. Sci. 2021, 9, 713148. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Alrasheed, A.M.; Al-Mundarij, A.A.; Omar, A.F.; Alhewairini, S.S.; Al-Jamhan, K.A. Levels of residues and dietary risk assessment of the fungicides myclobutanil, penconazole, tebuconazole, and triadimenol in squash. Biomed. Chromatogr. 2021, 35, e5126. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Y.; Wang, X.; Xu, J.; Zheng, X.; Sui, C. Residue analysis and risk assessment of tebuconazole in jujube (Ziziphus jujuba Mill). Biomed. Chromatogr. 2017, 31, e3917. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Xue, J.; Pan, D.; Wu, X.; Li, Q.X.; Hua, R. Simultaneous determination of dimethenamid, saflufenacil and their metabolites in maize using a modified QuEChERS method and liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2018, 11, 3396–3405. [Google Scholar] [CrossRef]
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, R.; del Mar Bernal-Suárez, M.; Luzardo, O.P. Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci. Total Environ. 2021, 753, 142015. [Google Scholar] [CrossRef]
- Zhan, X.P.; Ma, L.; Huang, L.Q.; Chen, J.B.; Zhao, L. The optimization and establishment of QuEChERS-UPLC–MS/MS method for simultaneously detecting various kinds of pesticides residues in fruits and vegetables. J. Chromatogr. B 2017, 1060, 281–290. [Google Scholar]
- Soliman, A.S.; Helmy, R.; Nasr, I.N.; Abbas, M.S.; Mahmoud, H.A.; Jiang, W. Behavior of Thiophanate Methyl and Propiconazole in Grape and Mango Fruits under the Egyptian Field Conditions. Bull. Environ. Contam. Toxicol. 2017, 98, 720–725. [Google Scholar] [CrossRef]
- Dong, B.; Yang, Y.; Pang, N.; Hu, J. Residue dissipation and risk assessment of tebuconazole, thiophanate-methyl and its metabolite in table grape by liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 260, 66–72. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, S.; Chen, X.; Hu, J. Simultaneous determination of pyraclostrobin, prochloraz, and its metabolite in apple and soil via RRLC–MS/MS. Food Anal. Methods 2018, 11, 1312–1320. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, S.; Li, X.; He, L.; Zhu, J.; Mu, W.; Liu, F. Residue determination of pyraclostrobin, picoxystrobin and its metabolite in pepper fruit via UPLC-MS/MS under open field conditions. Ecotoxicol. Environ. Saf. 2019, 182, 109445. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Zheng, Q.; Wang, Y.; Cheng, D.; Zhang, Z. Dissipation and distribution of pyraclostrobin in bananas at different temperature and a risk assessment of dietary intake. Int. J. Environ. Anal. Chem. 2020, 1–12. [Google Scholar] [CrossRef]
- GB 2763. Maximum Residue Limit Standard for Pesticides in Food. 2021. Available online: http://2763.foodvip.net/ (accessed on 7 July 2021).


| Matrix | Compounds | Liner Range (mg/kg) | Calibration Curves | Correlation Coefficients | Slop Ration | ME (%) | LOD (ng/kg) | LOQ (ng/kg) |
|---|---|---|---|---|---|---|---|---|
| Acetonitrile | Carbendazim | 0.001–0.1 | Y = 105045X − 31881 | 0.9997 | / | / | / | / |
| Tebuconazole | 0.001–0.1 | Y = 110317X + 50545 | 0.9993 | / | / | / | / | |
| Thiophanate-methyl | 0.001–0.1 | Y = 34102X − 10859 | 0.9996 | / | / | / | / | |
| Pyraclostrobin | 0.001–0.1 | Y = 88561X + 157793 | 0.9991 | / | / | / | / | |
| Difenoconazole | 0.001–0.1 | Y = 84024X + 54151 | 0.9994 | / | / | / | / | |
| Pear | Carbendazim | 0.001–0.1 | Y = 4654.6X + 49335 | 0.9992 | 0.044 | 95.57 | 1.5 | 5.1 |
| Tebuconazole | 0.001–0.1 | Y = 3898.9X + 14377 | 0.9992 | 0.035 | 96.47 | 0.16 | 0.6 | |
| Thiophanate-methyl | 0.001–0.1 | Y = 3157.8X + 34032 | 0.9993 | 0.093 | 90.74 | 2.5 | 8.4 | |
| Pyraclostrobin | 0.001–0.1 | Y = 3381.1X + 16999 | 0.9994 | 0.038 | 96.18 | 0.24 | 0.8 | |
| Difenoconazole | 0.001–0.1 | Y = 5667.4X + 4252.6 | 0.9990 | 0.067 | 93.26 | 2.0 | 6.5 |
| Sample | Temperature (°C) | Dilution Ratio | Dynamic Equation | T1/2 (d) | R2 |
|---|---|---|---|---|---|
| Thiophanate-methyl | 25 | 1200-fold | Ct = 0.2193 × e−0.0626t | 11.1 | 0.9542 |
| 800-fold | Ct = 0.6472 × e−0.0960t | 7.2 | 0.9381 | ||
| 4 | 1200-fold | Ct = 0.5256 × e−0.0168t | 41.3 | 0.9570 | |
| 800-fold | Ct = 0.8516 × e−0.0219t | 31.6 | 0.9856 | ||
| Tebuconazole | 25 | 4000-fold | Ct = 0.4000 × e−0.0492t | 14.1 | 0.9913 |
| 2000-fold | Ct = 0.5474 × e−0.0451t | 15.4 | 0.9730 | ||
| 4 | 4000-fold | Ct = 0.3675 × e−0.0065t | 106.6 | 0.9454 | |
| 2000-fold | Ct = 0.5686 × e−0.0054t | 128.3 | 0.9716 | ||
| Pyraclostrobin | 25 | 2400-fold | Ct = 0.6195 × e−0.0515t | 13.5 | 0.9728 |
| 1200-fold | Ct = 1.5527 × e−0.0481t | 14.4 | 0.9332 | ||
| 4 | 2400-fold | Ct = 0.6017 × e−0.0082t | 84.5 | 0.9593 | |
| 1200-fold | Ct = 1.4310 × e−0.0040t | 173.3 | 0.9068 | ||
| Difenoconazole | 25 | 4000-fold | Ct = 0.4221 × e−0.09247t | 7.5 | 0.8918 |
| 3000-fold | Ct = 0.5646 × e−0.03282t | 21.1 | 0.8147 | ||
| 4 | 4000-fold | Ct = 0.3459 × e−0.01575t | 44.0 | 0.9675 | |
| 3000-fold | Ct = 0.6327 × e−0.00513t | 135.1 | 0.9291 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Hu, K.; Li, X.; Liu, C.; Xu, Y.; Zhang, Z.; Wu, X. Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear. Agriculture 2022, 12, 630. https://doi.org/10.3390/agriculture12050630
Tang Y, Hu K, Li X, Liu C, Xu Y, Zhang Z, Wu X. Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear. Agriculture. 2022; 12(5):630. https://doi.org/10.3390/agriculture12050630
Chicago/Turabian StyleTang, Yongfeng, Kuikui Hu, Xiaomeng Li, Chaogang Liu, Yanhui Xu, Zhaoxian Zhang, and Xiangwei Wu. 2022. "Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear" Agriculture 12, no. 5: 630. https://doi.org/10.3390/agriculture12050630





