Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan
Abstract
:1. Introduction
2. Materials and Methods
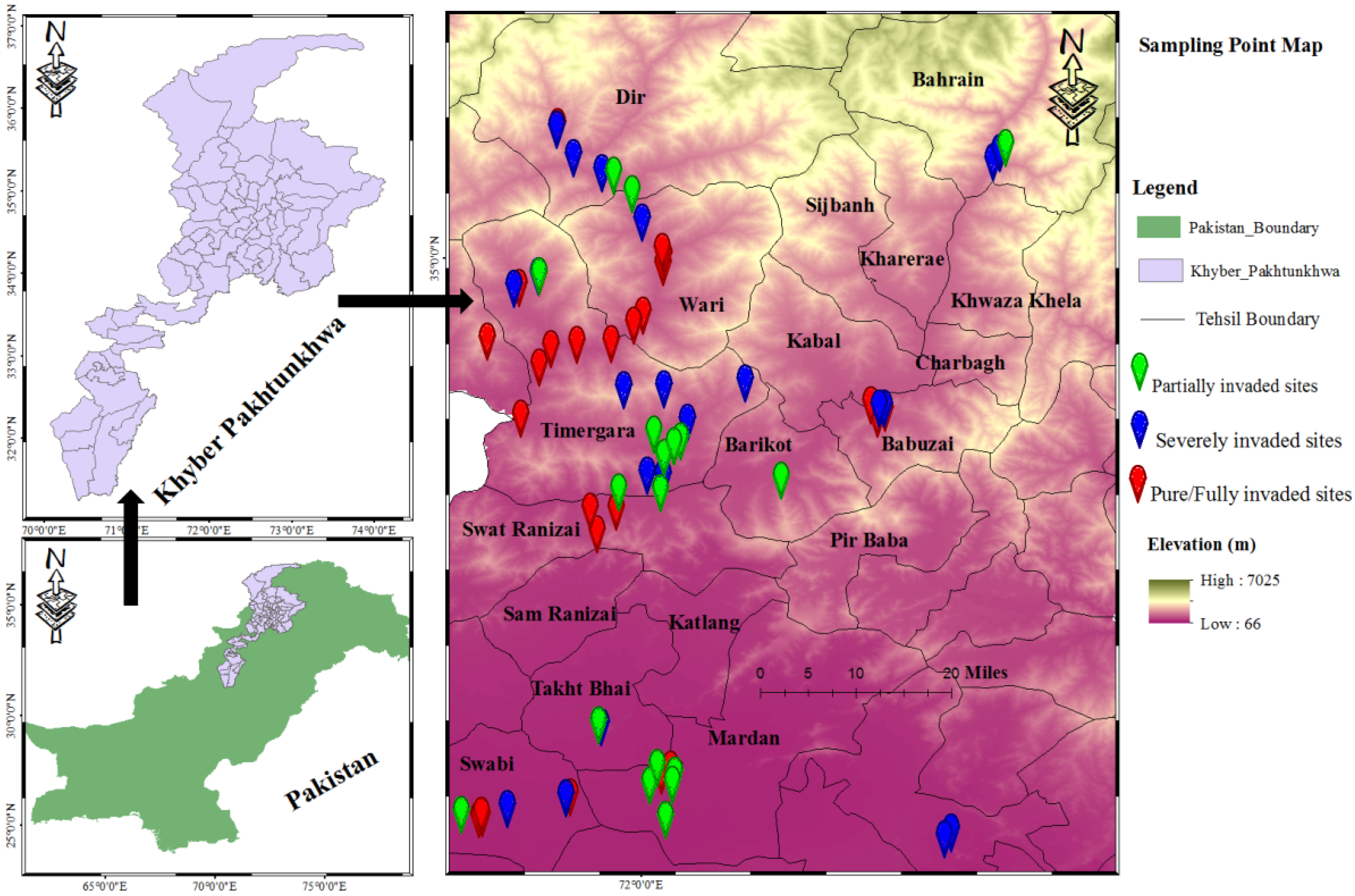
2.1. Study Area and Sampling Sites
2.2. Vegetation Sampling
2.3. Environmental and Soil Variables
2.4. Data Analyses
3. Results
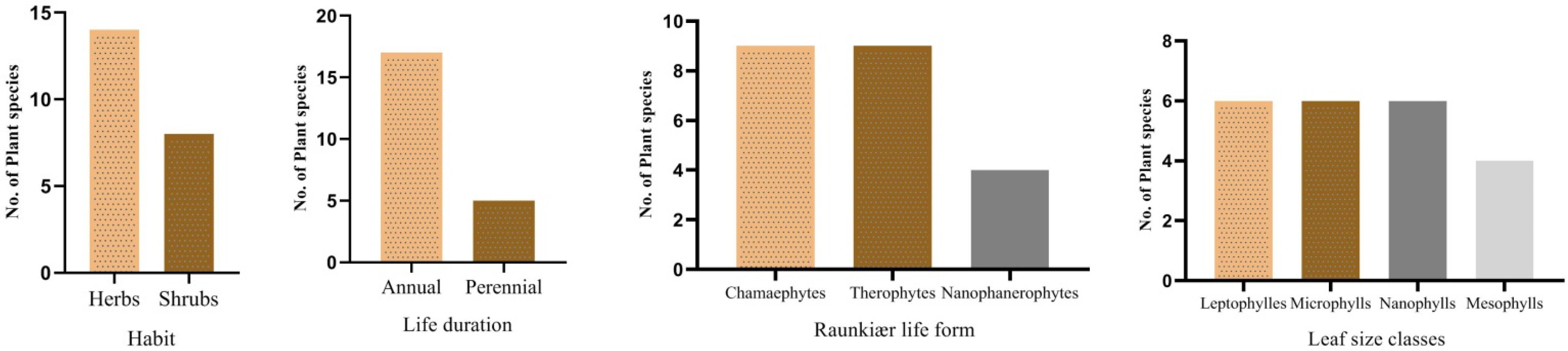
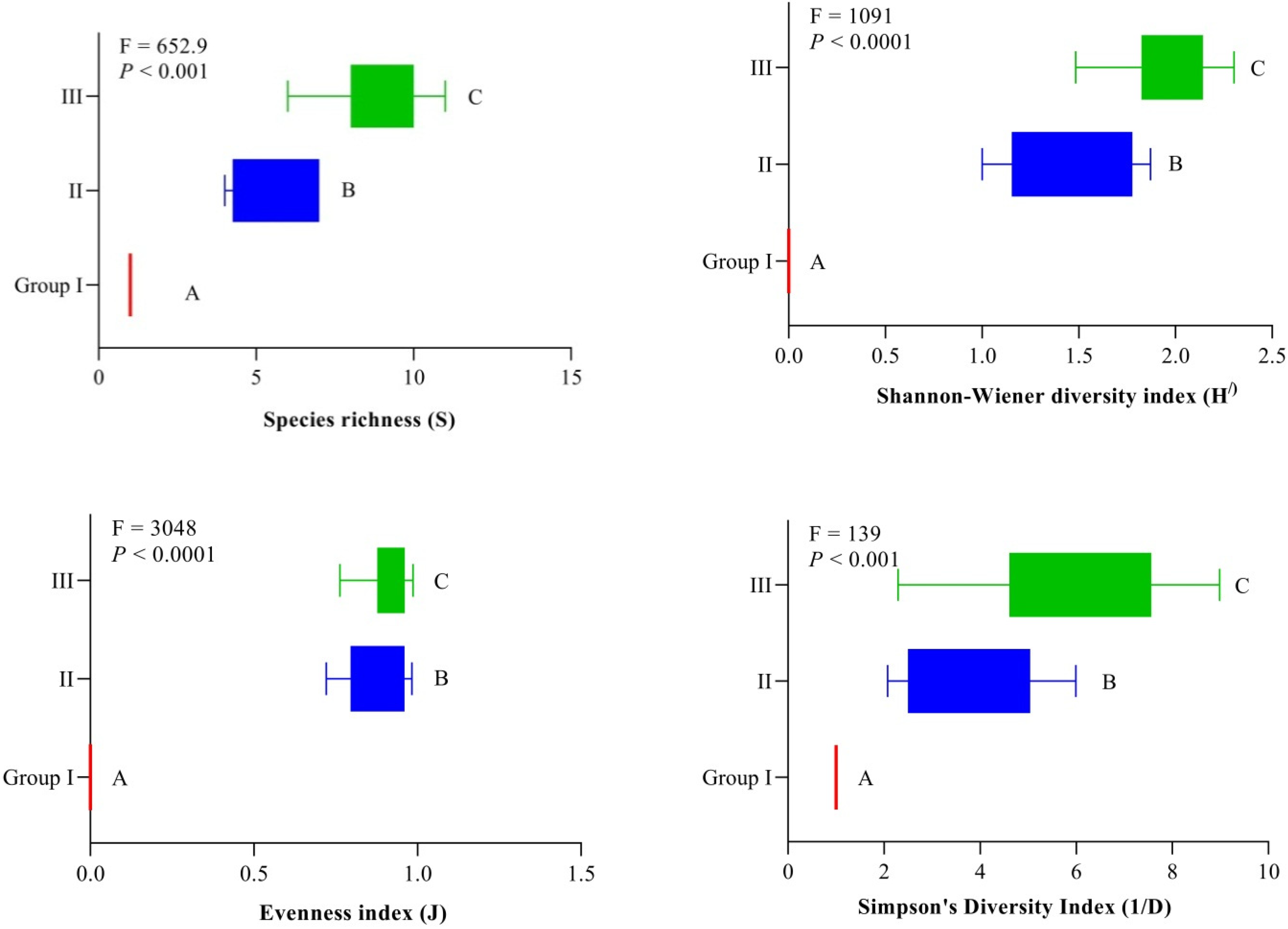
3.1. Phytosociological/Vegetation Traits
3.2. Environmental Variables
4. Discussion
5. Conclusions
- The findings show that S. marianum is found in low-elevation pure communities, and progressive spreading to higher elevations significantly influences local diversity and homogenizes the region.
- There was a clear correlation between the S. marianum importance value index and the diversity indices, with the latter decreasing as the former increased, and this is a possible hazard to the biodiversity of the native region.
- Communities’ responses were found to be complemented by changes in environmental variables. The results revealed that the nutrient concentrations in the severely invaded sites were more significant than those in the pure and partially invaded sites.
- The management and control of this species are thus necessary for conserving and maintaining natural vegetation.
- These findings open up many possibilities for further research. It can forecast not just the behavior of S. marianum but also the behavior of other invasive plant species with phylogenetic or morphological similarities with S. marianum.
- This research will be extremely important in increasing our understanding of the distribution, development, and spread of alien invasive species along various climatic gradients in observed and predicted fast climate change.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latombe, G.; Roura-Pascual, N.; Hui, C. Similar compositional turnover but distinct insular environmental and geographical driv-ers of native and exotic ants in two oceans. J. Biogeogr. 2019, 46, 2299–2310. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Jia, G.; Epstein, H. Recent changes in phenology over the northern high latitudes detected from multi-satellite data. Environ. Res. Lett. 2011, 6, 045508. [Google Scholar] [CrossRef] [Green Version]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Minfared, H.H.; Khalafi, J.; Taghiloo, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Stohlgren, T.; Rejmánek, M. No universal scale-dependent impacts of invasive species on native plant species richness. Biol. Lett. 2014, 10, 2013093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stohlgren, T.J.; Barnett, D.T.; Jarnevich, C.S.; Flather, C.; Kartesz, J. The myth of plant species saturation. Ecol. Lett. 2008, 11, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, A.; Shea, K. Integrating the study of non-native plant invasions across spatial scales. Biol. Invasions 2006, 8, 399–413. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot. 2011, 98, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Price, E.P.F.; Spyreas, G.; Matthews, J.W. Biotic homogenization of regional wetland plant communities within short time-scales in the presence of an aggressive invader. J. Ecol. 2018, 106, 1180–1190. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [Green Version]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B Biol. Sci. 2006, 273, 2659–2665. [Google Scholar] [CrossRef] [Green Version]
- Morri, C.; Montefalcone, M.; Gatti, G.; Vassallo, P.; Paoli, C.; Bianchi, C.N. An Alien Invader is the cause of homogenization in the recipient ecosystem: A simulation-like approach. Diversity 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.; Richardson, D.M.; Rouget, M.; Procheş, Ş.; Amis, M.A.; Henderson, L.; Thuiller, W. Residence time and potential range: Crucial considerations in modelling plant invasions. Divers. Distrib. 2007, 13, 11–22. [Google Scholar] [CrossRef]
- Obiri, J.F. Invasive plant species and their disaster-effects in dry tropical forests and rangelands of Kenya and Tanzania. J. Disaster Risk Stud. Jàmbá 2011, 3, 417–428. [Google Scholar] [CrossRef]
- Hamouda, M. Molecular analysis of genetic diversity in population of Silybum marianum (L.) Gaertn in Egypt. J. Genet. Eng. Biotechnol. 2019, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Groves, R.H.; Kaye, P.E. Germination and phenology of seven introduced thistle species in Southern Australia. Aust. J. Bot. 1989, 37, 351–359. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Shokrpour, M.; Moghaddam, M.; Javanshir, A. AFLP-based molecular characterization and population structure analysis of Silybum marianum L. Plant Genet. Resour. 2011, 9, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Omidbaigi, R.; Nobakht, A. Nitrogen fertilizer affecting growth, seed yield and active substances of milk thistle (Silybum marianum). Pak. J. Biol. Sci. 2001, 4, 1345–1349. [Google Scholar]
- Rafizadeh, A.; Koohi-Dehkordi, M.; Sorkheh, K. Molecular insights of genetic variation in milk thistle (Silybum marianum [L.] Gaertn.) populations collected from southwest Iran. Mol. Biol. Rep. 2018, 45, 601–609. [Google Scholar] [CrossRef]
- Holm, L.G.; Doll, J.; Holm, E.; Pancho, J.; Herberger, J. World Weeds. In Natural Histories and Distribution; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Berner, D.K.; Paxson, L.K.; Bruckart, W.L.; Luster, D.G.; McMahon, M.B.; Michael, J.L. First report of Silybum marianum as a host of Puccinia punctiformis. Plant Distrib. 2002, 86, 1271. [Google Scholar] [CrossRef]
- Gabay, R.; Plitmann, U.; Danin, A. Factors affecting the dominance of Silybum marianum L. (Asteraceae) in its specific habitats. Flora 1994, 189, 201–206. [Google Scholar] [CrossRef]
- Marwat, K.B.; Hussain, Z.; Gul, B.; Saeed, M.; Din, S. Survey on weed problems in wheat crop in district Mardan. Pak. J. Weed Sci. Res. 2006, 12, 353–358. [Google Scholar]
- Saeed, A. Milk Thistle Seeds. Ray’s Herbs Trading Company, Karachi, Pakistan. 2008. Available online: http://itrademarket.com/rays (accessed on 22 August 2020).
- Rahman, A.; Dawood, M. Spatio-statistical analysis of temperature fluctuation using Mann–Kendall and Sen’s slope approach. Clim. Dyn. 2016, 48, 783–797. [Google Scholar] [CrossRef]
- Ali, A.; Khan, T.A.; Ahmad, A. Analysis of Climate Data of Khyber Pakhtunkhwa, Pakistan. Int. Res. J. Eng. Technol. 2018, 5, 4266–4283. [Google Scholar]
- Shah, A.A.; Ye, J.; Abid, M.; Ullah, R. Determinants of flood risk mitigation strategies at household level: A case of Khyber Pakhtunkhwa (KP) province, Pakistan. Nat. Hazards 2017, 88, 415–430. [Google Scholar] [CrossRef]
- Deo, R.C.; Şahin, M. Application of the Artificial Neural Network model for prediction of monthly Standardized Precipitation and Evapotranspiration Index using hydrometeorological parameters and climate indices in eastern Australia. Atmos. Res. 2015, 161, 65–81. [Google Scholar] [CrossRef]
- Ali, F.; Khan, N.; Ahmad, A.; Khan, A.A. Structure and biomass carbon of Olea ferruginea forests in the foot hills of Malakand division, Hindukush Range Mountains of Pakistan. Acta Ecol. Sin. 2019, 39, 261–266. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland management and ecological adaptation analysis model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Ali, S.I.; Nasir, E. Flora of West Pakistan; Department of Botany, University of Karachi: Karachi, Pakistan, 1971. [Google Scholar]
- Rahman, A.; Khan, N.; Ali, K.; Ullah, R.; Khan, M.E.H.; Jones, D.A.; Rahman, I.U. Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan. Appl. Sci. 2021, 11, 11372. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Li, X. Effects of soil pH and texture on soil carbon and nitrogen in soil profiles under different land uses in Mun River Basin, Northeast Thailand. PeerJ 2019, 7, e7880. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.W. Exchangeable cations. In Methods of Soils Analysis; Page, A.L., Millar, R.H., Keeney, D.R., Eds.; Part 1; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Stubbins, A.; Dittmar, T. Low volume quantification of dissolved organic carbon and dissolved nitrogen. Limnol. Oceanogr. Methods 2012, 5, 347–352. [Google Scholar] [CrossRef]
- Orlóci, L. An agglomerative method for classification of plant communities. J. Ecol. 1967, 55, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Ter Braak, C.J. The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetation 1987, 69, 69–77. [Google Scholar] [CrossRef]
- Skinner, W.R.; Jefferies, R.L.; Carleton, T.J.; Abraham, R.R.D.K. Prediction of reproductive success and failure in lesser snow geese based on early season climatic variables. Glob. Change Biol. 1998, 4, 3–16. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Pino, J.; Font, X.; de Cáceres, M.; Molowny-Horas, R. Floristic homogenization by native ruderal and alien plants in north-east Spain: The effect of environmental differences on a regional scale. Glob. Ecol. Biogeogr. 2009, 18, 563–574. [Google Scholar] [CrossRef]
- McKinney, M.L. Species introduced from nearby sources have a more homogenizing effect than species from distant sources: Evidence from plants and fishes in the USA. Divers. Distrib. 2005, 11, 367–374. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E. The role of exotic species in homogenizing the north American flora. Ecol. Lett. 2006, 9, 1293–1298. [Google Scholar] [CrossRef]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Guo, Q.; Qian, H. Growth form and distribution of introduced plants in their native and non-native ranges in Eastern Asia and North America. Divers. Distrib. 2008, 14, 381–386. [Google Scholar] [CrossRef]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Salsa, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 1988, 132, 87–106. [Google Scholar] [CrossRef]
- Tappeiner, J.; Zasada, J.; Ryan, P.; Newton, M. Salmonberry clonal and population structure: The basis for a persistent cover. Ecology 1991, 72, 609–618. [Google Scholar] [CrossRef]
- Timsina, B.B.B.; Shrestha, M.B.; Rokaya, Z.M.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grass land communities in Nepal. Flora 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Khan, N.; Bibi, K.; Ullah, R. Distribution pattern and ecological determinants of an invasive plant Parthenium hysterophorus L., in Malakand division of Pakistan. J. Mt. Sci. 2020, 17, 1670–1683. [Google Scholar] [CrossRef]
- Paterson, I.D.; Hoffmann, J.H.; Klein, H.; Neser, S.; Mathenge, C.W.; Zimmermann, H.G. Biological control of cactaceae in South Africa. Afr. Entomol. 2011, 19, 230–246. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Oduor, A.M.; Long, H.; Fandohan, A.B.; Liu, J.; Yu, X. An invasive plant provides refuge to native plant species in an intensely grazed ecosystem. Biol. Invasions 2018, 20, 2745–2751. [Google Scholar] [CrossRef]
- Ogbazghi, W.; Stillhardt, B. Sustainable Land Management a Textbook with a Focus on Eritrea; Geographica Bernensia and Hamelmalo Agricultural College: Bern, Switzerland, 2011. [Google Scholar]
- Bein, E.; Habte, B.; Jaber, A.; Birnie, A.; Tengnas, B. Useful Trees and Shrubs in Eritrea; Regal Press Limited: Nairobi, Kenya, 1996. [Google Scholar]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Novoa, A.; Le Roux, J.J.; Robertson, M.P.; Wilson, J.R.; Richardson, D.M. Introduced and invasive cactus species: A global review. AoB Plants 2015, 7, plu078. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Eaton, W.D.; Farrell, R.E. Catabolic and genetic microbial indices, and levels of nitrate, ammonium and organic carbon in soil from the black locust (Robinia pseudo-acacia) and tulip poplar (Liriodendron tulipifera) trees in a Pennsylvania forest. Biol. Fertil. Soils 2004, 39, 209–214. [Google Scholar] [CrossRef]
- Morgan, E.C.; Overholt, W.A.; Sellers, B. Wildland Weeds: Paper Mulberry, Broussonetia Papyrifera. Publication #ENY-702. Series of the Entomology and Nematology Department, Florida Co-Operative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. 2019. Available online: https://edis.ifas.ufl.edu/in498 (accessed on 22 August 2020).
- Ghersa, C.M.; de la Fuente, E.; Suarez, S.; Leon, R.J.C. Woody species in the Rolling Pampa grasslands, Argentina. Agric. Ecosyst. Environ. 2002, 88, 271–278. [Google Scholar] [CrossRef]
- Bosu, P.P.; Apetorgbor, M.M.; Nkrumah, E.E.; Bandoh, K.P. The impact of Broussonetia papyrifera (L.) Vent. on community characteristics in the forest and forest–savannah transition ecosystems of Ghana. Afr. J. Ecol. 2013, 51, 528–535. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z. Broussonetia papyrifera (L.) L’h´er. Ex Vent.: An environmental constraint on the Himalayan foothills’ vegetation. Pak. J. Bot. 2007, 39, 1045–1053. [Google Scholar]
- Monemizadeh, Z.; Ghaderi-Far, F.; Sadeghipour, H.R.; Siahmarguee, A.; Soltani, E.; Torabi, B.; Baskin, C.C. Variation in seed dormancy and germination among populations of Silybum marianum (Asteraceae). Plant Species Biol. 2021, 36, 412–424. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phenotypic variations alter the ecological impact of invasive alien species: Lessons from Parthenium hysterophorus. J. Environ. Manag. 2019, 241, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Batish, D.R.; Singh, H.P.; Jaryan, V.; Kohli, R.K. The impact of invasive Hyptis suaveolens on the floristic composition of the peri urban ecosystems of Chandigarh, northwestern India. Flora 2017, 233, 156–162. [Google Scholar] [CrossRef]
- Anning, A.K.; Gyamfi, B.; Effah, A.T. Broussonetia papyrifera controls nutrient return to soil to facilitate its invasion in a tropical forest of Ghana. J. Plant Ecol. 2018, 11, 909–918. [Google Scholar] [CrossRef]
- McEwan, R.W.; Arthur, M.A.; Alverson, S.E. Throughfall chemistry and soil nutrient effects of the invasive shrub Lonicera maackii in deciduous forests. Am. Midl. Nat. J. 2012, 168, 43–55. [Google Scholar] [CrossRef]
- Yapi, T.S.; O’Farrell, P.J.; Dziba, L.E.; Esler, K.J. Alien tree invasion into a South African montane grassland ecosystem: Impact of Acacia species on rangeland condition and livestock carrying capacity. J. Biodivers. Ecosyst. Serv. Environ. Manag. 2018, 14, 105–116. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Hernea, C.; Bakti, B.; Keserű, Z.; Antal, B.; Rédei, K. Black locust (Robinia pseudoacacia L.) as a multi-purpose tree species in Hungary and Romania: A review. J. For. Res. 2018, 29, 1449–1463. [Google Scholar] [CrossRef]
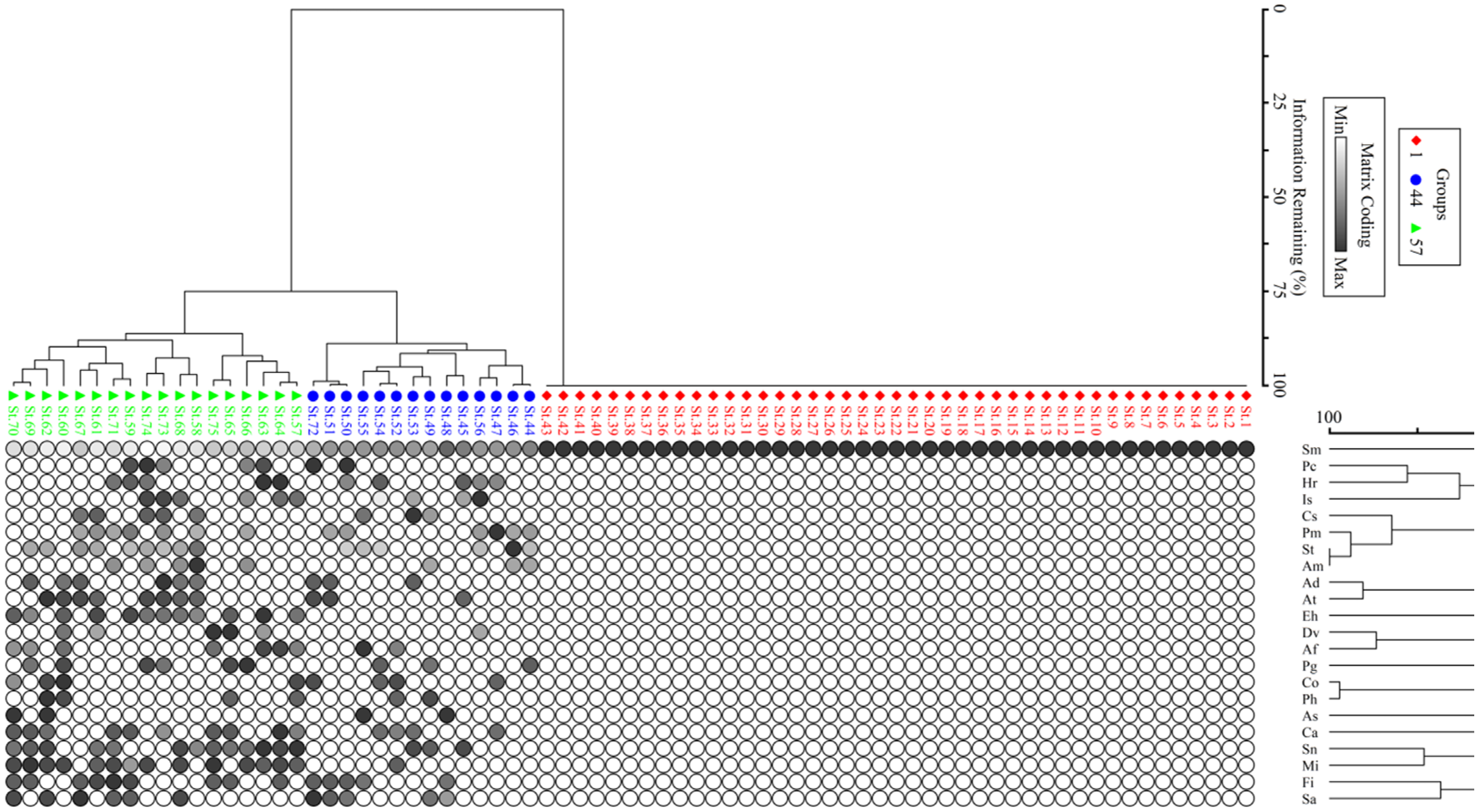

| Group-I | Group-II | Group-III |
|---|---|---|
|
|
|
| Species | Ac | Group I | Group II | Group III | ||
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | F | p-Value | ||
| Silybum marianum (L.) Gaernt | Sm | 100 ± 0 a | 62.44 ± 1.91b | 30.44 ± 1.75 c | 1451.3 | 6.61 × 10−59 |
| Amaranthus viridis L. | Am | 0 ± 0 a | 1.03 ± 0.70 b | 3.1 ± 1.28 c | 7.11 | 0.001514 |
| Artemisia scoparia Waldst. & Kit. | As | 0 ± 0 a | 1.58 ± 0.84 b | 3.35 ± 0.91 c | 13.63 | 9.50 × 10−6 |
| Asphodelus tenuifolius Cav. | Ad | 0 ± 0 a | 1.71 ± 0.91 b | 2.62 ± 0.91 c | 8.59 | 0.0004 |
| Avena fatua L. | Af | 0 ± 0 a | 1.27 ± 0.88 b | 3.07 ± 0.96 c | 10.16 | 0.00012 |
| Avena sativa L. | As | 0 ± 0 a | 1.03 ± 0.70 b | 0.77 ± 0.53 c | 3.07 | 0.052573 |
| Cannabis sativa L. | Cs | 0 ± 0 a | 1.64 ± 0.90 b | 2.22 ± 0.87 c | 6.94 | 0.0017 |
| Carthamus oxyacantha M.Bieb. | Co | 0 ± 0 a | 2.18 ± 0.96 b | 1.67 ± 0.77 c | 6.99 | 0.0016 |
| Chenopodium murale L. | Cm | 0 ± 0 a | 2.11 ± 0.92 b | 5.06 ± 1.08 c | 21.18 | 5.84 × 10−8 |
| Cortaderia selloana (Schult. & Schult.f.) Asch. & Graebn. | Pg | 0 ± 0 a | 1.64 ± 0.87 b | 2.85 ± 0.99 c | 9.04 | 0.000315 |
| Dodonaea viscosa Jacq. | Dv | 0 ± 0 a | 0.51 ± 0.51 b | 3.26 ± 1.37 c | 7.91 | 0.0007 |
| Euphorbia helioscopia L. | Eh | 0 ± 0 a | 0 ± 0 a | 5.12 ± 0.91 b | 50.89 | 1.67 × 10−14 |
| Fumaria indica Hausskn. | Fi | 0 ± 0 a | 2.66 ± 0.99 b | 4.3 ± 1.04 c | 17.89 | 4.91 × 10−7 |
| Hypochaeris radicata L. | Hr | 0 ± 0 a | 2.38 ± 0.91 b | 2.36 ± 0.94 c | 8.78 | 0.000387 |
| Lathyrus sativus L. | Ls | 0 ± 0 a | 1.61 ± 0.88 b | 2.94 ± 1.01 c | 10.77 | 8.08 × 10−5 |
| Melilotus indicus (L.) All. | Mi | 0 ± 0 a | 0.57 ± 0.57 b | 6.75 ± 0.90 c | 68.9 | 1.90 × 10−17 |
| Parthenium hysterophorus L. | Ph | 0 ± 0 a | 1.06 ± 0.73 b | 1.69 ± 0.77 c | 5.123 | 0.00831 |
| Phalaris caroliniana Walter | Pc | 0 ± 0 a | 1.13 ± 0.77 b | 1.85 ± 0.73 c | 6.24 | 0.0031 |
| Phalaris minor Retz. | Pm | 0 ± 0 a | 4.47 ± 1.55 b | 3.41 ± 1.07 c | 12.91 | 1.61 × 10−5 |
| Solanum nigrum L. | Sn | 0 ± 0 a | 1.89 ± 1.01 b | 5.81 ± 0.90 c | 35.39 | 1.97 × 10−11 |
| Spergula arvensis L. | Sa | 0 ± 0 a | 2.69 ± 1.04 b | 2.97 ± 1.02 c | 10.38 | 0.000109 |
| Stylosanthes humilis Kunth | Sh | 0 ± 0 a | 3.72 ± 1.56 b | 4.65 ± 1.19 c | 13.78 | 8.52 × 10−6 |
| Factors | Group 1 | Group II | Group III | F-Value | p-Value |
|---|---|---|---|---|---|
| Elevation | 340.15 ± 4.84 a | 859.38 ± 13.75 b | 1548.64 ± 35.37 c | 50.81 | 0.000 |
| Latitude (°) | 34.76 ± 0.11 | 34.67 ± 0.1 | 34.66 ± 0.86 | 0.365 | 0.69 |
| Longitude (°) | 71.86 ± 0.81 | 72.09 ± 0.06 | 71.94 ± 0.05 | 0.365 | 0.69 |
| Aspect degree | 93.79 ± 6.3 | 118.15 ± 13 | 111.6 ± 10.1 | 2.13 | 0.12 |
| Clay (%) | 31.58 ± 1.2 | 31.96 ± 2.9 | 34.55 ± 2.2 | 0.784 | 0.46 |
| Silt % | 36.59 ± 1.4 | 36.25 ± 2.6 | 32.76 ± 2.3 | 1.07 | 0.35 |
| Sand (%) | 31.94 ± 1.4 | 31.77 ± 2.5 | 32.68 ± 1.8 | 14.03 | 7.11 × 10−6 |
| pH (1:5) | 6.75 ± 0.10 | 5.45 ± 0.05 | 5.73 ± 0.04 | 45.64 | 1.57 × 10−13 |
| Organic matter (%) | 0.55 ± 0.04 | 0.61 ± 0.07 | 0.57 ± 0.05 | 0.793 | 3.124 |
| Lime % | 11.21 ± 0.5 | 11.63 ± 1.9 | 12.1 ± 0.08 | 0.419 | 0.66 |
| Nitrogen % | 2.114 ± 1.2 | 4.77 ± 4.3 | 3.39 ± 2.2 | 0.382 | 0.68 |
| Conductivity (µs/cm) | 37.74 ± 1.7 | 42.07 ± 2.7 | 57 ± 18.49 | 1.43 | 0.24 |
| Phosphorus (mg/kg) | 6.726 ± 6.3 | 6.6 ± 0.72 | 6.34 ± 0.49 | 0.25 | 0.78 |
| Potassium (mg/kg) | 137.7 ± 4.9 | 145.84 ± 13 | 157.2 ± 8.3 | 1.92 | 0.15 |
| Axis 1 | Axis 2 | Axis 3 | |
|---|---|---|---|
| Eigenvalue | 0.384 | 0.075 | 0.047 |
| Data variance | |||
| Explained variance in % | 22.8 | 4.4 | 2.8 |
| Explained cumulative % | 22.8 | 27.2 | 29.9 |
| Pearson Correlation | 0.888 | 0.65 | 0.676 |
| Kendall (Rank) Correlation | 0.684 | 0.376 | 0.363 |
| Variable | Correlation | Biplot Scores | |||||
|---|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 3 | Axis 1 | Axis 2 | Axis 3 | ||
| 1 | Latitude (°) | 0.104 | 0.015 | 0.023 | 0.081 | 0.014 | 0.023 |
| 2 | Longitude (°) | 0.104 | 0.015 | 0.023 | 0.081 | 0.014 | 0.023 |
| 3 | Elev. | 0.638 | −0.178 | 0.005 | 0.001 | 0.001 | 0.012 |
| 3 | AA (°) | −0.237 | −0.002 | 0.316 | −0.186 | −0.002 | 0.309 |
| 4 | CLY (%) | 0.912 | −0.091 | 0.075 | 0.716 | −0.088 | 0.073 |
| 5 | SLT % | 0.777 | −0.086 | −0.057 | 0.61 | −0.083 | −0.055 |
| 6 | SND (%) | −0.528 | −0.082 | 0.202 | −0.415 | −0.078 | 0.198 |
| 7 | pH (1:5) | 0.176 | 0.15 | −0.125 | 0.138 | 0.144 | −0.122 |
| 8 | Organic matter (%) | −0.217 | 0.769 | 0.12 | −0.17 | 0.74 | 0.118 |
| 9 | Lime % | 0.882 | −0.02 | 0.068 | 0.692 | −0.02 | 0.066 |
| 10 | Nitrogen % | −0.41 | 0.646 | 0.098 | −0.322 | 0.622 | 0.095 |
| 11 | Conductivity (µs/cm) | −0.818 | 0.251 | −0.028 | −0.642 | 0.241 | −0.027 |
| 12 | Phosphorus (mg/kg) | −0.95 | 0.016 | −0.075 | −0.745 | 0.015 | −0.074 |
| 13 | Potassium (mg/kg) | −0.918 | 0.109 | 0.073 | −0.72 | 0.105 | 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Ullah, R.; Ali, K.; Jones, D.A.; Khan, M.E.H. Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan. Agriculture 2022, 12, 687. https://doi.org/10.3390/agriculture12050687
Khan N, Ullah R, Ali K, Jones DA, Khan MEH. Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan. Agriculture. 2022; 12(5):687. https://doi.org/10.3390/agriculture12050687
Chicago/Turabian StyleKhan, Nasrullah, Rafi Ullah, Kishwar Ali, David Aaron Jones, and Muhammad Ezaz Hasan Khan. 2022. "Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan" Agriculture 12, no. 5: 687. https://doi.org/10.3390/agriculture12050687
APA StyleKhan, N., Ullah, R., Ali, K., Jones, D. A., & Khan, M. E. H. (2022). Invasive Milk Thistle (Silybum marianum (L.) Gaertn.) Causes Habitat Homogenization and Affects the Spatial Distribution of Vegetation in the Semi-Arid Regions of Northern Pakistan. Agriculture, 12(5), 687. https://doi.org/10.3390/agriculture12050687








