Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Explant Selection
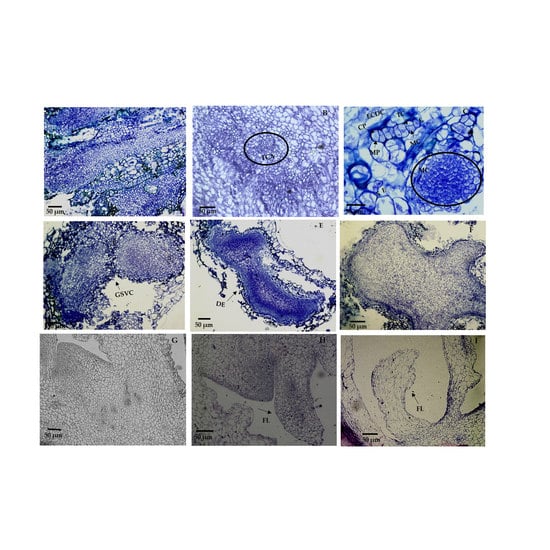
2.2. Histological Analysis of Morphogenic Structures That Originated in In Vitro Regeneration
2.3. Acclimatization of Plants In Vitro
2.4. Analysis of the Genetic Stability of the Plants Obtained from In Vitro Regeneration
2.5. Statistical Analysis
3. Results
3.1. Evaluation of the Treatments Established for In Vitro Regeneration of the Pineapple Hybrid ‘MD2′
3.1.1. Direct Somatic Embryogenesis (DSE)
3.1.2. Indirect Somatic Embryogenesis (ISE)
3.1.3. Indirect Organogenesis (IO) and Shoot Development
3.2. Histological Analysis
3.3. Acclimatization
3.4. Genetic Stability of the Plants Obtained from In Vitro Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaji, K.; Man, N.; Nawi, N.M. Factors affecting pineapple marker supply in Johor, Malaysia. Int. Food Res. J. 2018, 25, 365–371. [Google Scholar]
- Abdul, N.A.; Chin, B.; Razik, M.; Madon, M.; Khalid, N.; Syafawati, N. Abscisic acid and salinity stress induced somaclonal variation and increased histone deacetylase (HDAC) activity in Ananas comosus var. MD2. Plant Cell Tiss. Org. 2018, 133, 123–135. [Google Scholar] [CrossRef]
- Blanco, H.A.; Vargas, T.C.; García, T. Regeneración in vitro de plantas de piña (Ananas comosus, L. Merr.). Rev. Colomb. Biotechnol. 2017, 19, 7–20. [Google Scholar] [CrossRef]
- Hikal, W.M.; Mahmoud, A.A.; Hussein, A.H.; Ahl, S.A.; Bratovcic, A.; Tkachenko, K.G.; Kakaniova, M.; Maldonado, R. Pineapple (Ananas comosus L. Merr.) waste streams, characterization and valorization: An overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Russel, A. Piña miel: Un nuevo producto para aumentar la competitividad. 40 casos de éxitos. SAGARPA 2017, 1–18. Available online: https://www.redinnovagro.in/casosexito/2017/Pi%C3%B1a_Amador_Russell.pdf (accessed on 22 November 2021).
- Mercado, J.N.; Tortoledo, O.; García, J.M.; Báez, R.; García, B.Y.; Ávila, J.; Corella, D.A.; Cruz, M.C.; Velásquez, D.; Zuñiga, B.S. Calidad comercial de piña MD2 (Ananas comosus L.) tratada en postcosecha con ácido 2-hidroxibenzoico. Rev. Iberoam. Tec. Poscosecha 2019, 20, 1–15. Available online: https://www.redalyc.org/journal/813/81361553004/html/ (accessed on 22 November 2021).
- Nouri, F.; Farahani, F.; Noormohammadi, Z. Somaclonal variation in the in vitro regenerated pineapple (Ananas comosus): Investigation of the cellular characteristics, biochemical specificities and ISSR markers. Phytol. Balc. 2017, 23, 73–83. [Google Scholar]
- Garcidueñas, J.A. Caracterización Morfológica Y Molecular de Piña (Ananas Comosus, (L.) Merr.) Híbrido MD2 Y su Establecimiento in vitro. Master’s Thesis, Universidad Autónoma de Chapingo, Chapingo, Mexico, 2013. [Google Scholar]
- Guzmán, N.M. Obtención de Plantas de Piña (Ananas comosus, (L.) Merr) por Cultivo in vitro a partir de Yemas Axilares de La Corona. Master’s Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 1988. [Google Scholar]
- Souza, W.C.; Nascimento, M.; Oliveira, M.; Porcino, H.; Silva, A. Genetic diversity of Fusarium spp. in pineapple ‘Pérola’ cultivar. Fungal Biol. UK 2017, 120, 265–278. [Google Scholar] [CrossRef]
- Shamin, M.D.; Kumar, M.; Ranjan, T.; Ranjan, R.; Kumar, A.; Kumar, P.; Kumar, V.; Kumar, P. Importance of micropropagation in pineapple for disease free plantlets and rapid multiplication. J. Pharmacogn. Phytochem. 2016, 5, 359–362. [Google Scholar]
- Dhurve, L.; Kumar, K.A.; Bhaskar, J.; Sobhana, A.; Francies, R.M.; Mathew, D. Wide variability among the ‘Mauritius’ somaclones demonstrates somaclonal variation as a promising improvement strategy in pineapple (Ananas comosus L.). Plant Cell Tiss. Org. 2021, 145, 701–705. [Google Scholar] [CrossRef]
- Lin, W.; Xiao, X.; Zhang, H.; Li, Y.; Liu, S.; Sun, W.; Zhang, W.; Wu, Q. Whole-Genome Bisulfite Sequencing Reveals a Role for DNA Methylation in Variants from Callus Culture of Pineapple (Ananas comosus L.). Genes 2019, 10, 877. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Akdemir, H.; Suzerer, V.; Tilkat, E.; Onay, A.; Ozden, Y. Detection of variation in long term micro propagated mature pistachio via DNA based molecular markers. Appl. Biochem. Biotechnol. 2016, 180, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Piven, N.M.; Barredo, F.A.; Borges, I.A.; Herrera, M.A.; Mayo, A.; Herrera, J.L.; Robert, M.L. Reproductive Biology of Henequén (Agave fourcroydes) and its wild ancestor Agave angustifolia (Agavaceae). I. Gametophyte development. Am. J. Bot. 2001, 88, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198, p. 322. [Google Scholar]
- Tapia-Tussell, R.; Quijano-Ramayo, A.; Rojas-Herrera, R.; Larque-Saavedra, A.; Perez-Brito, D. A fast, simple and reliable high-yielding method for ADN extraction from different plant species. Mol. Biotechnol. 2005, 31, 137–139. [Google Scholar] [CrossRef]
- Wang, J.S.; He, J.H.; Chen, J.R.; Chen, Y.Y.; Qiao, F. Genetic Diversity in Various Accessions of Pineapple (Ananas comosus (L.) Merr.) using ISSR and SSR Markers. Biochem. Genet. 2017, 55, 347–366. [Google Scholar] [CrossRef]
- Weiguo, Z.; Zhihua, Z.; Xuexia, M.; Sibao, W.; Lin, Z.; Yile, P.; Yongping, H. Genetic relatedness among cultivated and wild mulberry (Moraceae: Morus) as revealed by inter-simple sequence repeat analysis in China. Can. J. Plant Sci. 2005, 86, 251–257. [Google Scholar] [CrossRef]
- Anderson, J.A.; Churcill, G.A.; Autrique, J.E.; Tanksley, S.D.; Sorrels, M.E. Optimizing parental selection for genetic linkage maps. Genome 1992, 36, 181–186. [Google Scholar] [CrossRef]
- Yancheva, S.D.; Golubowicz, S.; Fisher, E.; Lev-Yadun, S.; Flaishmanm, A. Auxin type and timing of application determine the activation of the developmental program during in vitro organogenesis in apple. Plant Sci. 2003, 165, 299–309. [Google Scholar] [CrossRef]
- Sripaoraya, S.; Power, B.J.; Davey, M.R. Plant regeneration by somatic embryogenesis and organogenesis in commercial pineapple (Ananas comosus L.). In Vitro Cell. Dev. Biol.-Plant 2003, 39, 450–454. [Google Scholar] [CrossRef]
- Firoozabady, E.; Moy, Y. Regeneration of pineapple plants via somatic embryogenesis and organogenesis. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 67–74. [Google Scholar] [CrossRef]
- Kouadio, O.; Kouadio, S.; Sopie, Y.; Kouassi, K.; Oumar, S.; Koffi, F.; Kouakou, T. Improved callogenesis and somatic embryogenesis using amino acids and plant growth regulators combination in pineapple [Ananas comosus (L.) Merr. (Bromeliaceae). Eur. J. Biotechnol. Biosci. 2017, 5, 6–16. [Google Scholar]
- Cisnero, A.; Daquinta, M.; Rodriguez, Y.; Escalona, M.; Luna, I.; Borroto, C. Influencia de los reguladores del crecimiento clorinados en la embriogénesis somática en piña. Rev. Bras. Frutic. 1996, 18, 269–274. [Google Scholar]
- Roostika, I.; Khumaida, N.; Wahyuning, S. RAPD analysis to detect somaclonal variation of pinneaple in vitro cultures during micropropagation. Biotropia 2015, 22, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Bennici, A.; Mori, B.; Tani, C.; Bussi, B. Callogenesis and organogenesis in pineapple: A histological and ultrastructural study of developing callus and morphogenic processes. Adv. Hortic. Sci. 2007, 21, 19–27. [Google Scholar]
- Paola, J.; Schmidt, C.E.; Guerra, M.P.; Bouzon, Z.; Dal Vesco, L.L.; Pescador, R. Histodifferentiation and ultrastructure of nodular cultures from seeds of Vriesea friburgensis Mez var. paludosa L.B. Smith and leaf explants of Vriesea reitzii Leme and A. Costa (Bromeliaceae). J. Microsc. Ultra. 2015, 3, 200–209. [Google Scholar]
- Paola, J.; De Conti, D.; Guerra, M.P.; Dal Vesco, L.L.; Pescador, R. Dynamics of proteins, carbohydrates and global DNA methylation patterns during induction of nodular cluster cultures from seeds of Vriesea reitzii. Acta Scientiarum. Agron. 2020, 42, 442–448. [Google Scholar] [CrossRef]
- Makaranga, A.; Songelael, M.; Ndee, A.; Mneney, E.; Mbwambo, G.; Lema, K.; Godfrey, A.; Kachiwile, M.; Mrema, M.; Theodosy, J. Diversity and genetic identity of pineapple [Ananas comosus (L.) Merr.] in Tanzania based on microsatellite markers. Afr. J. Biotechnol. 2018, 17, 811–817. [Google Scholar] [CrossRef]
- Leite, R.; Fortes, C.; Da Silva, C.A.; Hilo, E.; Henrique, P.; Pereira, M.A.; Duarte, F.V. Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tiss. Org. 2016, 127, 123–133. [Google Scholar] [CrossRef]
- Borsai, O.; Harța, M.; Szabo, K.; Kelemen, C.D.; Andrecan, F.A.; Codrea, M.M.; Clapa, D. Evaluation of genetic fidelity of in vitro propagated blackberry plants using RAPD and SRAP molecular markers. Hortic. Sci. 2020, 47, 21–27. [Google Scholar] [CrossRef]
- Norhayati, S.I.; Abdul, S.A.; Ab, S.F.; Zainal, R.A.; Mohd, M.F.; Zubir, N.M.; Zaynol, R. Genetic Diversity of Pineapple (Ananas comosus) Germplasm in Malaysia Using Simple Sequence Repeat (SSR) Markers. Trop. Life Sci. Res. 2020, 31, 15. [Google Scholar] [CrossRef]








| Treatments | NAA (mg L−1) | BAP (mg L−1) | 2,4-D (mg L−1) | P (mg L−1) |
|---|---|---|---|---|
| M1 | 5 | |||
| M2 | 2 | 5 | ||
| M3 | 2 | 2 | ||
| M4 | 1 | 1 | ||
| M5 | 1 | |||
| M6 | 1 | 1 | ||
| M7 | 2 | 2 | ||
| M8 | 3 | |||
| M9 | 0.05 | 3 | ||
| M10 | 0.05 | 5 |
| Treatments | Percentage Exp. Morph. Resp. | Morph. Resp. Type | Number of Embryos per Explant | Number of Shoots per Explant |
|---|---|---|---|---|
| M1 | 33.3 | DO | 20.0 ± 1.7 e | |
| M2 | 66.6 | IO | 30.0 ± 2.22 c | |
| M3 | 88.8 | DSE | 50.0 ± 2.63 b | 50.0 ± 2.63 b |
| M4 | 88.8 | DO | 25.0 ± 2.63 d | |
| M5 | 44.4 | IO | 20.0 ± 1.64 e | |
| M6 | 55.5 | IO | 25.0 ± 2.01 d | |
| M7 | 93.3 | ISE | 120.0 ± 8.18 a | 120.0 ± 8.18 a |
| M8 | 48.8 | IO | 22.0 ± 2.63 e | |
| M9 | 55.5 | IO | 25.0 ± 2.53 d | |
| M10 | 44.4 | IO | 20.0 ± 1.37 e | |
| Chi-square | 28.821 * | - | 18.778 * | 38.002 * |
| Primer | Sequences (5′-3′) | Alignment Temperature (°C) | Monomorphic Bands (%) |
|---|---|---|---|
| DT 338176 | F 5′-CTCCTCATCTACCGCACCTC-3′ R 5′-CCCTAGACGACGACGAAGAG-3′ | 60 | 1 |
| DT 336954 | F 5′-CATCCATCCATCCATCCAAT-3′ R 5′-GTCGTTGATCATTCGCAAAA-3′ | 60 | 2 |
| DT 3365 61 | F 5′-GCAAATGAGGCCACAAACTT-3′ R 5′-GGGTGGTGTGGACTTTCTCT-3′ | 60 | 1 |
| DT 336932 | F 5′-GCATGCCAAAGGAAAGAGTT-3′ R 5′-CCCTGAACAAATCACCCAAC-3′ | 60 | 2 |
| DT 337038 | F 5′-CCCTGAAGGTGGAGATTGTG-3′ R 5′-AAAAACCAAAACCCTGGACA-3′ | 60 | 1 |
| DT 338091 | F 5′-GCTGCTCTTGCTGCCAT-3′ R 5′-AAGCCATAGGACCACCAC-3′ | 60 | 2 |
| CO 731287 | F 5′-AGGGAAGCTTTGGAGGTCCAG-3′ R 5′-TGCAATAGCGATGATAAACCCCAG-3′ | 60 | 1 |
| CO 731629 | F 5′-AGAAGCGGAAGCGTGTTG-3′ R 5′-GCGGAGATCGAAGCACTC-3′ | 60 | 1 |
| CO 73 12 35 | F 5′-ATTTCGAGCCCTTGGTCG-3′ R 5′-TTTATGGGGTCGCGTCGG-3′ | 60 | 1 |
| CO 73 0888 | F 5′-CGCATCAGCGCCAAACGC-3′ R 5′-GGAAGCGAAAGGAGATCG-3′ | 60 | 2 |
| CO 73 18 16 | F 5′-CTCCTCAGCTTCGTCGCC-3′ R 5′-GACGAGATTGGCGTATCCC-3′ | 60 | 1 |
| AJ 845056 | F 5′-TGCTGGCTCTGTGGGATG-3′ R 5′-TTAGGTTTTCAGTGGAGAGAG-3′ | 60 | 1 |
| AJ 845081 | F 5′-ACATTCCTCAGAGTCACCAGC-3′ R 5′-CACTAATCCTTGACCCAGACC-3′ | 55 | 2 |
| AJ 845060 | F 5′-TGTAGGCATATGGTGGGTCTG-3′ R 5′-ATCTCTTAATCCAAGGGCCG-3′ | 60 | 2 |
| AY 098521 | F 5′-GTATATCGTGGATGCGGGAG-3′ R 5′-AGCATCAAGGGGTCCCGAGTT-3′ | 55 | 1 |
| Primer | Sequence (3′-5′) | Alignment Temperature (°C) | Total Number of Bands | Number of Polymorphic Bands | Number of Monomorphic Bands | PIC | Monomorphism (%) |
|---|---|---|---|---|---|---|---|
| IS 01 | (GACA)4 | 54 | 4 | 1 | 3 | 0.25 | 75 |
| IS 13 | (CT)8GT | 54 | 8 | 1 | 7 | 0.30 | 87.5 |
| IS 14 | (AG)8TA | 54 | 10 | 0 | 10 | 0 | 100 |
| IS 17 | (TG)8GT | 54 | 5 | 0 | 5 | 0 | 100 |
| IS 19 | (AG)8TC | 54 | 12 | 1 | 11 | 0.31 | 91.67 |
| Treatments | TBMT/TBM | Monomorphism (%) |
|---|---|---|
| M1 | 34/35 | 97.14 |
| M2 | 34/35 | 97.14 |
| M3 | 35/35 | 100 |
| M4 | 35/35 | 100 |
| M5 | 35/35 | 100 |
| M6 | 35/35 | 100 |
| M7 | 35/35 | 100 |
| M8 | 34/35 | 97.14 |
| M9 | 34/35 | 97.14 |
| M10 | 33/35 | 94.26 |
| Donor Plant | 35/35 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessel-Domini, A.; Pérez-Brito, D.; Guzmán-Antonio, A.; Barredo-Pool, F.A.; Mijangos-Cortés, J.O.; Iglesias-Andreu, L.G.; Cortés-Velázquez, A.; Canto-Flick, A.; Avilés-Viñas, S.A.; Rodríguez-Llanes, Y.; et al. Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture 2022, 12, 713. https://doi.org/10.3390/agriculture12050713
Kessel-Domini A, Pérez-Brito D, Guzmán-Antonio A, Barredo-Pool FA, Mijangos-Cortés JO, Iglesias-Andreu LG, Cortés-Velázquez A, Canto-Flick A, Avilés-Viñas SA, Rodríguez-Llanes Y, et al. Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture. 2022; 12(5):713. https://doi.org/10.3390/agriculture12050713
Chicago/Turabian StyleKessel-Domini, Argelys, Daisy Pérez-Brito, Adolfo Guzmán-Antonio, Felipe A. Barredo-Pool, Javier O. Mijangos-Cortés, Lourdes Georgina Iglesias-Andreu, Alberto Cortés-Velázquez, Adriana Canto-Flick, Susana A. Avilés-Viñas, Yaritza Rodríguez-Llanes, and et al. 2022. "Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”" Agriculture 12, no. 5: 713. https://doi.org/10.3390/agriculture12050713
APA StyleKessel-Domini, A., Pérez-Brito, D., Guzmán-Antonio, A., Barredo-Pool, F. A., Mijangos-Cortés, J. O., Iglesias-Andreu, L. G., Cortés-Velázquez, A., Canto-Flick, A., Avilés-Viñas, S. A., Rodríguez-Llanes, Y., & Santana-Buzzy, N. (2022). Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture, 12(5), 713. https://doi.org/10.3390/agriculture12050713